SUMMARY
The physiologic control of cytokine receptor activation is primarily mediated by reciprocal activation of receptor-associated protein tyrosine kinases and protein tyrosine phosphatases (PTPs). Here, we show that immediately following ligand-dependent activation, IL-4 receptor induces an intracellular calcium flux via IRS-PI3K-PLC-γ pathway which, in turn, induces PKC-dependent activation of NAD(P)H oxidase (NOX)5 that generates reactive oxygen species (ROS). IL-4 also induces NOX1-mediated ROS production via IRS-PI3K-RAC1 pathway. ROS, in turn, promote IL-4 receptor activation by oxidatively inactivating PTP1B that physically associates with and deactivates IL-4 receptor. However, ROS are not required for the initiation of IL-4 receptor activation. ROS generated by activated EPO-, TNF-α- or IL-3 receptor also promote IL-4 signaling. These data reveal that inactivation of receptor-associated PTP-activity by cytokine-generated ROS is a physiologic mechanism for the amplification of cytokine receptor activation in both cis and trans, unfolding a novel means of cytokine signaling cross-talk.
INTRODUCTION
Interleukin (IL)-4 is an immunomudulatory, type I cytokine secreted by activated Th2-lymphocytes, basophils and mast cells (Haque and Sharma, 2006; Nelms et al., 1999). It executes pleiotropic functions including induction of Th2-differentiation, immunoglobulin class-switching, B cell proliferation, suppression of Th1-differentiation and macrophage activation among others (Nelms et al., 1999). IL-4, like many other cytokines, initiates transmembrane signaling by tyrosine phosphorylation of cognate receptors. Protein tyrosine phosphorylation is a tightly regulated, reversible process in which the forward reaction is catalyzed by receptor-associated JAKs and the reverse reaction by protein tyrosine phosphatases (PTPs) (Haque and Sharma, 2006; Heldin, 1995). In the absence of cytokine engagement, the receptor-associated PTP dominates over the JAK, thereby holding the receptor in an inactive state (Fischer et al., 1991; Haque and Sharma, 2006). Binding of cytokine induces aggregation of receptor chains leading to the trans-phosphorylation of JAKs on signature tyrosine residues. This increases JAKs’ catalytic activities that promote the forward reaction, thereby stabilizing the receptor activation (Haque and Sharma, 2006; O'Shea et al., 2002).
This is an established mechanism for receptor activation by a majority of cytokines including IL-4 (Haque and Sharma, 2006). However, in principle, inactivation of receptor-associated PTP(s) could be an alternate means of receptor activation. As a proof of the principle, we have previously demonstrated that blockade of IL-4 receptor-associated PTP activity by pervanadate (PV) induces receptor activation in the absence IL-4 binding (Haque et al., 1997). However, PV is a non-physiologic agent that irreversibly inactivates PTPs. Recent studies have shown that catalytic cysteines of PTPs are reversibly oxidized and inactivated by reactive oxygen species (ROS) including superoxide and hydrogen peroxide (Rhee et al., 2000). ROS are produced by the NAD(P)H oxidase (NOX) family enzymes in response to cytokine or growth factor stimulation of cells (Rhee et al., 2000). The NOX family is comprised of seven members, NOX1 through NOX5, DUOX1 and DUOX2, each exhibiting cell type-specific expression (Lambeth, 2004; Lambeth et al., 2007). Activation of NOX1, NOX2 and NOX3 requires specific regulatory subunits, whereas NOX5L (long form), DUOX1 and DUOX2 require calcium but no regulatory subunits, for activation (Lambeth, 2004; Lambeth et al., 2007). NOX4 activity is mostly constitutive but could be activated by p22phox (Lambeth, 2004; Lambeth et al., 2007).
IL-4 activates two types of receptors. The type I receptor is comprised of the JAK1-bound IL-4Rα nand JAK3-bound γc. Many non-hematopoietic cells that do not express γc and JAK3, utilize the type II receptor in which IL-4Rα associates with JAK2 (or TYK2)-bound IL-13R1 (Nelms et al., 1999). Binding of IL-4 to IL-4Rα induces JAK1-mediated phosphorylation of multiple tyrosine residues in the cytoplasmic region of IL-4Rα. This, in turn, activates two major downstream pathways, IRS-PI3K and STAT6 (Haque and Sharma, 2006; Nelms et al., 1999). Since signal transduction, in general, is limited in magnitude and duration, these pathways must be uncoupled by dephosphorylation of the activated receptor.
Here, we demonstrate that activated IL-4 receptor generates ROS by IRS-PI3-Kmediated, calcium-dependent and -independent activation of NOX5 and NOX1 respectively. We also show that IL-4 increases intracellular calcium flux that is required for NOX5 activation. ROS promote IL-4 receptor activation and subsequent signal transduction by oxidative inactivation of PTP1B, a ubiquitously expressed PTP that deactivates the IL-4 receptor. Further, we provide evidence that ROS generated by other activated cytokine receptors, like IL-3R, EPOR and TNFR markedly promote IL-4 receptor signaling in the same cells, unfolding a role for ROS in cytokine cross-talk.
RESULTS
IL-4 Induces ROS production which Promote Receptor Activation and Signal Transduction
We have previously demonstrated that IL-4 receptor-associated PTP activity is susceptible to inactivation by PV which is an exogenous oxidant (Haque et al., 1997). Here, we demonstrate, for the first time, that IL-4 stimulation of A549 cells generated endogenous oxidants, ROS, within 10 sec (Figures 1A & B), which reached a peak at ~15 min, and declined thereafter (Figure S1). Although the fluorescence probe, 5-(and-6)-chloromethyl-2',7'-dichloro-dihydrofluorescein diacetate (CM-H2DCFDA) used for ROS measurement, can also detect reactive nitrogen species (RNS), pretreatment of cells with an inhibitor of nitric oxide synthase, L-NAME (Kubes et al., 1991), did not reduce IL-4-generated fluorescence intensity, indicating that in response to IL-4, A549 cells did not generate RNS (Figure 1C). To examine if NOX-family members were involved in IL-4-induced ROS production, ROS were measured in A549 cells pretreated with diphenylene iodonuim (DPI), an inhibitor of flavoprotein (Cross and Jones, 1986), and apocynin that inhibits NOX activity (Fu et al., 2006). Both inhibitors completely blocked IL-4-induced ROS production (Figure 1C) suggesting that NOX-family enzymes were involved in this process. Importantly, these inhibitors significantly reduced IL-4-dependent STAT6 activation measured by EMSA (Figure 1D), and subsequent gene expression assessed by Stat6-responsive promoter-driven luciferase activity (Figure 1E) (Haque et al., 2000). Similar observations were made in mouse primary splenocytes (Figures 1F & G), and in other cells of both human and mouse origins (data not shown). The specificity of DNA-protein complexes in EMSA was confirmed by competition with an excess of unlabeled DNA probe, and by super-shift with STAT6-specific antibody (Figure S2). Collectively, these data suggest that IL-4 induces ROS production in all cell types examined, and ROS induce amplification but not initiation of IL-4 signaling.
Figure 1. IL-4 Generates ROS that Promote STAT6 Activation and Gene Expression.
(A) IL-4 generates ROS in A549 cells. Cells were loaded with 5 µM CM-H2DCFDA, stimulated with IL-4 (20 ng/ml) under fluorescence microscope, and pictures taken.
(B) ROS generation is rapid in the early phases of IL-4 action. CM-H2DCFDA loaded A549 cells were stimulated with IL-4 (20 ng/ml) and fluorescence intensities were quantified. Values in relative fluorescence units (RFU) represent mean ± SE, (n=3).
(C) IL-4 generates ROS by NOX activation. A549 cells were pretreated for 2 hr with apocynin (500 µM), DPI (10 µM), L-NAME (50 µM) or DMSO, and ROS measured following IL-4 stimulation. RFU represent mean ± SE, (n=3) (** indicates P< 0.01).
(D) Blockade of ROS generation inhibits IL-4-dependent STAT6 activation. A549 cells were pretreated with apocynin, DPI or DMSO as described above, and treated with IL-4 (20 ng/ml) for indicated times. Cell extracts were subjected to EMSA, and relative signal intensities (RSI) were quantified using ImageQuant software.
(E) Blockade of ROS generation inhibits IL-4-dependent gene expression. A549 cells were transfected with 4.0 µg DNA of STAT6-responsive luciferase construct. After 48 hr, cells were pretreated with 500 µM apocynin (or DMSO) for 2 hr, stimulated with IL-4 (20 ng/ml) for indicated periods, and luciferase activity measured. Luciferase activities in arbitrary units (AU) are plotted as mean ± SE, (n=3) (** = P < 0.01; NS = not significant).
(F) IL-4 generates ROS by NOX activation in mouse primary splenocytes. Splenocytes were pretreated for 2 hr with apocynin (500 µM) or DMSO, and ROS were measured following IL-4 stimulation. RFU represent mean ± SE, (n=3) (** indicates P< 0.01).
(G) Blockade of NOX activity inhibits IL-4-dependent STAT6 activation in mouse primary splenocytes. Cells were pretreated with apocynin (500 µM) for 2 hr, and treated with IL-4 (20 ng/ml) for indicated times. Cell extracts were subjected to EMSA, and RSI quantified.
IL-4 Activates NOX-family Enzymes through the IRS-PI3K Pathway
To identify the biochemical pathways through which IL-4 induces ROS production, A549 was used as a model cell line. Binding of IL-4 to its receptor activates IRS-PI3K, STAT6 and RAS-MAPK (in some cells) pathways (Haque and Sharma, 2006; Nelms et al., 1999). Pretreatment with JAK inhibitor, AG490 (Meydan et al., 1996), or PI3K inhibitors, LY294002 and wortmannin (Fukuchi et al., 2000) but not MEK1 inhibitor, U0126 (DeSilva et al., 1998), completely blocked ROS production by IL-4, suggesting that JAK and PI3K activities were required for IL-4-induced ROS production (Figure 2A). AG490, LY294002 and wortmannin, but not U0126, also significantly inhibited IL-4-dependent STAT6 activation (Figure 2B), further suggesting that ROS promote IL-4-dependent signal transduction.
Figure 2. PI3K Activation Is Required for IL-4-induced ROS Generation.
(A) IL-4-dependent ROS generation requires JAK and PI3K activities. A549 cells were pretreated with AG490 (50 µM for 6 hr), LY294002 (20 µM for 2 hr), wortmannin (10 nM for 2 hr), U0126 (25 µM for 2 hr), or DMSO, and IL-4-induced ROS production measured. RFU are plotted as mean ± SE, (n=3).
(B) Blockade of JAK or PI3K activity inhibits IL-4-induced STAT6 activation. A549 cells were pretreated with the inhibitors described above, and stimulated with IL-4 (20 ng/ml) for 5 min. Cell extracts were subjected to EMSA, and RSI quantified.
(C) PI3K activation is indispensable for IL-4-induced ROS generation. A549 cells were transfected with 1.0 µg of murine wild-type IL-4Rα, mutant IL-4Rα (Y500F), or vector. After 48 hr, IL-4-generated ROS were measured, and RFU plotted as mean ± SE, (n=3) (** indicates P < 0.01).
(D) Inhibition of PTEN expression increases IL-4-induced ROS generation. A549 cells were transfected with 4.0 µg of shRNA (or scrambled) construct for PTEN. After 48 hr, IL-4 generated ROS were measured, and RFU plotted as mean ± SE, (n=3) (** indicates P < 0.01).
(E) Inhibition of PTEN expression increases IL-4-dependent STAT6 activation. A549 cells were transfected as described in (D). After 48 hr, total RNA was isolated and steady-state PTEN mRNA levels determined by RT-PCR (upper panel). Alternatively, 48 hr post-transfection, cells were stimulated with IL-4 (20 ng/ml) for 5 min. Cell extracts were subjected to EMSA, and RSI quantified.
To confirm the role of PI3K, and to determine if STAT6 has a role, in IL-4-induced ROS generation, we undertook the following approach. IL-4 signals A549 cells through the type II receptor (Haque and Sharma, 2006; Nelms et al., 1999). Since the binding of IL-4 to its primary receptor, IL-4Rα is species-specific (Ohara and Paul, 1987), A549 (human) cells did not respond to murine IL-4 (data not shown). IL-13Rα1 that serves as a secondary receptor chain in type II IL-4 receptor complex in A549 cells, does not exhibit species specificity (Nelms et al., 1999). We isolated cDNA of murine IL-4Rα, confirmed its functionality (Figure S3), and generated a mutant receptor (Y500F) deficient in activating the IRS-PI3K pathway (Nelms et al., 1999). When expressed in A549 cells, the wild-type murine IL-4Rα efficiently supported murine IL-4-induced ROS generation, whereas the mutant IL-4Rα (Y500F) failed to do so (Figure 2C), confirming that IRS-PI3K couples the IL-4 receptor to the ROS-generating complex. In addition, inhibition of PTEN expression by shRNA (Figure 2E, upper panel) significantly enhanced IL-4-induced ROS production (Figure 2D), and STAT6 activation (Figure 2E, lower panel) in A549 cells. Further, overexpression of wild-type, but not a catalytically inactive mutant PTEN (C124S) (Myers et al., 1997), significantly inhibited IL-4-induced ROS generation in A549 cells (Figure S4). Taken together, these results confirm the requirement of PI3K activity in IL-4-induced ROS generation.
Murine IL-4Rα did not support the activation of human STAT6, in 293T cells (Figure S3), suggesting that IL-4Rα-STAT6 interaction is also species-specific. In consistent with this, in response to murine IL-4 treatment, A549 cells expressing the murine IL-4Rα did not activate endogenous (human) STAT6 but efficiently supported ROS generation (Figure 2C). Moreover, cycloheximide did not alter IL-4-induced ROS generation in A549 and other cells (data not shown). Taken together, these data clearly indicate that IL-4Rα-mediated ROS generation does not require either STAT6 activation or new protein synthesis.
IL-4 Activates NOX1 and NOX5
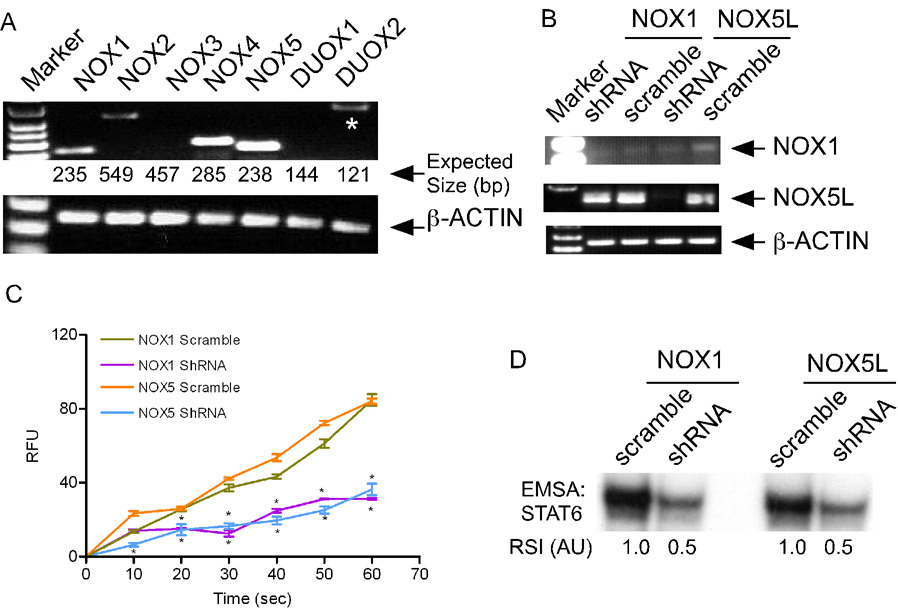
The next question was: which of the NOX-family members were involved in IL-4-induced ROS production? We found that NOX1, NOX4 and NOX5L (long forms) were predominantly expressed in A549 cells (Figures 3A, and S5A). Overexpression of NOX1 and NOX5L but not NOX4 in A549 cells significantly increased IL-4-induced ROS generation (Figure S5B) as well as STAT6 activation (Figure S5C). Further, inhibition of NOX1 expression by shRNA (Figure 3B) significantly compromised IL-4-induced ROS generation (Figure 3C), and STAT6 activation (Figure 3D) in A549 cells. NOX1 activation requires the regulatory subunits, p22phox, NOXA1, NOXO1 and RAC1 (Cheng et al., 2006; Frey et al., 2006; Lambeth et al., 2007). We found that IL-4-induced ROS generation in A549 cells was significantly increased by overexpression of p22phox (Figure S6A) and markedly compromised by either overexpression of a dominant-negative mutant p22phox (P156Q) (Kawahara et al., 2005) or shRNA-mediated inhibition of p22phox expression (Figure S6A). In addition, reconstitution of NOX1 complex in A549 cells by overexpression of NOX1, NOXO1 and NOXA1 significantly increased IL-4-induced ROS generation (Figure S6B). Moreover, IL-4-induced ROS generation was inhibited by overexpression of a dominant negative mutant (N17) RAC1 (Cool et al., 1998) (Figure S6C). Further, IL-4 stimulation of A549 cells significantly increased RAC1 activation, which was markedly compromised by inhibition of PI3K activity (Figure S6D). Collectively, these data demonstrate that IL-4 activates NOX1 complex through the IRS-PI3K-RAC1 pathway.
Figure 3. NOX1 and NOX5 Are Involved in IL-4-dependent ROS Production.
(A) Different NOX genes are expressed in A549 cells. Total RNA from was used for RT-PCR using NOX-specific primers (upper panel), or for β-actin (lower panel). The expected sizes (bp) of PCR products are indicated below, and non-specific products are indicated by asterisks.
(B) Inhibition of NOX1 and NOX5 expression by shRNA. A549 cells were transfected with 4.0 µg of shRNA constructs for NOX1, NOX5 or their scrambled versions. After 48 hr, steady-state levels of NOX1 and NOX5L mRNAs were determined by RT-PCR.
(C) Inhibition of NOX1 and NOX5 expression reduces IL-4-dependent ROS generation. A549 cells were transfected as in (B). After 48 hr, IL-4 generated ROS were measured, and RFU plotted as mean ± SE, (n=3). (* = P < 0.05).
(D) Inhibition of NOX1 and NOX5 expression reduces IL-4-dependent STAT6 activation. A549 cells were transfected as in (B). After 48 hr, cells were stimulated with murine IL-4 (20 ng/ml) for 5 min. Cell extracts were subjected to EMSA, and RSI quantified.
Next, we demonstrated the involvement of NOX5 in IL-4-induced ROS production and STAT6 activation by overexpression (Figures S5B & C) and silencing of NOX5 gene, in A549 cells (Figures 3B–D). Since NOX5L activation requires calcium binding (Banfi et al., 2004; Lambeth et al., 2007), we examined if IL-4-induced ROS generation was dependent on intracellular calcium flux. Pretreatment of A549 cells with BAPTA-AM, a general calcium chelator (Tsien, 1980), or heparin, an inhibitor of inositol 1,4,5-triphosphate (IP3)-receptor-mediated calcium flux (Seuwen and Boddeke, 1995) but not nifedipine, a blocker of L-channel-mediated calcium flux (Reid et al., 1997), significantly inhibited IL-4-induced ROS generation (Figure 4A) and STAT6 activation (Figure 4B). Therefore, it was important to determine if IL-4 stimulation of cells increased cytoplasmic calcium flux. Using Fluo-4AM (Murata et al., 2004), an increase in cytoplasmic calcium flux was detected by confocal microscopy within 5 sec of IL-4 stimulation of A549 cells, which continued to increase for ~180 sec, and reached a plateau thereafter (Figures 4C & D). The kinetics of this calcium flux correlated with that of IL-4-induced ROS generation (Figure 1B).
Figure 4. IL-4 Induces Calcium Flux that Increases ROS production.
(A) IL-4-dependent ROS generation is inhibited by blocking IP3-receptor-mediated calcium release. A549 cells were treated for 2 hr with BAPTA/AM (30 µM), heparin (1 µM), nifedipine (0.1 nM) or DMSO, and IL-4-generated ROS measured in RFU are plotted as mean ± SE, (n=3) (** = P < 0.01).
(B) Inhibitors of IP3-receptor-mediated calcium release compromise IL-4-dependent STAT6 activation. A549 cells were pretreated as in (A), treated with IL-4 (20 ng/ml) for 5 min. Cell extracts were subjected to EMSA and RSI quantified.
(C) IL-4-stimulation increases cytoplasmic calcium flux. A549 cells were loaded with 1 µM Fluo4-AM and changes in cytoplasmic calcium levels measured using live-cell confocal microscopy. The arrow indicates the time when the medium was replaced by fresh medium containing 20 ng/ml of IL-4 or 1 µM of ionomycin (as positive control). The experiment was performed five times and data from one representative experiment are shown.
(D) IL-4-stimulation increases cytoplasmic calcium flux. A549 cells were processed as in (C), and confocal microscopic pictures taken. The experiment was performed five times and data from one representative experiment is shown.
(E) Inhibition of PLC-γ1 or PLC-γ2 expression by shRNA reduces IL-4-dependent ROS generation. A549 cells were transfected with 4.0 µg of shRNA or scrambled constructs for PLC-γ1 or PLC-γ2. After 48 hr, IL-4 generated ROS were measured, and RFU plotted as mean ± SE, (n=3) (* = P < 0.05; ** = P < 0.01).
(F) Inhibition of PLC-γ1 or PLC-γ2 expression increases IL-4-dependent STAT6 activation. A549 cells were transfected as in (E). After 48 hr, PLC-γ1 or PLC-γ2 transcript levels were determined by RT-PCR (upper panel). Alternatively, 48 hr posttransfection, cells were stimulated with IL-4 for 5 min. Cell extracts were subjected to EMSA, and RSI quantified.
To examine the role of PLCγ that catalyzes the production of IP3 and diacylglycerol (DAG), in IL-4-induced NOX5 activation, pretreatment of A549 cells with PLC-γ inhibitor, U73122 (Stam et al., 1998), or inhibitors of DAG-dependent PKC, calphostin C (Jarvis et al., 1994) and Go6976 (Gschwendt et al., 1996), significantly inhibited IL-4-induced ROS generation (Figure S7). Further, shRNA-mediated inhibition of PLCγ1 and PLCγ2 expression significantly reduced IL-4-induced ROS production (Figure 4E), and STAT6 activation (Figure 4F), in A549 cells. Collectively, these results demonstrate that activated IL-4 receptor induces an intracellular calcium flux via IRS-PI3K-PLC-γ pathway that likely induces DAG- and calcium-dependent PKC-mediated activation of NOX5L to generate ROS in A549 cells.
Mouse genome does not possess a NOX5 gene but encodes DUOX1 and DUOX2 (Lambeth, 2004; Lambeth et al., 2007). We found that mouse CD4+ naïve T cells but not MEFs expressed DUOX1 (Figure S8A) that requires calcium for activation. However, BAPTA-AM and heparin failed to inhibit IL-4-induced ROS production in mouse T cells (Figure S8B) and in MEFs (as expected) (Figure S8C). Since NOX1 was predominantly expressed in both these cell types, IL-4-induced ROS production was likely mediated by NOX1 in these cells.
PTP1B Downregulates IL-4 Receptor Activation
To understand the biochemical basis of ROS-mediated amplification of IL-4 signaling, we wished to examine if ROS generated by activated IL-4 receptor oxidatively inactivate the IL-4 receptor-associated PTP activity. Prior to addressing this, it was necessary to know the molecular identity of the PTP that deactivates IL-4 receptor. Previously we and others have identified SHP-1 and CD45 that are exclusively expressed in hematopoietic cells, as negative regulators of IL-4 signaling (Haque et al., 1998; Yamada et al., 2002). Since IL-4 induces ROS generation in all cell types examined, we sought to identify a ubiquitously expressed PTP that deactivates IL-4 receptor. Screening of a panel of such PTPs by overexpression in 293T cells, identified PTP1B as a potential candidate that significantly inhibited IL-4-dependent STAT6 activation (Figure S9A). Consistently, overexpression of wild-type but not catalytically inactive mutant PTP1B (C215S) markedly inhibited IL-4-dependent STAT6 activation (Figure S9B) and subsequent gene expression in 293T cells (Figure S9C). These observations were confirmed in immortalized embryonic fibroblasts derived from PTP1B−/− mice (Buckley et al., 2002; Elchebly et al., 1999) which exhibited significant increases in magnitude and duration of IL-4-induced activation of STAT6 (Figure 5A). Further, IL-4-dependent STAT6 activation was markedly inhibited when PTP1B was knocked-in to PTP1B−/− MEFs (Figure 5B). Moreover, IL-13 that utilizes the type II IL-4 receptor for cell signaling (Nelms et al., 1999), also induced significantly higher levels of STAT6 activation in PTP1B−/− MEFs compared with wild-type MEFs (Figure S9D).
Figure 5. PTP1B Negatively Regulates IL-4 Signaling.
(A) PTP1B-deficiency increases IL-4-dependent STAT6 activation in MEFs. Immortalized MEFs derived from PTP1B+/+ and PTP1B−/− mice were treated with IL-4 (20 ng/ml) for the indicated periods, and cell extracts were subjected to EMSA or Western analyses, and RSI quantified.
(B) Knocking-in of PTP1B to PTP1B−/− MEFs reduces IL-4-dependent STAT6 activation. Immortalized PTP1B−/− MEFs were stably transfected with either vector or PTP1B, and clones were selected in the presence of hygromycin B. Selected pools were treated with IL-4 (20 ng/ml) for 30 min, and cell extracts were subjected to Western analyses and EMSA.
(C) PTP1B-deficiency increases IL-4-dependent STAT6 activation in mouse primary splenocytes. Splenocytes from PTP1B+/+ and PTP1B−/− mice were treated with IL-4 (20 ng/ml) for the indicated periods, cell extracts were subjected to EMSA, and RSI quantified.
(D) PTP1B-deficiency increases IL-4-dependent ROS generation in MEFs. Immortalized MEFs derived from PTP1B+/+ and PTP1B−/− mice, and representative clones of PTP−/− MEFs stably transfected with PTP1B (or vector) were examined for IL-4-induced ROS production. RFU are plotted as mean ± SE, (n=3) (* = P < 0.05; ** = P < 0.01).
(E) PTP1B-deficiency increases IL-4-dependent ROS generation in mouse primary splenocytes. Splenocytes isolated from PTP1B+/+ and PTP1B−/− mice were examined for IL-4-induced ROS production in RFU plotted as mean ± SE, (n=3) (** = P < 0.01).
(F) PTP1B-deficiency increases number of IL-4-producing CD4+ T cells. Naïve CD4+ T cells were purified from lymph nodes of PTP1B+/+ and PTP1B−/− mice, and cultured with anti-CD3/CD28 antibodies plus T-cell depleted syngenic irradiated spleen cells as a source of antigen-presenting cells, in a ratio of 1:5, for 72 hr under conditions to induce Th0, Th1 or Th2 differentiation. Intracellular IL-4 and IFN-γ levels were determined by flow cytometry following re-stimulation with anti-CD3 and anti-CD28 antibodies.
PTPs may exhibit functional redundancy in the regulation of cytokine signaling pathways. Since SHP-1 and CD45 inhibit IL-4 signaling in hematopoietic cells (Haque et al., 1998; Yamada et al., 2002), it was important to know if PTP1B negatively regulates IL-4 signaling in hematopoietic cells. As shown in Figure 5C IL-4-dependent STAT6 activation was markedly increased in primary splenocytes derived from PTP1B−/− mice. Importantly, PTP1B-deficiency also increased IL-4-induced ROS production in both MEFs (Figure 5D) and splenocytes (Figure 5E). Further, when PTP1B was knocked-in to PTP1B−/− MEFs, IL-4-induced ROS production was markedly reduced (Figure 5D). PTP1B-deficiency also increased ROS production, by IL-4 in mouse primary macrophages, mast cells and T cells, and by IL-13 in MEFs, splenocytes and macrophages (Figure S10). Collectively, these data demonstrate that PTP1B functions as a non-redundant, negative regulator of IL-4 and IL-13 signaling in hematopoietic and non-hematopoietic cells.
Next, we asked whether PTP1B-deficiency favors the differentiation of naïve CD4+T cells to Th2 effector cells. Highly purified CD44low naïve CD4+ T cells were obtained from lymph nodes of wild-type and PTP1B−/− mice by cell sorting, and subsequently stimulated with anti CD3/CD28 antibodies in the presence of irradiated T cell depleted splenocytes. Cytokines and antibodies were included in the culture to induce Th1 and Th2 differentiation. As shown in Figure 5F, enhanced IL-4 producing Th2 cells were found in PTP1B−/− CD4+ T cells. Enhanced IL-4 production was also observed when T cells were stimulated in Th neutral condition (data not shown). These data suggest that PTP1B negatively controls Th2 differentiation of naïve CD4+ T cells in vitro. We also noted that PTP1B−/− CD4+ T cells produced more IFN-γ when stimulated under Th1 condition. Th1 differentiation is controlled by IL-12- and IFN-γ signaling (Nelms et al., 1999). PTP1B binds to, and dephosphorylates JAK2, thereby attenuates IFN-γ signaling (Myers et al., 2001). Although PTP1B-mediated downregulation of IL-12 signaling has not been directly demonstrated, JAK2 and TYK2, which are required for IL-12-mediated cell signaling (Haque and Sharma, 2006), are shown to be potential substrates for PTP1B. Therefore, it was not unexpected that PTP1B-deficiency would increase the number of IFN-γ producing cells under Th1-skewed condition (Figure 5F).
Next, to determine if PTP1B dephosphorylates IL-4 receptor, we used a chimeric receptor, EPOR-IL-4R-α, composed of the extracellular and transmembrane domains of the murine EPOR and the cytoplasmic domain of the human IL-4Rα (Figure S11A). This receptor, when co-expressed with STAT6 in 293T cells (which do not express endogenous, functional STAT6) becomes activated, upon EPO binding, and induces STAT6 activation (Haque et al., 2000). PTP1B significantly inhibited EPO-dependent tyrosine phosphorylation of EPOR-IL-4Rα, and subsequent activation of STAT6 in 293T cells (Figure 6A), suggesting that PTP1B may interact with IL-4Rα, JAK1 or STAT6. However, a previous study has shown that JAK1 does not bind to PTP1B (Myers et al., 2001). To determine if PTP1B binds to IL-4Rα and/or STAT6, either wild-type PTP1B or substrate-trapping mutant PTP1B (D181A) (Flint et al., 1997), was co-expressed with EPOR-IL-4Rα and/or STAT6, in 293T cells. Employing conventional co-immunoprecipitation technique, we could not detect physical association of PTP1B with either EPOR-IL-4Rα̣ or STAT6 (data not shown). However, when cell lysates were prepared in the presence of the chemical cross-linking agent, dithio[succimidyl propionate] (DSP) (Maiti et al., 2005), PTP1B was found to form a complex with IL-4Rα but not STAT6 (Figure 6B) suggesting that the interaction between PTP1B and IL-4Rα was weak and dynamic in nature. Further, using deletion mutants of EPOR-IL-4Rα (Figure S11B), the PTP1B-interacting motif in IL-4Rα was mapped to a region encompassing the STAT6-docking sites (Nelms et al., 1999) (Figure S11C).
Figure 6. PTP1B Binds to, and Dephosphorylates IL-4Rα.
(A) PTP1B deactivates IL-4Rα. 293T cells were transfected with 50 ng of STAT6 and 5.0 µg of chimeric EPOR-IL-4Rα-V5, along with 5.0 µg of PTP1B or vector. After 48 hr, cells were treated with EPO (5 U/ml) for 5 min, and cell extracts were subjected to EMSA. Immunoprecipitate derived from 1.0 mg proteins or 50 µg of cell lysate proteins were analyzed by Western blotting. The experiment was repeated two times and similar results were obtained.
(B) PTP1B physically associates with the cytoplasmic domain of IL-4Rα: In lanes 1 to 6, 293T cells were transfected with EPO-IL-4Rα-V5 (2 µg), untagged STAT6 (2 µg) and 4 µg of wild-type PTP1B, mutant PTP1B (D181A) or empty vector. In lanes 7 to 12, cells were transfected with 2 µg of STAT6-V5 and 4 µg wild-type PTP1B, mutant PTP1B (D181A) or empty vector. After 48 hr, cells were treated with EPO (5 U/ml) or IL-4 (20 ng/ml) for 5 min. Cell lystaes were prepared in the presence of DSP and immunoprecipitated (IP) with anti-FLAG antibody, decross-linked and subjected to Western analyses along with decross-linked total lysates.
(C) PTP1B interacts with IL-4Rα nin live cells. For BRET assay, 293T cells were transfected with 1.0 µg of the donor plasmid, pIL-4Rα-Luc-N3 and 3.0 µg of an acceptor plasmid pGFP2-N2 (empty vector), STAT6-GFP2-N2 or PTP1B-D181A-GFP2-N2 using Lipofectamine 2000 (Invitrogen). After 48 hr, cells were used for BRET assay, and the calculated BRET ratios are plotted as mean ± SE, (n=3).
Bioluminescence resonance energy transfer (BRET) is a powerful tool for the detection of weak and dynamic protein-protein interactions in live cells (Pfleger and Eidne, 2006). Employing this technique, an interaction between PTP1B (D181A) and IL-4Rα was detected even in the absence of IL-4 stimulation, which was increased by IL-4 stimulation of cells (Figure 6C). These results confirmed the in vitro cross-linking data that PTP1B physically associates with IL-4Rα
ROS Inactivate PTP1B by Oxidation of Its Catalytic Cysteine, and Serve as a Mediator of Cytokine Cross-talk
ROS-mediated oxidative inactivation of PTP1B has been demonstrated both in vitro (Salmeen et al., 2003; van Montfort et al., 2003), and in vivo by insulin and EGF (Lee et al., 1998; Mahadev et al., 2001; Meng et al., 2002). Since we found that IL-4 induced ROS production (Figure 1), and that PTP1B deactivated IL-4 receptor (Figure 6A), it was important to examine if IL-4-generated ROS could cause oxidative inactivation of PTP1B. Using a monoclonal antibody against oxidized PTP-active site (Persson et al., 2004), we observed a time-dependent oxidation of the catalytic cysteine215 of PTP1B in A549 cells after stimulation with IL-4 (Figure 7A) or IL-13 (Figure 7B). Further, pretreatment of these cells with apocynin or LY294002 that completely inhibited IL-4-induced ROS production (Figure 1C, 1F & Figure 2A), significantly reduced the oxidation of PTP1B (Figure 7C). Moreover, shRNA-mediated reduction of NOX1 or NOX5 expression, which significantly decreased IL-4-induced ROS generation (Figure 3C), significantly inhibited IL-4-induced oxidation of PTP1B (Figure 7D). IL-4 also induced a time-dependent oxidation of PTP1B in primary mouse splenocytes (Figure 7E), and primary bone marrow-derived macrophages (Figure 7F). These results clearly demonstrate that ROS-mediated amplification of IL-4 (or IL-13) signaling is, in part, due to oxidative inactivation of PTP1B, in both primary and immortalized cells.
Figure 7. ROS Induce PTP1B Oxidation and Cytokine Cross-talk.
(A, B) Oxidation of PTP1B in IL-4/IL-13-stimulated A549 cells. Cells were treated with 20 ng/ml of IL-4 (A) or 10 ng/ml of IL-13 (B) for indicated lengths of time, with 3 mM H2O2 (positive control) for 5 min, or left untreated. Cell lysates were prepared in the presence (upper panel) or absence (lower panel) of iodoacetic acid (IAA), immunoprecipitated using anti-PTP1B antibody, and the immune complexes were subjected to Western analysis using a monoclonal antibody that recognizes oxidized PTP1B.
(C) Blockade of ROS production inhibits PTP1B oxidation in IL-4-stimulated cells. A549 cells were treated with 500 µM apocynin, 20 µM LY294002 or DMSO for 2 hr prior to measuring IL-4-mediated oxidation of PTP1B. The upper and lower panels represent results derived from cell lysates prepared in the presence and absence of IAA respectively. The experiment was repeated two times and similar results were obtained.
(D) Inhibition of NOX1 and NOX5 expression reduces IL-4-induced PTP1B oxidation. A549 cells were transfected with 4.0 µg of shRNA or scrambled constructs for NOX1 or NOX5. After 48 hr, cells were treated with IL-4 (20 ng/ml) or H2O2 (3 mM) for 5 min or left untreated. Cell lysates were prepared in the presence or absence of IAA, and PTP1B oxidation measured.
(E, F) IL-4-generated ROS induce PTP1B oxidation in mouse primary hematopoietic cells. Splenocytes (E) and bone marrow-derived macrophages (F) were stimulated with IL-4 for indicated times or with 3 mM H2O2 for 5 min. Cell lysates were prepared in the presence or absence of IAA, and PTP1B oxidation measured.
(G) EPO-generated ROS promote IL-4-dependent STAT6 activation. A549 cells were transfected with 1.0 µg of murine IL-4Rα (Y500F), along with 3.0 µg of murine STAT6 plasmid. After 48 hr, cells were treated for 5 min with EPO (5U/ml) and murine IL-4 (20 ng/ml), either singly or in combination. Cell extracts were subjected to EMSA and RSI quantified.
(H) TNF-α- and EPO-generated ROS promote endogenous IL-4 receptor activation in A549 cells. Cells pretreated with LY294002 (20 µM) or DMSO for 2 hr, were stimulated for 3 min with EPO (5U/ml) or TNF-α (10 ng/ml) followed by treatment with human IL-4 (20 ng/ml) for 2 min. Cell extracts were subjected to EMSA, and RSI quantified.
(I, J) TNF-α- and IL-3-generated ROS promote endogenous IL-4 receptor activation in mouse primary splenocytes. After pretreatment for 2 hr with LY294002 (20 µM) (I) or apocynin (500 µM) (J), cells were treated for 3 min with TNF-α (10 ng/ml) or IL-3 (10 ng/ml) followed by treatment with mouse IL-4 (20 ng/ml) for 2 min. Cell extracts were subjected to EMSA, and RSI quantified.
(K) Proposed mechanism of ROS-mediated cross-talk among cytokine receptors. IL-4R, EPOR and TNFR produce ROS in response to cognate cytokine stimulation. In accordance with our hypothesis, if a given cell is simultaneously stimulated with more than one cytokine, ROS produced by one cytokine would not only activate its own receptor or downstream targets (NF-κB in case of TNFR) in cis, but also would activate other receptors in trans in the same cell. EPOR- and IL-4R-produced ROS would inactivate PTP (both in cis and trans) that deactivate the receptor, and TNFR-produced ROS would do the same in trans. The cis target (X) of TNFR-produced ROS is not known.
Under physiologic settings, multiple cytokines may act on a single cell that expresses the cognate receptors. Since ROS are small and diffusible radicals or molecules, oxidative inactivation of PTP1B by other cytokine-generated ROS may amplify the activation of IL-4 receptor in the same cell. To examine this possibility, the mutant murine IL-4Rα (Y500F) that did not generate ROS (Figure 2C) but supported STAT6 activation, in response to murine IL-4 stimulation (Figure 7G), was expressed in A549 cells. As shown in Figure 7G, a simultaneous stimulation of these cells with EPO and murine IL-4 significantly increased the STAT6 activation. EPO did not activate STAT6 (Figure 7G) but induced ROS production in these cells (Figure S12A). These results suggest that EPO-generated ROS can act in trans to promote IL-4 signaling in the same cell. Further, to examine if EPO or other cytokines can promote IL-4 signaling through its endogenous receptor, A549 cells were pretreated with EPO or TNF-α followed by treatment with IL-4, in the presence or absence of PI3K inhibitor, LY294002. Figure 7H shows that both EPO and TNF-α markedly promoted the activation of endogenous IL-4 receptors in A549 cells. Similar observations were made in mouse primary splenocytes where ROS generated by IL-3 and TNF-α (Figures S12B & C) significantly increased IL-4-dependent STAT6 activation (Figures 7I & 7J). Of note, LY294002 completely blocked EPO- and TNF-α-induced ROS generation in A549 cells, but failed to completely compromise IL-3- and TNF-α-induced ROS production in primary splenocytes (Figures S12), suggesting that IL-3 and TNF-α may also induce ROS production by PI3K-independent mechanism(s). Importantly, the levels of ROS produced by IL-3 or TNF-α correlated with the trans-activation levels of IL-4 signaling (Figures 7G–J).
Collectively, these results demonstrate, for the first time, that ROS produced by activation of other cytokine receptors were able to promote the activation of IL-4 receptor in the same cells, suggesting that ROS can serve as a physiologic mediator of cross-talk between different cytokine receptors (Figure 7K).
DISCUSSION
In this study, we have demonstrated for the first time that immediately following IL-4 engagement, activated receptor produced ROS that, in turn, increased the magnitudes of receptor activation and consequent signal transduction in all IL-4-resonsive cells examined, in the absence of de novo protein synthesis and STAT6 activation. Subsequently, undertaking multiple, complementary approaches, we have demonstrated that IL-4 induces NOX1 and NOX5 through PI3K-RAC1 and PI3K-PLC-γ respectively.
The mechanisms of NOX1 activation is characterized in a number of cell types. NOX1 requires p22phox, NOXA1, RAC1 and NOXO1, for activation (Lambeth et al., 2007). Typically, NOXO1 is recruited to NOX1-p22phox complex to which NOXA1 is recruited by activated RAC1 (Lambeth et al., 2007). An earlier report shows that IL-4 activates RAC1 in human keratinocytes (Wery-Zennaro et al., 2000), but it was not known if IL-4-activted RAC1-induced NOX1 activation. Our results clearly demonstrate that phosphorylation of specific tyrosine (Y497 in human and Y500 in mouse) in IL-4Rα that recruits IRS-PI3K, is required for IL-4-dependent ROS production by NOX1 and NOX5 (in human cells). PI3K-dependent regulation of NOX-mediated ROS production has previously been demonstrated in EGF- and TNF-α-stimulated cells (Frey et al., 2006; Park et al., 2004). We found that IL-4-activated PI3K was indispensable for ROS production as well as IL-4-induced RAC1 activation in A549 cells, suggesting that IL-4 activates RAC1 through PI3K activation, and RAC1 is involved in ROS production by NOX1, since dominant negative mutant RAC1 significantly compromised ROS production by IL-4.
IL-4-dependent ROS generation was also significantly reduced by inhibitors of cytoplasmic calcium flux, suggesting that calcium flux is required for IL-4-induced NOX5 activation. It was not known if IL-4 induced calcium flux. Using Fluo-4AM, whose fluorescence intensity increases >100-fold, upon calcium binding (Harkins et al., 1993), here we demonstrated, for the first time, that IL-4 induced an immediate cytoplasmic calcium flux in A549 cells. IL-4-induced ROS generation was inhibited by shRNA or small molecule inhibitor of PLC-γ1 and PLC̣-γ 2. Previously two studies have demonstrated the role for PLC-γ1 in IL-4 signaling (Ikizawa et al., 1994; Ikizawa and Yanagihara, 2000), whereas another report has implicated a role of phosphatidylcholine-specific PLC, but not PLC-γ, in IL-4 signaling (Zamorano et al., 2003). This discrepancy may be due to the use of mouse cells in the later study, which do not express NOX5 (Banfi et al., 2001). Interestingly, IL-4-dependent ROS generation was significantly reduced by the inhibition of DAG-dependent PKCs. A recent study shows that activation of NOX5 is regulated by unidentified PKC-mediated phosphorylation of a serine and a threonine located in the FAD-binding domain of NOX5 (Jagnandan et al., 2007). Previous studies have focused on DAG- and calciumin-dependent PKC-mediated regulation of IL-4 signaling (Duran et al., 2004; Ho et al., 1994). Our results suggest a role for classical PKCs that depend on both DAG and calcium, in IL-4-mediated cell signaling. The mouse genome does not contain the NOX5 gene but encodes DUOX1 and DUOX2, which require calcium for activation. We noted that mouse T cells but not MEFs expressed DUOX1; however, calcium blockers did not inhibit IL-4-induced ROS production, suggesting that IL-4-induced ROS production was catalyzed by NOX1 which was predominantly expressed in both the mouse cell types. Further, studies are necessary to confirm whether IL-4-induces calcium flux and it is required for DUOX1- or DUOX2-catalyzed ROS production in other murine cell types.
We found that IL-4-generated ROS promoted IL-4-dependent signal transduction and gene expression. As an underlying mechanism, we demonstrate, for the first time, that PTP1B physically interacted with IL-4Rα and deactivated it, and that IL-4-generated ROS inactivated its catalytic cysteine215 by oxidation, in both hematopoietic and non-hematopoietic cells. Since IL-13Rα1, the second chain of type II IL-4 receptor, constitutively associates with JAK2 or TYK2, PTP1B-mediated downregulation of type II IL-4 receptor activation may attributed to PTP1B-catalyzed deactivation of JAK2 or TYK2 (Myers et al., 2001).
Both oxidative inactivation and genetic inactivation of PTP1B were found to upregulate IL-4 receptor activation and signal transduction. However, the former is a reversible and physiologic process, whereas the later is a non-physiologic and irreversible depletion of PTP1B function. PTP1B inactivation by ROS occurs locally at the site of IL-4 receptor activation and its immediate vicinity, whereas genetic inactivation of PTP1B occurs globally, and this unleashes PTP1B-dependent restraints on all the signaling pathways that are regulated by PTP1B under normal physiologic conditions. As a consequence, an in vitro differentiation of PTP1B-deficient naïve Th cells can be expected to be skewed to both Th1 and Th2 lineages, since PTP1B functions as negative regulators of IL-4 (the current study) and IFN-γ (and likely IL-12) signaling pathways (Myers et al., 2001).
Our data also revealed that PTP1B played a non-redundant role in hematopoietic cells in which both SHP-1 and CD45 are found to downregulate IL-4-dependent signal transduction (Haque et al., 1998; Yamada et al., 2002). Therefore, it remains to be examined if SHP-1 and CD45 are also oxidatively inactivated by IL-4-generated ROS, given the catalytic cysteine is conserved in all PTPs and susceptible to oxidation by ROS (Rhee et al., 2000).
ROS are small and diffusible radicals/molecules that inactivate PTPs including PTP1B (Rhee et al., 2000). Using IL-4 as a model cytokine, we uncover a novel cellular mechanism underlying the homeostatic control of cytokine receptor activation and signal transduction, by addressing how an activated cytokine receptor induces ROS generation by activating NOX enzymes to promote the receptor’s own activation as well as the activation of other cytokine receptors in the same cells. ROS-mediated cytokine signaling cross-talk may be dependent on the proximity of the different cytokine (or growth factor) receptors, and their susceptibility to regulation by oxidative inactivation of common PTPs. Another level of regulation may be provided by the specificity and abundance of the antioxidant proteins utilized by different receptors for elimination of cytokine-generated ROS, thus allowing the regeneration of reversibly inactivated PTPs and restoration of normal homeostasis of cellular cytokine signaling. Unfolding of the ROS-mediated cytokine signaling cross-talk may explain the molecular basis of side effects of cytokine therapies in a variety of human diseases. It has been believed that the cytokine-activated JAK-STAT pathway operates directly from the cell surface to the nucleus via protein-protein and DNA-protein interactions, without involving any second messengers. This study unfolds a role for ROS, as a second messenger, in the amplification of IL-4-mediated signal transduction both in cis and in trans.
EXPERIMENTAL PROCEDURES
Cells and Reagents
Reagents and methods for isolation and culture of cells are described in Supplemental Procedures. All animal experiments were conducted according to the guidelines of our Institutional Animal Care and Use Committee.
Intracellular ROS Assays
ROS were measured fluorimetrically using CM-H2DCFDA probe (Choi et al., 2005; Myhre et al., 2003), as detailed in Supplemental Procedures.
EMSA and Luciferase Assay
EMSA was performed using 10 µg proteins of whole cell extracts and 0.2 ng of 32P-labeled high-affinity STAT6-specific probe (Haque et al., 2000), as detailed in Supplemental Procedures. Luciferase assay was performed as described (Haque et al., 1997).
cDNA Cloning, Expression Vector Construction and Site-directed Mutagenesis
Cloning of murine IL-4Rα cDNA and various shRNAs and preparation of different mutant constructs are detailed in Supplemental Procedures
Transfection and Generation of Stable Clones
A549 and 293T cells were transfected using Lipofectamine 2000 (Invitrogen) and calcium phosphate co-precipitation respectively (Haque et al., 2000). Stable clones of immortalized PTP1B−/− MEFs were generated by selection with 150 µg/ml hygromycin B (Invitrogen), for 2 weeks.
RT-PCR
For determination of mRNA levels 1.0 µg of total RNA was used for first-strand cDNA synthesis according to the manufacturer’s protocol (Invitrogen). One-tenth of cDNA was used as a template for 35 cycles of PCR using specific primer sets detailed in Supplemental Procedures.
Measurement of Cytoplasmic Calcium Flux
The cytoplasmic calcium flux was measured using Fluo4-AM, (Molecular Probes) according to the manufacturer’s instructions, and the method is detailed in Supplemental Procedures.
In Vitro T Cell Differentiation
Mouse lymph node naïve CD4+ T cells were purified by MACS, followed by cell sorting and culturing in vitro for differentiation followed by intracellular cytokine staining (Min et al., 2004), as detailed in Supplemental Procedures.
Chemical Cross-linking, Immunoprecipitation and Immunoblotting
Chemical cross-linking, immunoprecipitation and immunoblotting of proteins were performed as described (Maiti et al, 2005). Other details are described in Supplemental Procedures.
BRET Assay
BRET assay was performed following the method of Pfleger and Eidne (Pfleger and Eidne, 2006), as detailed in Supplemental Procedures.
ROS-mediated Oxidation of PTP1B
PTP1B oxidation was measured as described (Persson et al., 2004), with minor modifications described in Supplemental Procedures.
Statistical and Densitometric Analyses
All experiments were performed at least three times, and data of one representative experiment are shown. Statistical differences between different groups were determined using paired Student’s t-test. EMSA and Western blot signals were quantified using ImageQuant (Molecular Dynamics).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs. Tom Hamilton, Shyamasree Datta, Jaydip Dasgupta, Charis Eng, Olga Stenina and Jack Dixon for sharing of reagents, Dr. Judith Drazba for assistance in imaging, and Drs. Thomas Koeck and Kulwant Aulak for technical assistance. We also thank Drs. Janet Houghton, Serpil Erzurum, Fred Hsieh, Ganes Sen, Robert Silverman and Baisakhi Raychaudhuri for comments. This work was supported by grants R01 GM060533 and R01 CA095006 from the National Institutes of Health to SJH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, Krause KH. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- Banfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnar GZ, Krause KH, Cox JA. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5) J Biol Chem. 2004;279:18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- Buckley DA, Cheng A, Kiely PA, Tremblay ML, O'Connor R. Regulation of insulin-like growth factor type I (IGF-I) receptor kinase activity by protein tyrosine phosphatase 1B (PTP-1B) and enhanced IGF-I-mediated suppression of apoptosis and motility in PTP-1B-deficient fibroblasts. Mol Cell Biol. 2002;22:1998–2010. doi: 10.1128/MCB.22.7.1998-2010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Diebold BA, Hughes Y, Lambeth JD. Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem. 2006;281:17718–17726. doi: 10.1074/jbc.M512751200. [DOI] [PubMed] [Google Scholar]
- Choi MH, Lee IK, Kim GW, Kim BU, Han YH, Yu DY, Park HS, Kim KY, Lee JS, Choi C, et al. Regulation of PDGF signalling and vascular remodeling by peroxiredoxin II. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- Cool RH, Merten E, Theiss C, Acker H. Rac1, and not Rac2, is involved in the regulation of the intracellular hydrogen peroxide level in HepG2 cells. Biochem J. 1998;332(Pt 1):5–8. doi: 10.1042/bj3320005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AR, Jones OT. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem J. 1986;237:111–116. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSilva DR, Jones EA, Favata MF, Jaffee BD, Magolda RL, Trzaskos JM, Scherle PA. Inhibition of mitogen-activated protein kinase kinase blocks T cell proliferation but does not induce or prevent anergy. J Immunol. 1998;160:4175–4181. [PubMed] [Google Scholar]
- Duran A, Rodriguez A, Martin P, Serrano M, Flores JM, Leitges M, Diaz-Meco MT, Moscat J. Crosstalk between PKCzeta and the IL4/Stat6 pathway during Tcell-mediated hepatitis. Embo J. 2004;23:4595–4605. doi: 10.1038/sj.emboj.7600468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- Fischer EH, Charbonneau H, Tonks NK. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991;253:401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Flint AJ, Tiganis T, Barford D, Tonks NK. Development of "substrate-trapping" mutants to identify physiological substrates of protein tyrosine phosphatases. Proc Natl Acad Sci U S A. 1997;94:1680–1685. doi: 10.1073/pnas.94.5.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey RS, Gao X, Javaid K, Siddiqui SS, Rahman A, Malik AB. Phosphatidylinositol 3-kinase gamma signaling through protein kinase Czeta induces NADPH oxidase-mediated oxidant generation and NF-kappaB activation in endothelial cells. J Biol Chem. 2006;281:16128–16138. doi: 10.1074/jbc.M508810200. [DOI] [PubMed] [Google Scholar]
- Fu X, Beer DG, Behar J, Wands J, Lambeth D, Cao W. cAMP-response element-binding protein mediates acid-induced NADPH oxidase NOX5-S expression in Barrett esophageal adenocarcinoma cells. J Biol Chem. 2006;281:20368–20382. doi: 10.1074/jbc.M603353200. [DOI] [PubMed] [Google Scholar]
- Fukuchi K, Watanabe H, Tomoyasu S, Ichimura S, Tatsumi K, Gomi K. Phosphatidylinositol 3-kinase inhibitors, Wortmannin or LY294002, inhibited accumulation of p21 protein after gamma-irradiation by stabilization of the protein. Biochim Biophys Acta. 2000;1496:207–220. doi: 10.1016/s0167-4889(00)00018-5. [DOI] [PubMed] [Google Scholar]
- Gouilleux F, Wakao H, Mundt M, Groner B. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. Embo J. 1994;13:4361–4369. doi: 10.1002/j.1460-2075.1994.tb06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwendt M, Dieterich S, Rennecke J, Kittstein W, Mueller HJ, Johannes FJ. Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase c isoenzymes. FEBS Lett. 1996;392:77–80. doi: 10.1016/0014-5793(96)00785-5. [DOI] [PubMed] [Google Scholar]
- Haque SJ, Harbor P, Tabrizi M, Yi T, Williams BR. Protein-tyrosine phosphatase Shp-1 is a negative regulator of IL-4- and IL-13-dependent signal transduction. J Biol Chem. 1998;273:33893–33896. doi: 10.1074/jbc.273.51.33893. [DOI] [PubMed] [Google Scholar]
- Haque SJ, Harbor PC, Williams BR. Identification of critical residues required for suppressor of cytokine signaling-specific regulation of interleukin-4 signaling. J Biol Chem. 2000;275:26500–26506. doi: 10.1074/jbc.275.34.26500. [DOI] [PubMed] [Google Scholar]
- Haque SJ, Sharma P. Interleukins and STAT Signaling. Vitam Horm. 2006;74C:165–206. doi: 10.1016/S0083-6729(06)74007-9. [DOI] [PubMed] [Google Scholar]
- Haque SJ, Wu Q, Kammer W, Friedrich K, Smith JM, Kerr IM, Stark GR, Williams BR. Receptor-associated constitutive protein tyrosine phosphatase activity controls the kinase function of JAK1. Proc Natl Acad Sci U S A. 1997;94:8563–8568. doi: 10.1073/pnas.94.16.8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkins AB, Kurebayashi N, Baylor SM. Resting myoplasmic free calcium in frog skeletal muscle fibers estimated with fluo-3. Biophys J. 1993;65:865–881. doi: 10.1016/S0006-3495(93)81112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin CH. Dimerization of cell surface receptors in signal transduction. Cell. 1995;80:213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- Ho JL, Zhu B, He S, Du B, Rothman R. Interleukin 4 receptor signaling in human monocytes and U937 cells involves the activation of a phosphatidylcholine-specific phospholipase C: a comparison with chemotactic peptide, FMLP, phospholipase D, and sphingomyelinase. J Exp Med. 1994;180:1457–1469. doi: 10.1084/jem.180.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikizawa K, Kajiwara K, Koshio T, Yanagihara Y. Possible role of tyrosine kinase activity in interleukin 4-induced expression of germ-line C epsilon transcripts in a human Burkitt lymphoma B-cell line, DND39. J Allergy Clin Immunol. 1994;94:620–624. doi: 10.1016/0091-6749(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Ikizawa K, Yanagihara Y. Possible involvement of Shc in IL-4-induced germline epsilon transcription in a human B cell line. Biochem Biophys Res Commun. 2000;268:54–59. doi: 10.1006/bbrc.2000.2080. [DOI] [PubMed] [Google Scholar]
- Jagnandan D, Church JE, Banfi B, Stuehr DJ, Marrero MB, Fulton DJ. Novel mechanism of activation of NADPH oxidase 5. calcium sensitization via phosphorylation. J Biol Chem. 2007;282:6494–6507. doi: 10.1074/jbc.M608966200. [DOI] [PubMed] [Google Scholar]
- Jarvis WD, Turner AJ, Povirk LF, Traylor RS, Grant S. Induction of apoptotic DNA fragmentation and cell death in HL-60 human promyelocytic leukemia cells by pharmacological inhibitors of protein kinase C. Cancer Res. 1994;54:1707–1714. [PubMed] [Google Scholar]
- Kawahara T, Ritsick D, Cheng G, Lambeth JD. Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J Biol Chem. 2005;280:31859–31869. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem. 2001;276:21938–21942. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- Maiti NR, Sharma P, Harbor PC, Haque SJ. Serine phosphorylation of Stat6 negatively controls its DNA-binding function. J Interferon Cytokine Res. 2005;25:553–563. doi: 10.1089/jir.2005.25.553. [DOI] [PubMed] [Google Scholar]
- Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, Leeder JS, Freedman M, Cohen A, Gazit A, et al. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996;379:645–648. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, Urban JF, Jr, Dvorak AM, Finkelman FD, LeGros G, Paul WE. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Hori M, Sakamoto K, Karaki H, Ozaki H. Dexamethasone blocks hypoxia-induced endothelial dysfunction in organ-cultured pulmonary arteries. Am J Respir Crit Care Med. 2004;170:647–655. doi: 10.1164/rccm.200309-1311OC. [DOI] [PubMed] [Google Scholar]
- Myers MP, Andersen JN, Cheng A, Tremblay ML, Horvath CM, Parisien JP, Salmeen A, Barford D, Tonks NK. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J Biol Chem. 2001;276:47771–47774. doi: 10.1074/jbc.C100583200. [DOI] [PubMed] [Google Scholar]
- Myers MP, Stolarov JP, Eng C, Li J, Wang SI, Wigler MH, Parsons R, Tonks NK. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci U S A. 1997;94:9052–9057. doi: 10.1073/pnas.94.17.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhre O, Andersen JM, Aarnes H, Fonnum F. Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol. 2003;65:1575–1582. doi: 10.1016/s0006-2952(03)00083-2. [DOI] [PubMed] [Google Scholar]
- Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109 Suppl:S121–S131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- Ohara J, Paul WE. Receptors for B-cell stimulatory factor-1 expressed on cells of haematopoietic lineage. Nature. 1987;325:537–540. doi: 10.1038/325537a0. [DOI] [PubMed] [Google Scholar]
- Park HS, Lee SH, Park D, Lee JS, Ryu SH, Lee WJ, Rhee SG, Bae YS. Sequential activation of phosphatidylinositol 3-kinase, beta Pix, Rac1, and Nox1 in growth factor-induced production of H2O2. Mol Cell Biol. 2004;24:4384–4394. doi: 10.1128/MCB.24.10.4384-4394.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson C, Sjoblom T, Groen A, Kappert K, Engstrom U, Hellman U, Heldin CH, den Hertog J, Ostman A. Preferential oxidation of the second phosphatase domain of receptor-like PTP-alpha revealed by an antibody against oxidized protein tyrosine phosphatases. Proc Natl Acad Sci U S A. 2004;101:1886–1891. doi: 10.1073/pnas.0304403101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleger KD, Eidne KA. Illuminating insights into protein-protein interactions using bioluminescence resonance energy transfer (BRET) Nat Methods. 2006;3:165–174. doi: 10.1038/nmeth841. [DOI] [PubMed] [Google Scholar]
- Reid K, Guo TZ, Davies MF, Maze M. Nifedipine, an L-type calcium channel blocker, restores the hypnotic response in rats made tolerant to the alpha-2 adrenergic agonist dexmedetomidine. J Pharmacol Exp Ther. 1997;283:993–999. [PubMed] [Google Scholar]
- Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000;2000 doi: 10.1126/stke.2000.53.pe1. PE1. [DOI] [PubMed] [Google Scholar]
- Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- Seuwen K, Boddeke HG. Heparin-insensitive calcium release from intracellular stores triggered by the recombinant human parathyroid hormone receptor. Br J Pharmacol. 1995;114:1613–1620. doi: 10.1111/j.1476-5381.1995.tb14947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam JC, Michiels F, van der Kammen RA, Moolenaar WH, Collard JG. Invasion of T-lymphoma cells: cooperation between Rho family GTPases and lysophospholipid receptor signaling. Embo J. 1998;17:4066–4074. doi: 10.1093/emboj/17.14.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- van Montfort RL, Congreve M, Tisi D, Carr R, Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature. 2003;423:773–777. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- Wery-Zennaro S, Zugaza JL, Letourneur M, Bertoglio J, Pierre J. IL-4 regulation of IL-6 production involves Rac/Cdc42- and p38 MAPK-dependent pathways in keratinocytes. Oncogene. 2000;19:1596–1604. doi: 10.1038/sj.onc.1203458. [DOI] [PubMed] [Google Scholar]
- Yamada T, Zhu D, Saxon A, Zhang K. CD45 controls interleukin-4-mediated IgE class switch recombination in human B cells through its function as a Janus kinase phosphatase. J Biol Chem. 2002;277:28830–28835. doi: 10.1074/jbc.M201781200. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Gurish MF, Friend DS, Austen KF, Boyce JA. Generation of a novel stem cell factor-dependent mast cell progenitor. J Immunol. 1998;161:5143–5146. [PubMed] [Google Scholar]
- Zamorano J, Rivas MD, Garcia-Trinidad A, Qu CK, Keegan AD. Phosphatidylcholine-specific phospholipase C activity is necessary for the activation of STAT6. J Immunol. 2003;171:4203–4209. doi: 10.4049/jimmunol.171.8.4203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.