Abstract
The 1, 3-dipolar cycloaddition of trimethylsiyl diazomethane with α, β-unsaturated esters was examined. The resulting 1-pyrazolines isomerize to regioisomeric 2-pyrazolines (a or b) or undergo desilylation (c). Acrylates yield only b or c. β-substituted dipolarophiles may yield all three types of products. This work demonstrates that the distribution of 2-pyrazoline products is highly dependant on the relative configuration of the substituents on the 1-pyrazoline intermediate.
Trimethylsilyldiazomethane has been most frequently used as a source of carbene for cyclopropanation reactions1 and has enjoyed only limited synthetic utility in 1, 3-dipolar cycloadditions. In fact, Seyferth reported that among a series of dipolarophiles examined, only acrylonitrile reacted with TMS diazomethane to produce a cycloadduct in a synthetically useful yield.2, 3 Recently, with its commercial availability, TMS diazomethane has become somewhat more popular as a synthetic reagent, particularly in the preparation of novel amino acid analogs.4,5
1, 3-Dipolar cycloadditions of diazoalkanes and alkenes yield 1-pyrazolines. In most instances, when the dipolarophile is an α,β-unsaturated ester, HOMO-LUMO interactions dictate that the regioselectivity is such that the carbon atom of the diazoalkane attacks the β-carbon of the ester (Scheme 1). 1-Pyrazolines tend to be unstable and isomerize to the 2-pyrazolines with the regioselectivity of the isomerization dependent on the substituents. When the dipolarophile is an α,β-unsaturated carbonyl compound, isomerization typically yields the conjugated 2-pyrazoline.6, 7
Scheme 1.
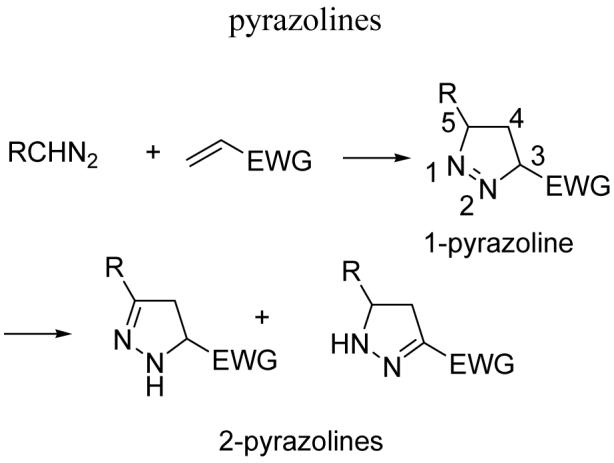
1, 3-Dipolar cycloaddition of diazoalkanes followed by isomerization yields 2-pyrazolines
A survey of the recent literature indicates that the regioselectivity of the double bond isomerization of TMS-substituted 1-pyrazolines does not always follow the typical course and is, at first glance, unpredictable. Carreira and Kanemasa have reported the 1, 3-dipolar cycloaddition of TMSCHN2 with camphorsultam and oxazolidinone derivatives, respectively.4, 5, 8, 9. These 1-pyrazoline products undergo proteodesilylation to yield 2-pyrazolines (1 and 2) or loss of the proton α to TMS (3). On the other hand, Barluenga et al.10 recently described the cycloaddition of the menthol ester of trans-cinnamic acid to produce the conjugated 2-pyrazoline, with retention of the TMS group (4).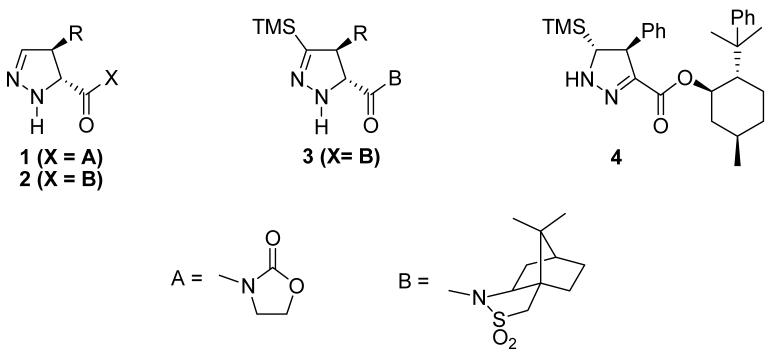
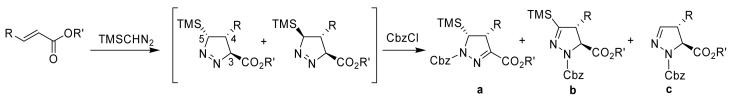
In an effort to develop a mechanistic rational for the cycloaddition/ isomerization process, which could account for the products obtained, we undertook a systematic survey of 1, 3-dipolar cycloadditions between TMS diazomethane and α, β-unsaturated esters. The dipolarophiles vary in terms of the size of the ester group and the substituents at the β-carbon. The results of these studies are summarized in Table 1. Because 1 and 2-pyrazolines oxidize readily to pyrazoles, the cycloaddition products were immediately converted to the Cbz (benzyloxycarbonyl) derivatives of the 2-pyrazolines for characterization. However, we were able to observe the 1-pyrazolines or 2-pyrazolines by 1H NMR. This allowed us to make assignments of relative configurations of the cycloadducts.
Table 1.
1, 3-Dipolar cycloadditions of TMS diazomethane and various dipolarophiles
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Cmpd. | R | R’ | Cycloaddition Conditions | %Yielda | Product Ratio a:b:c | Relative configuration of 1-pyrazoline (3, 5 trans: 3, 5-cis) |
| 1 | 5 | H | Me | 2 eq. TMSCHN2, PhCH3 / hexane (1:1), rt, 8.5 hr | 20b | 0:60:40 | Not determined |
| 2 | 6 | H | Et | 1.5 eq. TMSCHN2, C6H6/ hexane (1:1), rt, 24 hr | 90 | 0:35:65 | 50:50 |
| 3 | 7 | H | t-Bu | 1.2 eq. TMSCHN2, C6H6/ hexane (1:1), rt, 24 hr | 71 | 0:44:56 | 50:50 |
| 4 | 8 | H | Mtc | 1.5 eq. TMSCHN2, C6H6/ hexane (1:1), rt, 24 hr | 50 | 0:45:55 | 50:50 |
| 5 | 9 | Me | Et | 3 eq. TMSCHN2, C6H6/ hexane (1:1), reflux, 8.5 hr | 40 | 0:37:63 | 6:94 |
| 6 | 9 | Me | Et | 3 eq. TMSCHN2, Toluene, reflux, 8.5 hr | 49 | 16:16:68 | 50:50 |
| 7 | 10 | Me | t-Bu | 2 eq. TMSCHN2, C6H6/ hexane (1:1), reflux, 8 hr | 47 | 0:76:24 | Not determined |
| 8 | 11 | Me | Mt | 2 eq. TMSCHN2, C6H6/ hexane (1:1), reflux, 8 hr | 62 | 0:5050 | Not determined |
| 9 | 12 | Ph | Et | 2 eq. TMSCHN2, C6H6/ hexane (1:1), rt, 8 hr | 7.5 | 5:20:75 | 7:93 |
| 10 | 12 | Ph | Et | 2 eq. TMSCHN2, C6H6/ hexane reflux, 8 hr | - | Complex mixture | Not determined |
| 11 | 13 | Ph | t-Bu | 2 eq. TMSCHN2, C6H6/ hexane (1:1), reflux, 8 hr | 35 | 11:33:56 | 10:90 |
| 12 | 13 | Ph | t-Bu | 2 eq. TMSCHN2, Toluene, reflux, 8.5 hr (1:1), reflux, 8 hr | 45 | 43:43:14 | 50:50 |
| 13 | 14 | Ph | Mt | 2 eq. TMSCHN2, C6H6/ hexane (1:1), reflux, 8 hr | 71 | 24:0:76 | 100:0 (2-pyrazoline) |
Isolated yield.
Yield based on acryloyl chloride.
Mt = menthyl.
As shown in Table 1, three products may be obtained from the isomerization of the intermediate 1-pyrazoline: the conjugated 2-pyrazoline (a), isomerization with loss of the proton α to the TMS group (b), or the desilylation product (c). In contrast to earlier reports, we found that the acrylates react with TMSCHN2 at ambient temperature to provide cycloadducts in good to excellent yield (Entries 1-4). Products b and c were formed exclusively and with little selectivity. The β-substituted dipolarophiles (Entries 5-13) require elevated temperatures or extended reaction times to provide cycloadducts in moderate yields. Previous reports have indicated that the 1, 3-dipolar cycloaddition with TMS diazomethane is suprafacial with respect to the dipolarophile4, 5, 8, 9 and our data is consistent with this as well. The crotonate esters, like the acrylates, yield products b and c when the cycloaddition reaction is performed in refluxing benzene/hexane (Entries 5, 7 and 8). However, the product distribution appears to be highly sensitive to the temperature of the cycloaddition, as performing the reaction with ethyl crotonate in refluxing toluene yields a mixture of 9a, 9b, and 9c upon protection (Entry 6). Under reflux in benzene/hexane, ethyl cinnamate produces a complex mixture of at least eight products (Entry 10). However, a mixture of 12a, 12b and 12c could be isolated in poor yield after performing the reaction at room temperature (Entry 9). Ethyl and t-butyl cinnamates (Entries 9-12) behave like the crotonates, in that products 12b/13b and 12c/13c are formed predominantly with the proportion of 12a and 13a increasing with increasing temperature (Entries 11 and 12). Surprisingly, in refluxing benzene/hexane, menthyl cinnamate yields a mixture of 14a and 14c with no 14b observed (Entry 13).
While we were unable to isolate 1-pyrazolines, in most cases they could be observed by 1H NMR immediately after the cycloaddition step. The only exception to this was menthyl cinnamate, in which we observed a 2-pyrazoline (vide infra). Therefore, we can conclude that (with this single exception) the isomerization does not take place until the protection step. Furthermore, as the isomerization/protection step was performed under identical conditions in all examples, we must conclude that the regioselectivity of the isomerization is dependant on the distribution of the stereoisomeric 1-pyrazolines formed in the cycloaddition step. These observations prompted us to examine the distribution of 1-pyrazolines more carefully.
Analysis of the crude reaction mixtures of acrylates (Entries 2-4) with TMSCHN2 by 1H NMR revealed the presence of 3, 5-cis and 3, 5-trans 1-pyrazoline cycloadducts, in an approximate 1:1 ratio. Apparently, the size of the ester group has little influence on the relative configuration of the newly generated stereocenters, at least not for the acrylates. We assume that both the cis and trans isomers lead to products b and c, although we cannot be certain about this as we were unable to either separate the cis and trans 1-pyrazolines or to prepare one isomer selectively.
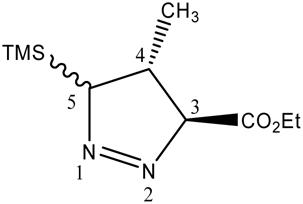
In contrast to the acrylates, the 1H NMR of the 1-pyrazoline derived from the cycloaddition of ethyl crotonate and TMSCHN2 in refluxing benzene/hexane indicated the presence of a mixture of cycloadducts in a ratio of 6:94 (Entry 5). It is often difficult to assign relative configurations of five-membered rings on the basis of coupling constants alone. In fact, Macromodel 11 predicts nearly the same 1H coupling constants for the 4, 5-cis and 4, 5-trans 1-pyrazolines. The assignment of relative configurations for the major 1-pyrazoline was based on difference NOE (Table 2). Irradiation of H-4 produces an NOE of 5% at the TMS group and the same NOE (2%) at H-3 and H-5. Since H-3 and H-4 must be trans, this suggests that H-4 and H-5 are also trans. Additionally, irradiation of the methyl group produces essentially the same NOE (3.8 and 3.3%) at both H-3 and H-5 and results in no NOE for TMS. These observations indicate that the relative configuration of the major 1-pyrazoline is 3, 4-trans-4, 5-trans and the minor 1-pyrazoline as 3, 4-trans-4, 5-cis. When the cycloaddition is performed with ethyl crotonate in refluxing toluene (Entry 6), the stereoisomeric 1-pyrazoline cycloadducts are observed, in a ratio of 1:1. Based on the isolation of 9a from this mixture of 1-pyrazolines, we conclude that only the 3, 4-trans-4, 5-cis isomer gives rise to product 9a. Like ethyl crotonate, 1H NMR revealed that a major 1-pyrazoline cycloadduct is produced with ethyl cinnamate at room temperature (Entry 9). By analogy with ethyl crotonate, and because this also yields predominantly protected 2-pyrazolines 12b and 12c, the major cycloadduct was assigned the 3, 4-trans-4, 5-trans relative configuration. Both ethyl and t-butyl cinnamate produce predominantly one stereoisomeric 1-pyrazoline (Entries 9 and 11) and for t-butyl cinnamate, the percentage of the minor cycloadduct increases at elevated temperature (Entry 12) as does the proportion of 13a.
Table 2.
Difference NOE of 1-pyrazoline, ethyl 4-methyl-5-(trimethylsilyl)-4,5-dihydro-3H-pyrazole-3-carboxylate (the major isomer from the cycloaddition in refluxing benzene/ hexane)
 | ||
|---|---|---|
| Irradiate | ||
| Observe | H-4 | 4-CH3 |
| H-3 (δ 4.73) | 2% | 4% |
| H-4 (δ 2.16) | - | 4% |
| H-5 (δ 3.95) | 2% | 3% |
| 4-CH3 (δ 1.03) | 15% | - |
| TMS (δ 0.14) | 5% | 0% |
Menthyl cinnamate behaved differently from all other dipolarophiles. We were unable to observe the 1-pyrazoline cycloadduct. Instead, the 1H NMR taken immediately after the cycloaddition step indicated that isomerization to the 2-pyrazolines had occurred. Due to the additional stereocenter in the menthyl group, two stereoisomeric 2-pyrazolines were formed in a ratio of 1.7:1. While we were unable to assign relative configurations for any of the 1-pyrazolines or protected 2-pyrazolines based on coupling constants, we were able to make the assignment from the unprotected 2-pyrazoline which corresponds to 14a. A 12 Hz coupling constant between H-4 and H-5 in both isomers indicated that these cycloadducts had the 3, 4-trans-4, 5-cis relative configuration. Additionally, hydrogenolysis of purified 14a was identical to the 2-pyrazoline observed after the cycloaddition.
From our observations, we conclude that the cycloaddition of acrylates is not stereoselective. However, the introduction of a β-substituent on the dipolarophile increases steric demand in the transition state and dictates that TMS be trans to the β-substituent in the preferred cycloadduct. The selectivity for the 4, 5-trans cycloadduct is diminished with elevated temperature and as the size of the ester group increases. The influence of the increasing size of the ester group is seen earlier for the cinnamates when compared to the crotonates, as the planar phenyl group is less sterically demanding. For menthyl cinnamate, the influence of the phenyl group is entirely overcome by the large menthyl ester resulting in the exclusive formation of the 4, 5-cis relative configuration.
It is also interesting to note that when the cycloaddition of ethyl cinnamate is attempted in refluxing benzene/ hexane, a complex mixture of at least eight products is obtained. Yet, both t-butyl and menthyl cinnamate gave the typical two or three products. While we did not attempt to purify the products from the mixture obtained from ethyl cinnamate, we suggest that the additional products arise from alternative regioisomers. These different behaviours may be attributed to a competition between stereoelectronic and steric influences. FMO calculations demonstrate that having a phenyl group as β-substituent on the dipolarophile can diminish the regioselectivity of cycloaddition. Attack by the carbon atom of the diazoalkane at the α-carbon of the ester becomes competitive with attack at the β-carbon. Tables 3 and 4 give energies and atomic contributions for the HOMO and LUMO of dipolarophiles ethyl acrylate, ethyl crotonate, and ethyl cinnamate and of diazomethane and TMSCHN2 calculated at the B3LYP/TZVP level. In all cases, the dominant interaction is between the HOMO of the diazo compound and the LUMO of the dipolarophile. The LUMO energies are relatively insensitive to dipolarophile’s substituents. However, the atomic contributions to the LUMO are sensitive to the dipolarophile’s β-substituent. For 6 and 9, the contribution of C-2 to the LUMO is about twice that of C-3. Since the dominant contribution to the HOMO of TMSCHN2 is from C-3, the principle of maximum overlap leads to the observed regioselectivity. However, in the case of 12, the contributions of C-2 and C-3 to the LUMO are nearly equal, although C-2 still makes the larger contribution (0.47 vs 0.37). Thus at low temperature (Table 1, Entry 9), C-2 still dominates and the regioselectivity is the same as for 6 and 9; but at higher temperature (Entry 10), C-3 is able to compete with C-2 and both regioisomers are observed, leading to a large number of products. We note that when all orientations for cycloaddition are considered, eight distinct 2-pyrazolines can be formed upon isomeration and possible loss of TMS.
Table 3.
Energies (eV) of HOMO and LUMO and largest atomic contribution to LUMO for selected dipolarophiles
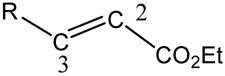 | ||||
|---|---|---|---|---|
| R | EHOMO | ELUMO | a(C-2) | a(C-3) |
| H | -7.8 | -1.6 | 0.79 | 0.36 |
| Me | -7.5 | -1.3 | 0.78 | 0.35 |
| Ph | -6.7 | -2.1 | 0.47 | 0.37 |
Table 4.
Energies of HOMO and LUMO and largest atomic contributions to HOMO for selected diazo compounds
| Cmpd. | EHOMO | ELUMO | a(N-1) | a(N-2) | a(C-3) |
|---|---|---|---|---|---|
| CH2N2 | -6.3 | -1.3 | 0.68 | 0.04 | 1.09 |
| TMSCHN2 | -6.1 | -1.6 | 0.75 | 0.04 | 1.14 |
This behavior is not observed for larger ester groups (O-t-Bu or OMt rather than OEt) in combination with a phenyl group as R. Apparently the steric interaction between TMS and the larger esters sufficiently disfavors alternative regioisomers that the stereoelectronic leveling influence of the phenyl substituent is not observed.
In summary, we have demonstrated that the steric demand of the dipolarophile influences the relative stereochemistry of 1, 3-dipolar cycloaddition reactions with TMSCHN2. In turn, the distribution of stereoisomeric 3, 4, 5-trisubstituted 1-pyrazolines has a profound influence on the outcome of the isomerization step. These steric effects are absent in the cycloaddition of acrylates and only products b and c are formed. One can rationalize that the isomerization to products of type a would be disfavored as this would increase torsional strain between the substituents. However, for β-substituted dipolarophiles, the β-substituent will generally prefer to be trans to TMS unless the ester group is large. The 3, 4-trans-4, 5-trans 1-pyrazolines isomerize to a mixture of the b and c type products, whereas the 3, 4-trans-4, 5-cis 1-pyrazolines isomerize to a mixture of a and c. The reasons for this preference are as yet unclear and are currently under investigation. However, it is now apparent that the product distribution may be controlled through the appropriate choice of ester.
Experimental
General procedure for cycloaddition/ protection reactions: Cycloaddition reactions were performed under the conditions described in Table 1. Upon completion, the solvent was removed in vacuo and the residue dissolved in CH2Cl2. Carbobenzyloxy chloride (1.1 equivalent) and a solution of NaHCO3 (1.5 equivalent) in H2O were sequentially added to the solution of the 1-pyrazoline at room temperature. The resulting mixture was stirred at room temperature overnight. The organic layer was extracted with CH2Cl2 (3 × 15mL), dried over Na2SO4, and filtered. Solvents were evaporated under reduced pressure and the residue was purified by automated flash, gravity or radial chromatography over silica gel with hexane/ethyl acetate 4:1 as eluants.
Supplementary Material
Acknowledgements
This is contribution number P200611 from the Center of Excellence in Biomedical and Marine Biotechnology. The work was supported in part by a Faculty Research Enhancement Award to K.S.R. funded by the NICHD/EARDA program (G11HD038341). The support of US ARO (W911NF-04-1-0022) for the purchase of 600 MHz NMR spectrometer is acknowledged. M.D. is grateful for a Presidential Enhanced Assistantship and a Dissertation Year Fellowship from the University Graduate School.
References and Footnote
- 1.Haszeldine RN, Scott DL, Tipping AE. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999) 1974;12:1440–1443. [Google Scholar]
- 2.Seyferth D, Dow AW, Menzel H, Flood TC. J. Am. Chem. Soc. 1968;4:1080–1082. [Google Scholar]
- 3.Seyferth D, Menzel H, Dow AW, Flood TC. Journal of Organometallic Chemistry. 1972;2:279–290. [Google Scholar]
- 4.Mish MR, Guerra FM, Carreira EM. J. Am. Chem. Soc. 1997;35:8379–8380. [Google Scholar]
- 5.Sasaki H, Carreira EM. Synthesis. 2000;1:135–138. [Google Scholar]
- 6.Galley G, Paetzel M, Jones PG. Tetrahedron. 1995;6:1631–1640. [Google Scholar]
- 7.Bartels A, Liebscher J. Tetrahedron: Asymmetry. 1994;8:1451–1452. [Google Scholar]
- 8.Kanemasa S, Kanai T. J. Am. Chem. Soc. 2000;43:10710–10711. [Google Scholar]
- 9.Whitlock GA, Carreira EM. Helv. Chim. Acta. 2000;8:2007-2007–2022. [Google Scholar]
- 10.Barluenga J, Fernandez-Mari F, Viado AL, Aguilar E, Olano B, Garcia-Granda S, Moya-Rubiera C. Chemistry--A European Journal. 1999;3:883–896. [Google Scholar]
- 11.Mohamadi F, Richards NGJ, Guida WC, Liskamp R, Lipton M, Caufield C, Chang G, Hendrickson T, Still WC. Journal of Computational Chemistry. 1990;4:440–467. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


