Abstract
Aged human skin is fragile because of fragmentation and loss of type I collagen fibrils, which confer strength and resiliency. We report here that dermal fibroblasts express increased levels of collagen-degrading matrix metalloproteinases-1 (MMP-1) in aged (>80 years old) compared with young (21 to 30 years old) human skin in vivo. Transcription factor AP-1 and α2β1 integrin, which are key regulators of MMP-1 expression, are also elevated in fibroblasts in aged human skin in vivo. MMP-1 treatment of young skin in organ culture causes fragmentation of collagen fibrils and reduces fibroblast stretch, consistent with reduced mechanical tension, as observed in aged human skin. Limited fragmentation of three-dimensional collagen lattices with exogenous MMP-1 also reduces fibroblast stretch and mechanical tension. Furthermore, fibroblasts cultured in fragmented collagen lattices express elevated levels of MMP-1, AP-1, and α2β1 integrin. Importantly, culture in fragmented collagen raises intracellular oxidant levels and treatment with antioxidant MitoQ10 significantly reduces MMP-1 expression. These data identify positive feedback regulation that couples age-dependent MMP-1-catalyzed collagen fragmentation and oxidative stress. We propose that this self perpetuating cycle promotes human skin aging. These data extend the current understanding of the oxidative theory of aging beyond a cellular-centric view to include extracellular matrix and the critical role that connective tissue microenvironment plays in the biology of aging.
Skin connective tissue (dermis) provides structural support for the skin’s vasculature, appendages, and epidermis, which are vital to the function of skin. Structural integrity and function of the dermis are primarily dependent on its extracellular matrix, which is primarily composed of type I collagen fibrils. Type I collagen is the most abundant structural protein in skin,1 and fragmented collagen fibrils are prominent, characteristic features of aged human skin in vivo.2,3,4 This fragmentation seriously impairs both the mechanical properties of skin, and the functions of cells that reside within the dermis.5 Clinically, this impairment manifests as delayed wound healing, reduced vascularization, propensity to bruise, and thin skin. Failure of normal functional interactions among dermal cells and their extracellular matrix microenvironment underlie these age-dependent phenotypic alterations.6
Damage to the collagenous extracellular matrix of the dermis can be observed at both the histological and ultrastructural level.5,7,8,9 In young dermis, intact, tightly packed, well-organized, long collagen fibrils are abundant. In contrast, in aged dermis, collagen fibrils are fragmented, disorganized, and sparse, resulting in the appearance of amorphous open space. Quantitative biochemical analysis reveals that the amount of fragmented collagen is 4.3-fold greater in aged (>80 years old) compared with young (21 to 30 years old) human dermis in vivo.10
Fibroblasts are the primary collagen-producing cells in the dermis. Fibroblasts bind to intact collagen fibrils through cell surface integrin receptors.11,12 Integrin binding is coupled with cytoskeletal contractile forces, which organize the intact collagen fibrils.13,14,15 This pulling is balanced by resistive forces of the collagen fibrils, creating a state of dynamic mechanical tension within fibroblasts and dermis.15,16 One obvious manifestation of this mechanical tension is flattening and spreading of fibroblasts, resulting from cytoskeletal reorganization and assembly. Mechanical tension and cell shape are critical determinants of cellular function.17,18,19 In young human dermis, fibroblasts appear flattened and spread, and are in intimate contact with numerous intact collagen fibrils. In contrast, in aged human dermis, fibroblasts have a collapsed appearance with little cytoplasm, and lack direct association with fragmented collagen fibrils.6,20 Quantitative morphometric analysis reveals that fibroblast contact with collagen fibrils is reduced 80%, and cross-sectional fibroblast surface area is reduced 75%, in aged human dermis in vivo.6 Reduced fibroblast spreading is indicative of reduced mechanical tension.
Normal collagen turnover is mediated by matrix metalloproteinases (MMPs), a family of zinc-containing proteinases that specifically degrade extracellular matrix proteins that comprise connective tissue.21,22 The human MMP gene family consists of more than 20 members, with distinct structural and substrate specificities. MMPs are involved in a variety of physiological and pathological processes related to extracellular matrix turnover, wound healing, angiogenesis, cancer, and development.23,24 A limited number of MMPs are able to initiate degradation of type I collagen; these include MMP-1, MMP-8, MMP-13, MT1-MMP (MMP-14), MT2-MMP (MMP-15), and MT3-MMP (MMP-16). Normal human skin expresses transcripts for MMP-1, MMP-14, MMP-15, and MMP-16. Among these, MMP-14 and MMP-15 are most highly expressed. In mouse, which does not express MMP-1, MMP-14 has been shown to be required for normal development and type I collagen turnover.25,26 However, in human skin MMP-1, but not MMP-14 or MMP-15, is highly induced by UV irradiation and appears to be responsible for collagen fragmentation associated with chronic sun exposure.20,27,28,29,30 In human skin, MMP-1 levels increase with age, presumably contributing to fragmented and disorganized collagen fibrils in the dermis.10,31 Mature collagen fibers contain many covalent cross-links, which are not susceptible to complete proteolytic cleavage. Consequently, age-dependent collagen fragmentation results in accumulation of fragmented collagen, which irreversibly impairs the functional and structural integrity of skin connective tissue. Evidence suggests that MMP-1-mediated cumulative collagen damage is a major contributor to the phenotype of aged human skin.32,33,34,35,36 However, molecular mechanisms underlying elevated expression of MMP-1 are not well understood.
The free radical theory of aging postulates that the aging process is primarily a consequence of aerobic metabolism, which produces reactive oxygen species (ROS) in excess of cellular anti-oxidant defenses.37,38 It is proposed that ROS oxidize cellular constituents (proteins, nucleic acids, lipids), and accumulation of oxidative cellular damage, which occurs during the passage of time, impairs cellular function to yield the aged phenotype. Age-related increase of oxidative damage has been reported in a variety of human and animal tissues, and a large body of evidence, obtained in simple organisms and mammals, supports an important role of oxidants as mediators of aging.39,40,41,42,43 However, aging of human skin does not only lead to cellular alterations, but also to substantial alterations of the collagenous extracellular matrix in the dermis, as described above. Normal function of fibroblasts in dermis requires appropriate interactions with collagen fibrils, and these interactions cannot be achieved when the fibrils are fragmented. Little is known regarding the impact of collagen fibril fragmentation on fibroblast function, and the role that altered fibroblast-collagen interactions play in human skin aging.
We report here that MMP-1 regulatory pathways and MMP-1 expression are elevated in fibroblasts in aged human skin in vivo, and that similar age-dependent alterations are induced in fibroblasts, obtained from young skin, by culture in three-dimensional collagen lattices containing fragmented collagen. These data reveal potential functional linkage between oxidant-driven cellular aging (ie, free radical theory of aging) and structural integrity of human skin connective tissue extracellular matrix. Expression of this linkage establishes a self-perpetuating cycle of damage whereby collagen fragmentation elevates cellular oxidation and MMP-1 expression, which in turn results in more collagen fragmentation. This mechanism of gradually escalating impairment of tissue structure and function is consonant with the natural course of the aging process.
Materials and Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum, trypsin solution, penicillin, and streptomycin were purchased from Invitrogen Life Technology (Carlsbad, CA). MMP-1 antibody and the Oxyblot protein oxidation detection kit were obtained from Chemicon Int. (Temecula, CA); human MMP-1 and intestinal collagenase were purchased from Calbiochem (La Jolla, CA); c-Jun antibody was obtained from BD Transduction Laboratories (Lexington, KY); RedoxSensor Red CC-1 and was purchased from Molecular Probes (Eugene, OR); fluorescein isothiocyanate (FITC)-labeled type I collagen was obtained from Elastin Product Co. (St. Louis, MO); rat tail type I collagen was purchased from BD Biosciences (San Jose, CA); TransAM AP-1 transcription factor assay kit was purchased from Active Motif (Carlsbad, CA); human MMP-1 and Biotrak enzyme-linked immunosorbent assay system were purchased from GE Health Care (Little Chalfont, UK); MitoQ5, MitoQ10, and MitoE2 were obtained from Antipodean Pharmaceuticals (San Francisco, CA); ECF Western blotting reagents were purchased from GE Health Care; and unless otherwise stated, all other reagents including NAC were purchased from the Sigma Chemical Company (St. Louis, MO).
Procurement of Human Skin
Young and aged volunteers were grouped according to age: 21 to 30 years for the young group and 80+ years for the aged group. Full-thickness human skin biopsies were obtained from sun-protected buttock skin of each subject by punch biopsy, as previously described.44,45 All procedures involving human patients were conducted in accordance with the regulations set forth by the University of Michigan Institutional Review Board, and all patients provided written informed consent.
Human Skin Organ Culture
Organ culture of human skin has been previously described.46,47,48 Briefly, replicate 2-mm punch biopsies were obtained from hip skin of volunteers 21 to 30 years of age. Skin samples were incubated in wells of a 24-well dish (one tissue piece per 500 μl of culture medium). Culture medium consisted of keratinocyte basal medium (Lonza, Walkersville, MD), supplemented with CaCl2 to a final concentration of 1.4 mmol/L. Skin samples were either treated with vehicle or purified, activated human MMP-1 (150 ng/ml), for 24 hours. At the end of the incubation period, organ culture-conditioned medium was collected and analyzed for the presence of collagen fragments by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Intact collagen and collagen that had been exposed to MMP-1 in vitro served as controls. At the end of the incubation period, tissue was fixed in 10% buffered formalin and processed for light microscopy. Additional tissue pieces were fixed in 2% glutaraldehyde and processed for scanning electron microscopy.
Three-Dimensional Collagen Lattice Fibroblast Cell Culture
Adult human dermal fibroblasts were isolated from 4-mm full-thickness punch biopsies of healthy patients, as previously described.49 Collagen lattices were prepared in a sterile tube, by mixing appropriate volume of rat tail type I collagen (BD Biosciences) to yield a final concentration of 1 mg/ml with medium cocktail [DMEM, NaHCO3 (44 mmol/L), l-glutamine (4 mmol/L), folic acid (9 mmol/L), and neutralized with 1 N NaOH to pH 7.2]. Collagen solution (0.5 ml per well of a 24-well plate) was placed in an incubator at 37°C for 30 minutes to allow polymerization of the collagen. Partially degraded collagen lattices were prepared by treatment with purified human MMP-1 (50 ng per well, Calbiochem) for 16 hours at 37°C. Before use full length MMP-1 was activated by trypsin treatment (1.25 ng per 100 ng MMP-1) for 1 hour at 37°C. Cleavage by trypsin converted full-length 52-kDa MMP-1 to a catalytically active 42-kDa form (see Supplemental Figure 1A at http://ajp.amjpathol.org). Trypsin activity was inhibited by addition of trypsin inhibitor (12.5 ng). Active MMP-1 released characteristic ¾ and ¼ length collagen fragments from the collagen lattices (see Supplemental Figure 1B at http://ajp.amjpathol.org). MMP-1 was inactivated by adding ethylenediaminetetraacetic acid (10 mmol/L) followed by addition of CaCl2 (15 mmol/L), and gels were washed three times with fresh DMEM. Control lattices were prepared as above, without addition of MMP-1. Early passage (fewer than nine passages) primary adult human dermal fibroblasts (5 × 104/well) were placed on top of each collagen lattice, and allowed to attach. After attachment, collagen lattices were covered with 1 ml of media (DMEM, 10% fetal bovine serum) and incubated 72 hours at 37°C, 5% CO2. For analyses, collagen lattices were either embedded in OCT and frozen for cryostat sectioning, or fibroblasts were harvested by digesting collagen with 1 mg/ml of bacterial collagenase (Sigma Chemical Co.) for 1 hour at 37°C. Fibroblasts were collected by centrifugation. Recovery of viable fibroblasts averaged greater than 90%.
Laser Capture Microdissection (LCM) Coupled with Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
LCM were performed as previously described.45,49,50 Briefly, human skin punch biopsies were embedded in OCT, sectioned (10 μm), and stained with the HistoGene LCM slide preparation kit (Arcturus, Mountain View, CA). Approximately 200 dermal fibroblasts were captured using LCM (Leica ASLMD system; Leica Microsystems, Wetzlar, Germany). Total RNA was extracted from LCM-captured dermal fibroblasts using a commercial kit (RNeasy micro kit; Qiagen, Chatsworth, CA), and was used for amplification of mRNA using the RiboAmp HS RNA amplification kit (Acturus). The quality and quantity of amplified mRNA were determined with the Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA). Typically, we were able to obtain 50 to 100 ng of amplified mRNA from LCM-captured 200 dermal fibroblasts (1 to 2 ng of total RNA). Only 5 ng of amplified mRNA is required for quantitative analysis of transcript levels by real-time RT-PCR. Therefore, we are able to quantify transcript levels of 10 to 20 different genes in one sample. We find that quantitation of transcript levels of several different genes in samples of total RNA and amplified mRNA yield essentially identical results. Quantitation of transcript levels were performed by real-time RT-PCR, as described previously.45,49,50 MMP-1, integrin α1, integrin α2, and integrin β1, PCR primers and probes were purchased from Applied Biosystems (Foster City, CA). Other PCR primers and probes including 36B4, an internal control, have been described previously.51
Immunofluorescence Staining
Immunohistology was performed as described previously.45,52 Briefly, OCT-embedded frozen tissue or three-dimensional collagen gels were sectioned, and the slides were fixed in 2% paraformaldehyde and permeabilized with for 0.5% Triton X-100 in phosphate-buffered saline (PBS) for 10 minutes at room temperature. The slides were blocked with appropriate serum (5% in PBS) for 1 hour at room temperature. Subsequently, the slides were incubated for 1 hour at room temperature with primary antibodies followed by incubation of appropriate secondary antibody for 1 hour at room temperature. After staining the slides were mounted with mounting media (Vector Laboratories, Burlingame, CA), and examined using a fluorescence microscope.
In Situ Zymography
In situ zymography was performed as previously described.29,53 Briefly, FITC-labeled calf skin type I collagen (50 μl, Elastin Products Co.) was coated onto glass slides. Cryostat skin sections (5 μm) were placed on top of the collagen coating, and incubated for 24 hours in a sealed, humidified chamber at 37°C. Sections were visualized by fluorescence microscopy. MMP-1-catalyzed degradation of FITC-collagen causes loss of fluorescence, which was visualized by fluorescent microscopy.
Western Analysis
Western blot was performed as previously described.45,51 Briefly, whole cell proteins were resolved on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membrane, and blocked with PBST (0.1% Tween 20 in PBS) containing 5% nonfat milk. Primary antibodies were incubated with polyvinylidene difluoride membrane for 1 hour at room temperature, and membranes were washed three times with PBST solution and incubated with appropriate secondary antibody for 1 hour at room temperature. After washing three times with PBST, the blots were developed with ECF (Vistra ECF Western blotting system, GE Health Care) following the manufacturer’s protocol. The Western bands were scanned with the STORM PhosphorImager (Molecular Dynamics, Sunnyvale, CA), and the intensities of each band were quantified by ImageQuant (GE Health Care), and normalized using β-actin as a marker for equal protein loading.
Measurement of ROS
Three-dimensional collagen lattice were cultured in DMEM containing 2,3,4,5,6-pentafluorodihydrotetramethylrosamine (1 mmol/L RedoxSensor Red CC-1, Molecular Probes), for 1 hour at 37°C, 5% CO2. After incubation, samples were embedded in OCT and frozen. Oxidation of RedoxSensor Red CC-1, an indicator of levels of ROS,54 were visualized in cryostat sections (10 μm) by confocal fluorescence microscopy using an Olympus (Tokyo, Japan) FluoView 500 laser-scanning confocal microscope, housed in the Morphology and Image Analysis Core, University of Michigan Diabetes Research and Training Center. Image analysis was performed using ImageJ software (National Institutes of Health, Bethesda, MD).
Activator Protein (AP-1) Activity Assay
AP-1 transcription activity was determined using TransAM AP-1 transcription factor assay kit (Active Motif) according to the manufacturer’s protocols. Briefly, the cells were harvested by digesting collagen gel with bacterial collagenase (Sigma), as described above. The nuclear extract was prepared using nuclear and cytoplasmic extraction reagents (NE-PER; Pierce, Rockford, IL), and c-Jun-dependent AP-1 transcriptional activation was determined by phospho-c-Jun antibody (New England Biolabs, Beverly, MA).
Statistical Analysis
Comparisons were made with the paired t-test (two groups) or the repeated measures of analysis of variance (more than two groups). Multiple pair-wise comparisons were made with the Tukey Studentized range test. All P values are two-tailed, and considered significant when <0.05.
Results
Protein Oxidation, a Marker of Oxidative Stress Is Increased in the Dermis of Aged Human Skin in Vivo
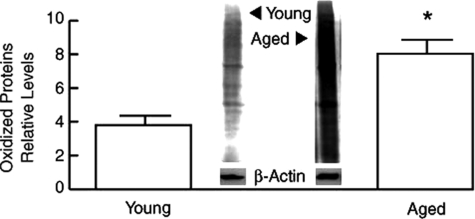
In model organisms, increased oxidative stress can result in phenotypic and functional changes that resemble aging. A large body of evidence indicates that damage to cellular constituents by ROS is a major driving force for the aging process. However, whether age-dependent alterations can promote oxidative stress is not established. To investigate this issue, we determined levels of protein carbonyls, a well-established biomarker of oxidative damage, in dermis from young and aged individuals. Figure 1 demonstrates that oxidative protein damage is significantly increased (twofold) in aged human dermis, compared with young dermis.
Figure 1.
Increased protein oxidation in aged human dermis in vivo. Dermis was obtained by dissection of full thickness human skin, from young (21 to 30 years old) and aged (>80 years old) individuals. Protein oxidation was determined by Western analysis of carbonyl residues. Results are means ± SEM. n = 7, *P < 0.05. Inset shows representative immunoblot.
Collagen-Degrading MMP-1 Is Elevated in the Dermis of Aged Human Skin in Vivo
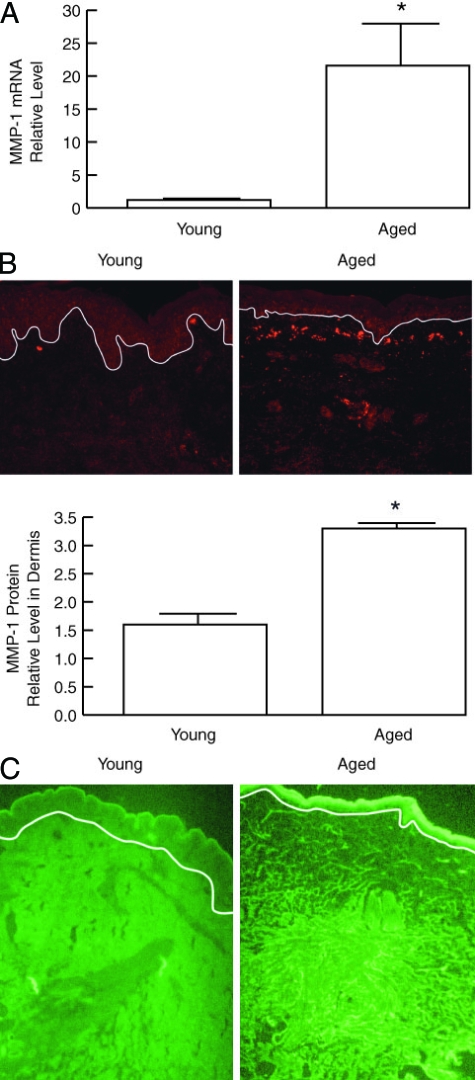
In human skin, degradation of mature collagen fibrils can be initiated by interstitial collagenase (MMP-1). Oxidative stress, shown above to be increased in aged dermis, has been shown to increase MMP-1 expression in cultured cell models. Therefore, we dissected dermis from whole skin and measured MMP-1 mRNA levels. As shown in Figure 2A, MMP-1 mRNA was increased eightfold in aged, compared with young dermis. Consistent with these data, MMP-1 protein levels, detected by immunofluorescence, were significantly elevated in aged, compared with young human dermis (Figure 2B). Increased MMP-1 expression in aged human skin was predominantly localized to fibroblasts in the upper dermis, where fibroblasts display increased levels of oxidants. Quantitation of immunostaining indicated twofold increase in MMP-1 protein in aged, compared with young dermis (Figure 2B). In addition, aged skin exhibited higher levels of collagenase activity as revealed by in situ zymography. This elevated activity, revealed as darkened areas resulting from hydrolysis of FITC-labeled collagen, was localized to the upper dermis, consistent with localization of MMP-1 protein (Figure 2C).
Figure 2.
MMP-1 mRNA, protein, and activity are increased in the dermis of aged human skin in vivo. A: MMP-1 mRNA levels in dermal fibroblasts in young (21 to 30 years old) and aged (>80 years old) human skin. Fibroblasts (150 cells) were obtained by LCM, total RNA was isolated, mRNA was amplified, and MMP-1 mRNA was quantified in 5 ng of amplified mRNA by real-time RT-PCR. Results are means ± SEM of MMP-1 mRNA normalized to 36B4 (housekeeping gene internal control) mRNA. n = 6, *P < 0.01. B: Quantitation of MMP-1 protein immunostaining in young and aged human skin. Figures show representative immunostaining. White line indicates dermal-epidermal boundary. n = 4, *P < 0.02. C: MMP-1 activity in young and aged human skin in vivo. MMP-1 activity was detected by in situ zymography, using FITC-labeled type I collagen as substrate (green fluorescence). MMP-1-catalyzed collagen cleavage causes loss of green fluorescence, resulting in darkened areas, which are most noticeable in the upper dermis of aged skin. Images are representative of three experiments.
Tissue Inhibitors of Matrix Metalloproteinases (TIMPs) Are Not Elevated in Aged Human Skin in Vivo
TIMPs are a family of proteins that bind directly to MMPs and inhibit their catalytic activity. MMPs and TIMPs are often coordinately regulated as a means to control excess MMP activity.55 We used LCM to obtain epidermis and dermis from skin of aged and young individuals. The upper half of the dermis, corresponding to the region where increased MMP-1 expression was observed (Figure 2), was captured for analyses. TIMP-1, -2, -3, and -4 mRNA levels in epidermis or dermis were not significantly different between young and aged skin (see supplemental Figure 2A at http://ajp.amjpathol.org). In both young and aged skin, transcripts for all four TIMPs were lower in epidermis than in dermis. TIMP-1, -2, -3, and -4 protein levels in whole dermis did not differ between young and aged skin (see supplemental Figure 2B at http://ajp.amjpathol.org).
c-Jun, a Major Regulator of MMP-1 Expression, Is Elevated in the Dermis of Aged Human Skin in Vivo
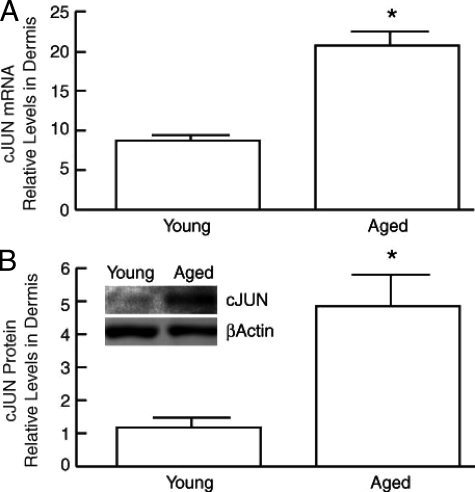
MMP-1 expression is primarily mediated by transcriptional regulation. Transcription factor AP-1, typically composed of c-Jun and c-Fos, is a major transcriptional regulator of MMP-1 expression. We have previously reported that in human skin in vivo, AP-1 activity is limited by the level of c-Jun, which can be induced, whereas c-Fos is constitutively expressed.56 c-Jun mRNA was increased 2.4-fold in aged (>80 years old) dermis, compared with young (21 to 30 years old) (Figure 3A). c-Fos mRNA expression was similar in the aged and young dermis (data not shown). In addition, c-Jun protein was elevated fivefold in the of aged (>80 years old) dermis, compared with young (21 to 30 years old) (Figure 3B). These data suggest that increased expression of c-Jun may contribute to increased levels of MMP-1 observed in aged human dermis in vivo.
Figure 3.
c-Jun mRNA and protein are elevated in aged human dermis in vivo. A: c-Jun mRNA levels in young (21 to 30 years old) and aged (>80 years old) human dermis were analyzed by real-time RT-PCR. Results are means ± SEM of c-Jun mRNA normalized to 36B4 (internal control) mRNA. n = 6, *P < 0.0001. B: c-Jun protein levels in young and aged human dermis was quantified by Western analysis. Inset shows representative immunoblot. Results are means ± SEM. n = 6, *P < 0.05.
α2β1 Integrin Is Elevated in the Dermis of Aged Human Skin in Vivo
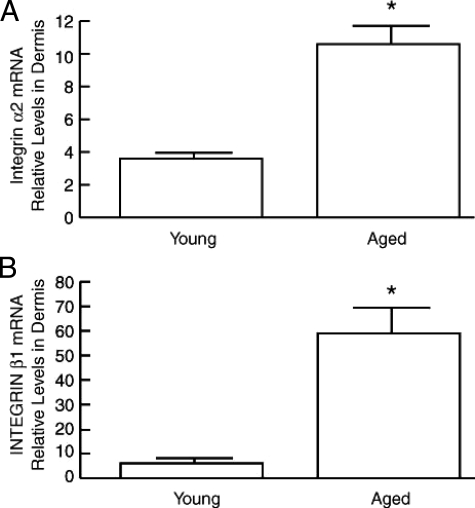
Integrins are the major cell surface receptors for extracellular matrix. Activation of α2β1 integrin activates a signal transduction cascade that results in increased expression of c-Jun and MMP-1. This MMP-1 induction via α2β1 integrin is thought to play an essential role in connective tissue remodeling and wound healing. Therefore, we examined α2 and β1 integrin expression in young and aged human dermis. α2 and β1 integrin mRNA levels were increased 2.9-fold and 9.8-fold, respectively, in aged (>80 years old) dermis, compared with young (21 to 30 years old) (Figure 4).
Figure 4.
α2 and β1 integrins are elevated in aged human dermis in vivo. α2 integrin (A) and β1 integrin (B) mRNA levels were determined in young (21 to 30 years old) and aged (>80 years old) human dermis by real-time RT-PCR. Results are means ± SEM of integrin α2 and β1 mRNA normalized to 36B4 mRNA (internal control). n = 3 to 6, *P < 0.05.
Exposure of Normal Human Skin to MMP-1 ex Vivo Generates Collagen Fragmentation that Resembles Aged Human Skin
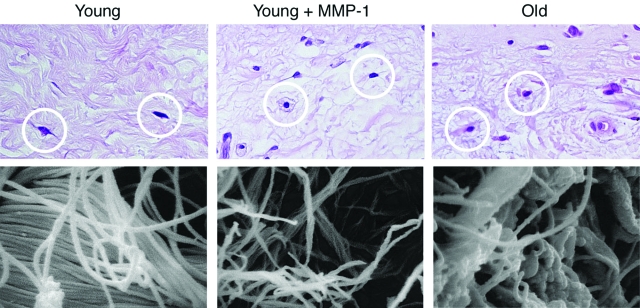
To directly investigate the ability of MMP-1 to degrade human skin collagen, sun-protected skin, from young individuals was exposed to purified human MMP-1 in organ culture. This treatment resulted in collagen fragmentation, as demonstrated by the release of characteristic three-quarter/one-quarter proteolytic fragments into the media (see supplemental Figure 3 at http://ajp.amjpathol.org). MMP-1 treatment caused alterations in the structure and organization of collagen fibrils, and fibroblast shape that were qualitatively similar to those observed in aged skin (Figure 5).6 These alterations included thinning and reduced density of collagen fibrils, and rounding of fibroblasts in areas of decreased collagen density, in comparison with the stretched morphology of fibroblasts in nontreated young skin. Scanning electron microscopy revealed fragmentation of collagen fibrils in MMP-1-treated young skin similar to that seen in aged human skin (Figure 5).
Figure 5.
MMP-1 treatment of young human skin in organ culture causes collagen fragmentation similar to that observed in aged human skin in vivo. Samples of young skin were treated with vehicle (left, top and bottom) or MMP-1 (middle, top and bottom). Top panels display paraffin-embedded sections stained with H&E. MMP-1 treatment causes fibroblasts (circled in white) to appear less stretched and more rounded. Bottom panels display scanning electron micrographs of dermal collagen, showing fragmentation of collagen fibrils in MMP-1-treated versus vehicle-treated young skin. Alterations of fibroblast morphology and collagen fragmentation observed in young skin after MMP-1 treatment resemble those observed in aged human skin (right, top and bottom).
Three-Dimensional Cell Culture Model to Study Regulation of MMP-1
Data presented above provide a foundation for investigating molecular mechanisms that lead to MMP-1-mediated collagen fragmentation in aged human dermis in vivo. Although observations of human skin in vivo are invaluable for understanding the biology of aging, in vivo observations alone generally do not reveal cause and effect mechanistic relationships. The reason for this limitation is the obvious restrictions on the type of experimental manipulations that are possible in humans in vivo.
Therefore, we sought to establish a fibroblast culture model that exhibited similar phenotypic and molecular alterations observed in aged human skin in vivo. We found that fibroblasts cultured from young and aged individuals were primarily indistinguishable (see supplemental Figures 4 to 7 at http://ajp.amjpathol.org). Additionally, we have been unable to observe increased expression of senescence markers in human dermal fibroblasts in vivo. Therefore, we have used dermal fibroblasts cultured in intact or partially MMP-1-degraded three-dimensional collagen lattices to model young and aged, respectively, human dermis. This model has been shown to mimic the fragmented collagen matrix that is observed in aged human skin.6,33 For these studies, we used fibroblasts cultured from the skin of younger individuals. We found that the age of the donor from which fibroblasts were obtained did not significantly influence their behavior in either monolayer or three-dimensional intact collagen gel culture (see supplemental Figures 8 at http://ajp.amjpathol.org). In other words, changes in fibroblast morphology and function, described below, result from culture in partially fragmented collagen lattice, rather than fibroblast donor age.
Dermal Fibroblasts Cultured in Fragmented Collagen Lattices Are Smaller, Generate Increased Levels of Oxidants, and Have Increased Levels of Oxidized Proteins
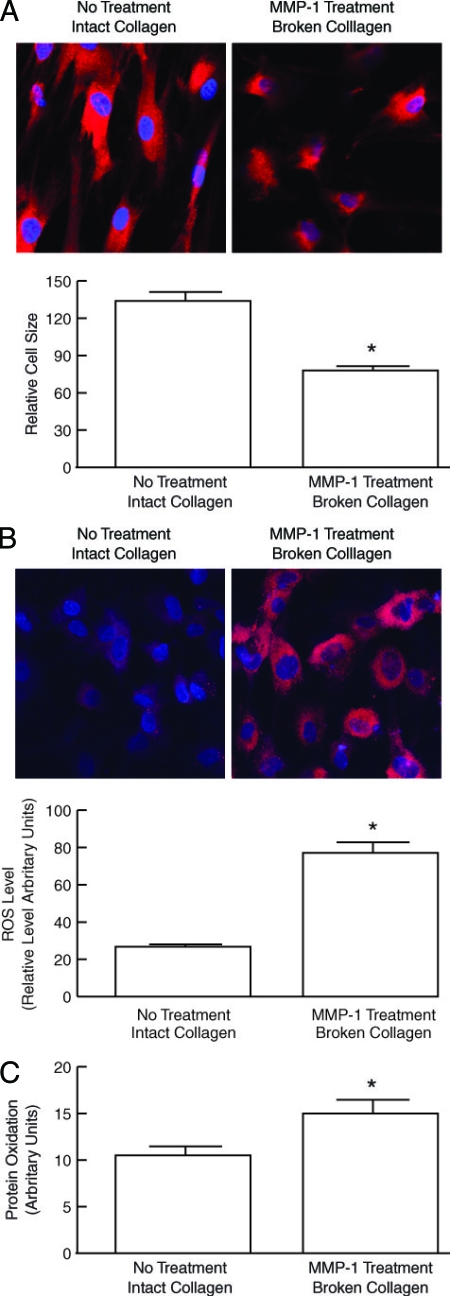
A red fluorescent dye (Cell Tracker), which is taken up into the cell cytoplasm, was used to assess the morphology of fibroblasts in three-dimensional collagen lattices. Dermal fibroblasts cultured in intact collagen lattices displayed spread flattened appearance (Figure 6A, left). In contrast, fibroblasts in MMP-1-treated collagen gels, had reduced cytoplasmic area, and a contracted appearance (Figure 6A, right), similar to fibroblasts in aged human skin in vivo.6 These data are consistent with reduced mechanical tension in fibroblasts in a three-dimensional lattice that contains fragmented collagen.
Figure 6.
Dermal fibroblasts cultured in MMP-1-fragmented three-dimensional collagen lattices have reduced spreading and increased levels of oxidants. Human skin dermal fibroblasts were cultured in intact or MMP-1-fragmented collagen lattices. A: Fibroblast morphology was assessed by incubation of cultures with CellTracker fluorescent dye for 1 hour. Fibroblasts were imaged by confocal microscopy. Reddish fluorescence delineates cell cytoplasm; blue fluorescence delineates nuclei. Results are means ± SEM. n = 5, *P < 0.05. Images are representative of three experiments. B: Oxidant levels were assessed by incubation of cultures with RedoxSensor Red CC-1 for 1 hour. Cells were imaged by confocal microscopy. Reddish fluorescence indicates relative oxidant levels; blue fluorescence delineates nuclei. Relative red fluorescence levels were quantified by ImageJ (NIH). Results are means ± SEM. n = 3, *P < 0.05. Top panels show representative images. C: Cellular lysates were analyzed for protein oxidation (carbonyls) by Western blot. Immunoreactive oxidized proteins were visualized by chemifluorescence, and quantified by ImageQuant software. Results are means + SEM of signal intensity in the entire lane. n = 3, *P < 0.05.
As discussed above, cellular damage from ROS likely plays a key role in the aging process. We therefore examined the relative oxidant levels in fibroblasts cultured in intact and partially-degraded collagen gels, using the redox-sensitive fluorescent dye RedoxSensor Red CC-1. Fibroblasts in intact collagen lattices displayed a relatively low level of oxidant-generated fluorescence (Figure 6B, left). In contrast, fibroblasts in MMP-1-treated, partially degraded collagen lattices displayed intense oxidant-generated fluorescence (Figure 6B, right). The level of oxidant-generated fluorescence was threefold greater in fibroblasts cultured in partially degraded collagen lattices, than in intact collagen lattices (Figure 6B). Increased oxidative stress in fibroblasts cultured in partially degraded collagen was associated with significantly more oxidation of intracellular proteins, as measured by Western analyses of protein carbonyls (Figure 6C). Taken together, these data indicate that reduced mechanical tension, brought about by culture in partially degraded collagen, induces oxidative stress and causes increased protein oxidation in human dermal fibroblasts.
MMP-1 Is Elevated in Fibroblasts Cultured in Fragmented Collagen Lattices
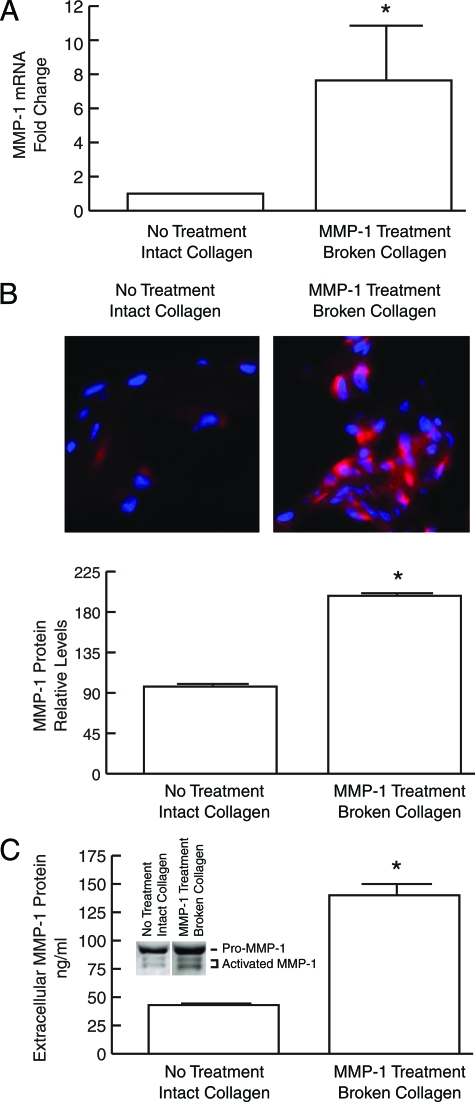
As described above, the collagenous extracellular matrix in aged dermis is partially fragmented, and fibroblasts residing in this fragmented extracellular matrix have increased levels of endogenous oxidants and express higher levels of MMP-1. MMP-1 mRNA and protein were also significantly increased in fibroblasts cultured in partially degraded collagen lattices, compared with fibroblasts cultured in intact collagen lattices. MMP-1 mRNA was elevated eightfold (Figure 7A), and immunostaining revealed twofold increased intracellular MMP-1 protein (Figure 7B). Secreted MMP-1 protein, quantified by enzyme-linked immunosorbent assay, was elevated threefold (Figure 7C). Western analysis revealed that expression of both full-length pro-MMP-1 and cleaved active MMP-1 were increased in partially degraded collagen lattice cultures (Figure 7C).
Figure 7.
MMP-1 is elevated in dermal fibroblasts cultured in MMP-1-fragmented three-dimensional collagen lattices. Human dermal fibroblasts were cultured in intact or MMP-1-fragmented, collagen lattices. A: Total RNA was extracted and MMP-1 mRNA levels were determined by real-time RT-PCR. Results are means ± SEM of MMP-1 mRNA normalized to 36B4 (internal control) mRNA. n = 4, *P < 0.05. B: MMP-1 protein expression in fibroblasts was determined by laser confocal immunofluorescence microscopy, and quantified by image analysis. Top panels show representative images. Cell nuclei are stained blue, and MMP-1 protein is stained red. Results are means ± SEM. n = 3, *P < 0.05. C: Secreted MMP-1 protein was quantified by enzyme-linked immunosorbent assay. Inset shows representative Western blot demonstrating increased levels of both full-length MMP-1 and cleaved activated MMP-1 secreted by fibroblasts in fragmented collagen lattices. n = 4, *P < 0.05.
Key Regulators of MMP-1 Expression c-Jun/AP-1 and α2β1 Integrin Are Elevated in Fibroblasts Cultured in Fragmented Collagen Lattices
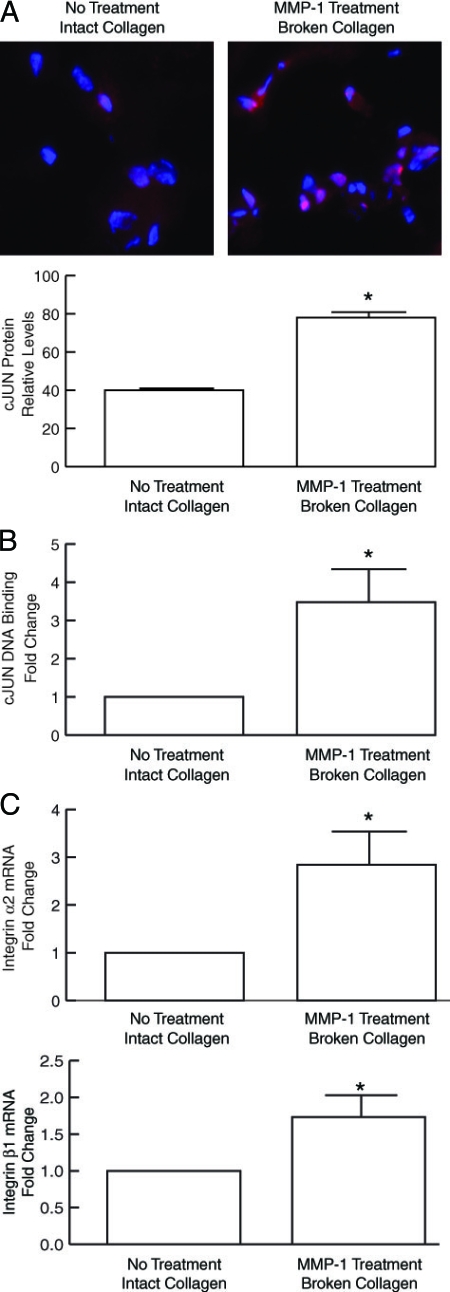
Transcription factors AP-1 and α2β1 integrin are major regulators of MMP-1 gene expression in human fibroblasts. As shown in Figure 8A, culture of fibroblasts in MMP-1-treated, partially degraded collagen-induced c-Jun protein expression twofold, compared with fibroblasts cultured in intact collagen lattices. Increased c-Jun was localized in the nucleus, and DNA binding of the phosphorylated, active form of c-Jun was increased 3.5-fold (Figure 8B). α2β1 integrin is the major cell surface extracellular matrix receptor that positively regulates expression of MMP-1. As shown in Figure 8C, α2 and β1 integrins mRNA were increased 2.8-fold and 1.7-fold, respectively, in dermal fibroblasts cultured in fragmented collagen lattices, compared with fibroblasts cultured in intact collagen lattices.
Figure 8.
c-Jun protein and DNA-binding are elevated in dermal fibroblasts cultured in MMP-1-fragmented three-dimensional collagen lattices. Human dermal fibroblasts were cultured in intact or MMP-1-fragmented lattices. A: c-Jun protein levels were determined by laser confocal immunofluorescence microscopy and quantified by image analysis. Top Panels show representative images. Cell nuclei are stained blue, and c-Jun protein is stained red. Results are means ± SEM. n = 3, *P < 0.05. B: Nuclear extracts were prepared and c-Jun DNA binding was determined using the TransAM AP-1 kit (Active Motif). Results are means ± SEM. n = 3, *P < 0.05. C: α2 and β1 integrins are elevated in dermal fibroblasts cultured in MMP-1-fragmented three-dimensional collagen lattices. Human dermal fibroblasts were cultured in intact and MMP-1-fragmented three-dimensional collagen lattices. Total RNA was extracted. α2 and β1 integrin mRNA levels were analyzed by real-time RT-PCR (10 ng total RNA). Results are mean ± SEM of α2 or β1 integrin mRNA normalized to 36B4 (internal control) mRNA. n = 3, *P < 0.03.
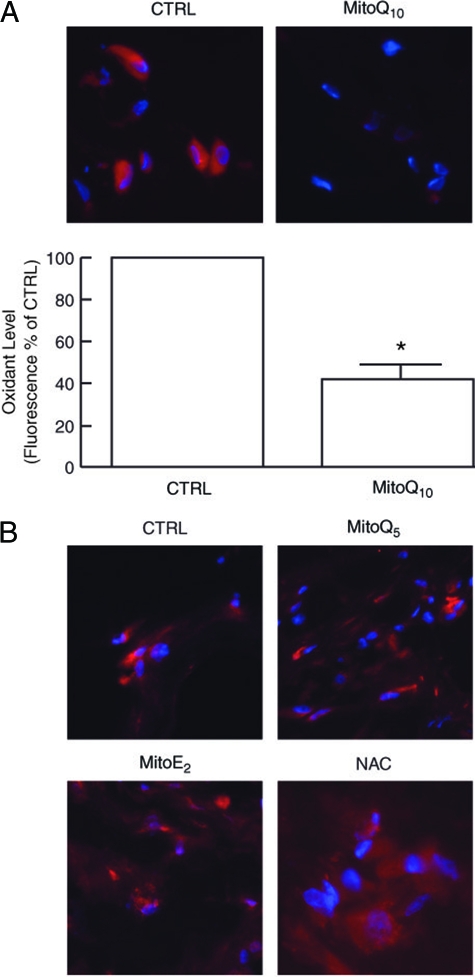
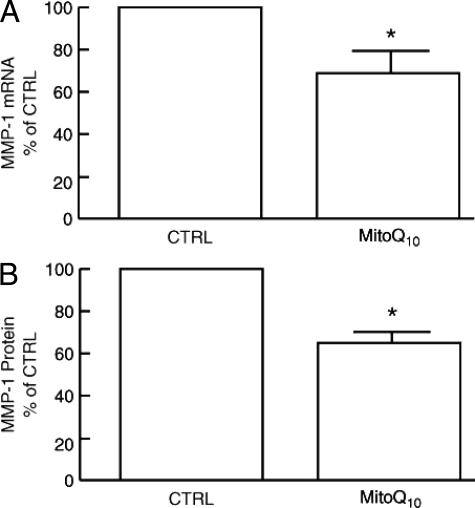
Mitochondria-Targeted Antioxidant MitoQ10 Reduces Oxidant Levels and MMP-1 Expression in Fibroblasts Cultured in Three-Dimensional Collagen Lattices
Data presented above indicate that collagen fragmentation elevates endogenous levels of oxidants, in human dermal fibroblasts. Therefore, we investigated the impact of antioxidants on oxidant levels and MMP-1 expression in fibroblasts. Low concentration (1 nmol/L) of mitochondria-targeted MitoQ10 (a derivative of ubiquinone or coenzyme Q) effectively reduced oxidant levels (Figure 9A). In contrast, the antioxidants N-acetyl cysteine, or MitoQ derivatives Q5 and E2, which localize to mitochondria but are less potent antioxidants than MitoQ10, were substantially less effective in reducing oxidant levels (Figure 9B). The impact of MitoQ10-mediated reduction of oxidant levels on MMP-1 expression is shown in Figure 10. MitoQ10 reduced MMP-1 mRNA levels 30% (Figure 10A) and MMP-1 protein levels 40% (Figure 10B). Consistent with this partial reduction of MMP-1 expression, c-Jun, and α2 integrin gene expression were reduced, 33% and 40%, respectively (data not shown).
Figure 9.
Antioxidant MitoQ10 reduces oxidant levels in human dermal fibroblasts cultured in MMP-1-fragmented collagen lattices. A: Fibroblast cultures were treated with vehicle (CTRL) or MitoQ10 (1 nmol/L) for 72 hours, followed by incubation with RedoxSensor Red for 1 hour. Lattices were embedded in OCT, sectioned, and imaged by fluorescence microscopy. Top panels show representative images. Nuclei are stained blue; oxidized RedoxSensor Red is stained red. Bottom panel is quantitative image analyses of RedoxSensor Red fluorescence. n = 5, *P < 0.01. B: MitoQ10 analogues, MitoQ5 and MitoE2, and N-acetyl cysteine (NAC) do not reduce oxidant levels in dermal fibroblasts cultured in MMP-1-fragmented collagen lattices. Fibroblast cultures were treated with vehicle (CTRL), MitoQ5 (100 nmol/L), MitoE2 (100 nmol/L), or NAC (10 mmol/L) for 72 hours, and analyzed for RedoxSensor Red fluorescence as described in A. Results are representative images from three experiments.
Figure 10.
Antioxidant MitoQ10 reduces MMP-1 expression in human dermal fibroblasts cultured in collagen lattices. Fibroblast cultures were treated with vehicle (CTRL) or MitoQ10 (1 nmol/L) for 72 hours. A: Fibroblasts were harvested and MMP-1 mRNA levels were determined by real-time RT-PCR. B: MMP-1 protein levels were determined by enzyme-linked immunosorbent assay. Results are means + SEM of five experiments. *P < 0.05.
Discussion
The free radical theory of aging was first proposed more than 60 years ago. Since that time, a growing body of evidence has revealed not only a central role of oxidative stress in natural aging, but also in a variety of age-related neurodegenerative, musculoskeletal, and inflammatory diseases.57,58,59,60,61 Age-related oxidative stress is generally thought to occur as a consequence of accumulated cellular damage primarily because of aerobic metabolism. As such, much of aging research has focused on mechanisms of cellular oxidant generation and anti-oxidant defenses. In general, these studies have primarily neglected the role of the extracellular matrix as an active participant in the aging process. It is becoming increasingly clear that the extracellular matrix is not just an inert substrate for cellular attachment, but rather is a critical regulator of many aspects of cellular function.13,62,63,64,65,66
The extracellular matrix influences cellular behavior through multiple mechanisms including, integrin-mediated cellular contacts that control cell shape and intracellular mechanical forces, conferring structural integrity and physical support to tissues, and acting as a depot for bioactive proteins. In this report, we describe functional linkage between fragmentation of the collagenous extracellular matrix in the skin and oxidative stress, which is associated with increased production of MMP-1. MMP-1 is elevated in aged human skin, and, is capable of initiating cleavage of type I collagen fibrils, as shown by addition of exogenous MMP-1 to skin in organ culture. Collagen fragmentation reduces fibroblast stretch and results in increased oxidative stress and MMP-1 expression. This self-perpetuating mechanism of extracellular matrix degradation, with attendant impairment of skin structure and function, may be considered a microcosm of the aging process.
Increased MMP-1 expression in aged skin was most prominent in the upper dermis. Localization of these alterations in the upper dermis may reflect greater collagen fragmentation and/or greater number/activity of fibroblasts. Collagen fibers in the lower dermis have larger diameter and are more highly organized than in the upper dermis. These factors may confer greater structural rigidity, which in turn may lessen the impact and/or degree of collagen fragmentation on fibroblasts in the lower dermis. Additionally, skin fibroblasts are heterogeneous, with distinct populations localized in the upper and lower dermis.67,68,69 The relative responsiveness of these different subpopulations to collagen fragmentation is not known.
Exposure of cells to exogenous oxidants, or raising endogenous levels of oxidants causes a multitude of responses, depending on the nature of the oxidant and cell type. Among these responses are activation of MAP kinase signaling pathways, induction of c-Jun and AP-1 activity, and expression of AP-1 target genes, including MMP-1. Alteration of endogenous oxidants is typically achieved by either overexpression or depletion of one or more antioxidant enzymes. For example, it has been shown that overexpression of mitochondrial superoxide dismutase leads to elevated levels of hydrogen peroxide, which can drive induction of AP-1 and its target gene MMP-1.70 In this case, increased hydrogen peroxide results from an imbalance in the levels of anti-oxidant enzymes. The capacity of superoxide dismutase to generate hydrogen peroxide, from mitochondrial superoxide, exceeds the capacity of catalase and glutathione peroxidase to reduce hydrogen peroxide to water. In contrast, overexpression of catalase in mitochondria has been shown to reduce endogenous levels of hydrogen peroxide and extend the lifespan of transgenic mice.71
Although we observed increased MMP-1 levels in dermal fibroblasts in aged skin in vivo, we found no consistent difference between fibroblasts cultured from young or aged individuals. These data suggest that tissue environment, rather than inherent, permanent cellular differences, predominantly influences oxidant levels in vivo, although we cannot rule out the possibility that adaptation to culture conditions masks inherent differences between young and aged fibroblasts. It should be noted that although fragmentation and disorganization of collagen fibers are prominent, consistent characteristics of aged skin, there exist many other differences in the dermal microenvironment between young and aged skin.72,73 Nevertheless, our finding that culture of fibroblasts in partially fragmented collagen lattices raises MMP-1 and cellular oxidant levels, independent of fibroblast age (ie, age of donor), supports the concept that the quality of the extracellular matrix is an important determinant of cellular aging.
Increased global protein oxidation, as measured by protein carbonyls, was evident in the dermis of aged skin and in fibroblasts cultured in fragmented collagen. An age-dependent increase of protein carbonyl content has been reported in other human tissues, including brain and eye lens.43,74 These data suggest that increased protein oxidation is a general feature of human aging that is not restricted to specific organs.
Accumulating evidence suggests that aging is associated with increased frequency of mitochondrial DNA mutations, and that such DNA damage can result in increased production of ROS.49,60,75,76,77,78,79 However, such observations have been made primarily in tissues with high rates of oxidative metabolism such as brain and muscle. The extent to which mitochondrial DNA damage contributes to age-related changes in skin dermis remains to be clarified. Although increased frequency of DNA mutations have been reported in skin chronically or repetitively exposed to UV irradiation, frequency of DNA mutations in naturally aged, sun-protected skin, has not been well documented.80 Our finding that fibroblasts cultured from aged individuals did not display increased levels of oxidants, compared with those from young individuals, suggests that mitochondrial mutations, with associated increased oxidant production, may not be a prominent feature of natural aging, as opposed to UV-induced aging, of fibroblasts in human skin. However, direct measurement of mitochondrial mutations in fibroblasts in aged versus young skin is needed to address this issue.
Although oxidized RedoxSensor Red is preferentially localized in mitochondria, initial oxidization can occur in the cytosol.81 NADPH oxidase and Dual oxidase, which are associated with surface membranes, are major sources of cellular ROS.82 It has recently been suggested that increased production of oxidants by these enzymes is a contributing factor to oxidative stress observed in many chronic diseases associated with aging.83,84 Although beyond the scope of the present studies, investigating the roles of NADPH oxidase and Dual oxidase in oxidant production by fibroblasts in fragmented collagen is of interest.
Aging is a complex process involving both cell intrinsic and extrinsic mechanisms. Our data suggest that in skin dermis, which is primarily composed of collagenous extracellular matrix, cell extrinsic factors play an important role in the aging process. However, our data do not exclude an important role for cell intrinsic alterations in skin aging. Although we did not observe differences in MMP-1 regulation in skin fibroblasts cultured from young or aged individuals, other differences may exist. In aged skin, fibroblasts adjacent to fragmented collagen are less stretched, and by inference have lower mechanical tension.6,85 Our results in collagen lattices demonstrate that collagen fragmentation causes loss of stretch, which results in increased levels of oxidants. Mechanism(s) by which loss of stretch (or mechanical tension) results in increased oxidative stress in fibroblasts is currently unknown. Mechanical tension impacts a multitude of cellular processes including signal transduction, gene expression, and metabolism. Recent evidence suggests that cytoskeletal tension plays a key role in translation of mechanical information into cell function.86,87,88,89 However, knowledge regarding mechano-sensing mechanisms and effector systems is in a nascent state. Therefore, understanding mechanisms that couple mechanical tension to oxidative stress in human dermal fibroblasts, their role in skin aging, and possible therapeutic implications must await further studies.
The antioxidant MitoQ10 has been shown to concentrate in the inner membrane of mitochondria, where it is effectively reduced by complex II of the electron transport chain.90,91 The 10 carbon alkyl chain, which connects the ubiquinone and triphenyl phosphonium moieties, confers optimal reduction efficiency, relative to MitoQ analogues with shorter alkyl chains. These data are consistent with our observations that MitoQ10 was substantially more potent than MitoQ5 or MitoE2, which have five and two carbon alkyl chains, respectively, in reducing fibroblast oxidant levels. Reduction of oxidant levels by MitoQ10 resulted in modest reduction of MMP-1 mRNA and protein levels. These results are consistent with the known ability of oxidative stress to stimulate MMP-1 production through activation of AP-1 activity.92 Failure of short-term MitoQ10 treatment to fully normalize MMP-1 expression indicates that although oxidative stress is a contributing factor, it is not the sole determinant of MMP-1 overexpression by fibroblasts in fragmented collagen lattices. It is possible that additional signals brought about by reduced mechanical tension contribute to altered MMP-1 regulation. This conclusion suggests that the benefit of antioxidant therapy alone may have limited potential, and better understanding of mechano-sensing mechanisms is needed to develop more effective therapies.
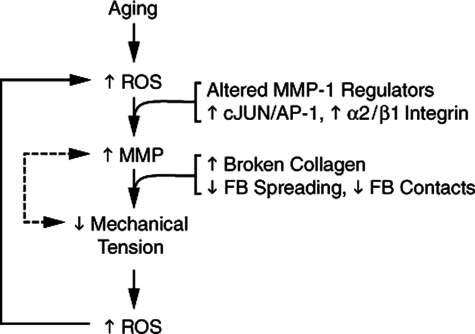
In summary, based on our findings in human skin in vivo, and collagen lattice cell culture model, we postulate that age-dependent collagen fragmentation alters fibroblast shape and mechanical tension. Reduced mechanical tension brings about numerous alterations that include integrin expression, signal transduction, cytoskeletal organization, gene expression, and oxidative stress, which promote expression of MMP-1. Up-regulation of MMP-1 in fibroblasts in aged human skin involves multiple alterations, acting in a complex regulatory network. We propose that coupling of collagen fragmentation, oxidative stress, and MMP-1 up-regulation forms a self-perpetuating cycle, which is a critical mechanism of human skin aging (Figure 11). This mechanism extends current understanding of the oxidative theory of aging beyond a cellular-centric view to include extracellular matrix and the critical role that connective tissue microenvironment plays in the biology of aging.
Figure 11.
Proposed model for self-perpetuating, MMP-1-mediated age-dependent collagen degradation in human skin connective tissue. MMP-1 induction in fibroblasts in aged dermis in vivo is mediated by coordinate alteration of MMP-1 regulatory pathways involving the transcription factor AP-1 and α2β1 integrin. MMP-1 breaks down collagen fibrils, thereby weakening the structural integrity of the extracellular matrix. The weakened extracellular matrix provides less resistance to mechanical forces exerted on it by dermal fibroblasts, thereby reducing mechanical tension in the fibroblasts. This reduced mechanical tension in the fibroblasts results in increased intracellular levels of oxidants, which in turn, through AP-1 and α2β1 integrin-dependent mechanisms, stimulate expression of MMP-1. Reduced mechanical tension may also stimulate MMP-1 expression through oxidant-independent pathways (dashed line). The positive feedback (or vicious cycle) nature of this model of age-dependent skin collagen connective tissue fragmentation is consistent with the biology of aging, which is epitomized by continual reduction of homeostatic control.
Supplementary Material
Acknowledgments
We thank Rui Wang for the technical contribution and Diane Fiolek and Laura Van Goor for graphic support.
Footnotes
Address reprint requests to Gary J. Fisher, Department of Dermatology, Med. Sci. I, R6447, 1150 W Medical Center Dr., Ann Arbor, MI 48109-5609. E-mail: gjfisher@umich.edu.
Supported in part by the National Institutes of Health (grant AG019364 to G.F.). This study used the Morphology and Image Analysis Core of the Michigan Diabetes Research and Training Center funded by the National Institute of Diabetes and Digestive Kidney Diseases (grant NIH5P60 DK20572).
G.J.F. and T.Q. contributed equally to this manuscript.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
Current address of M.K.C.: Soonchunhyang University College of Medicine, Seoul, Korea.
References
- Uitto J, Pulkkinen L, Chu M-L. Collagen. Fitzpatrick TB, Eisen AZ, Wolff K, Freedberg IM, Austen KF, editors. New York: McGraw-Hill,; Dermatology in General Medicine. 2003:pp 165–179. [Google Scholar]
- Smith J, Davidson E, Sams W, Clark R. Alterations in human dermal connective tissue with age and chronic sun damage. J Invest Dermatol. 1962;39:347–350. doi: 10.1038/jid.1962.122. [DOI] [PubMed] [Google Scholar]
- Uitto J. Biology of dermal cells and extracellular matrix. Fitzpatrick T, Eisen A, Wolff K, Freedberg I, Austen K, editors. New York: McGraw-Hill,; Dermatology in General Medicine. 1993:pp 299–314. [Google Scholar]
- Warren R, Gartstein V, Kligman A, Montagna W, Allendorf R, Ridder G. Age, sunlight, and facial skin: a histologic and quantitative study. J Am Acad Dermatol. 1991;25:751–760. doi: 10.1016/s0190-9622(08)80964-4. [DOI] [PubMed] [Google Scholar]
- West M. The cellular and molecular biology of skin aging. Arch Dermatol. 1994;130:87–95. [PubMed] [Google Scholar]
- Varani J, Dame MK, Rittie L, Fligiel SE, Kang S, Fisher GJ, Voorhees JJ. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861–1868. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreotti L, Bussotti A, Cammelli D, Aiello E, Sampognaro S. Connective tissue in aging lung. Gerontology. 1983;29:377–387. doi: 10.1159/000213148. [DOI] [PubMed] [Google Scholar]
- Rittié L, Fisher G. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002;1:705–720. doi: 10.1016/s1568-1637(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Uitto J, Bernstein E. Molecular mechanisms of cutaneous aging: connective tissue alterations in the dermis. J Investig Dermatol Symp Proc. 1998;3:41–44. [PubMed] [Google Scholar]
- Fisher G, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees J. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Fassler R. The integrin-action connection, an eternal love affair. EMBO J. 2003;22:2324–2333. doi: 10.1093/emboj/cdg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delon I, Brown NH. Integrins and the actin cytoskeleton. Curr Opin Cell Biol. 2007;19:43–50. doi: 10.1016/j.ceb.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Alenghat FJ, Ingber DE. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci STKE. 2002;2002:PE6. doi: 10.1126/stke.2002.119.pe6. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Ferara NS, Vernon RB. Impaired migration, integrin function, and actin cytoskeletal organization in dermal fibroblasts from a subset of aged human donors. Mech Ageing Dev. 2001;122:1203–1220. doi: 10.1016/s0047-6374(01)00260-3. [DOI] [PubMed] [Google Scholar]
- Wang N, Ingber DE. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys J. 1994;66:2181–2189. doi: 10.1016/S0006-3495(94)81014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;13:264–269. doi: 10.1016/s0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- Peyton SR, Ghajar CM, Khatiwala CB, Putnam AJ. The emergence of ECM mechanics and cytoskeletal tension as important regulators of cell function. Cell Biochem Biophys. 2007;47:300–320. doi: 10.1007/s12013-007-0004-y. [DOI] [PubMed] [Google Scholar]
- Eckes B, Zweers MC, Zhang ZG, Hallinger R, Mauch C, Aumailley M, Krieg T. Mechanical tension and integrin alpha 2 beta 1 regulate fibroblast functions. J Investig Dermatol Symp Proc. 2006;11:66–72. doi: 10.1038/sj.jidsymp.5650003. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore W, Bodden M, Windsor L, Birkedal-Hansen B, DeCarlo A, Engler J. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Kahäri V, Saarialho-Kere U. Matrix metalloproteinases in skin. Exp Dermatol. 1997;6:199–213. doi: 10.1111/j.1600-0625.1997.tb00164.x. [DOI] [PubMed] [Google Scholar]
- McCawley L, Matrisian L. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- Sounni NE, Noel A. Membrane type-matrix metalloproteinases and tumor progression. Biochimie. 2005;87:329–342. doi: 10.1016/j.biochi.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Choi HC, Bata-Csorgo Z, Shao Y, Datta S, Wang ZQ, Kang S, Voorhees JJ. Ultraviolet irradiation increases matrix metalloproteinase-8 protein in human skin in vivo. J Invest Dermatol. 2001;117:219–226. doi: 10.1046/j.0022-202x.2001.01432.x. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- Brennan M, Bhatti H, Nerusu K, Bhagavathula N, Kang S, Fisher G, Varani J, Voorhees J. Matrix metalloproteinase-1 is the collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem Photobiol. 2003;78:43–48. doi: 10.1562/0031-8655(2003)078<0043:mmitmc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung JH, Wang ZQ, Datta SC, Fisher GJ, Voorhees JJ. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;114:480–486. doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- Wang F, Garza LA, Kang S, Varani J, Orringer JS, Fisher GJ, Voorhees JJ. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143:155–163. doi: 10.1001/archderm.143.2.155. [DOI] [PubMed] [Google Scholar]
- Fligiel S, Varani J, Datta S, Kang S, Fisher G, Voorhees J. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J Invest Dermatol. 2003;120:842–848. doi: 10.1046/j.1523-1747.2003.12148.x. [DOI] [PubMed] [Google Scholar]
- Jacob M. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother. 2003;57:195–202. doi: 10.1016/s0753-3322(03)00065-9. [DOI] [PubMed] [Google Scholar]
- Lahmann C, Bergemann J, Harrison G, Young A. Matrix metalloproteinase-1 and skin ageing in smokers. Lancet. 2001;357:935–936. doi: 10.1016/S0140-6736(00)04220-3. [DOI] [PubMed] [Google Scholar]
- Toy LW. Matrix metalloproteinases: their function in tissue repair. J Wound Care. 2005;14:20–22. doi: 10.12968/jowc.2005.14.1.26720. [DOI] [PubMed] [Google Scholar]
- Harman D. The aging process. Proc Natl Acad Sci USA. 1981;78:7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Free radical theory of aging. Mutat Res. 1992;275:257–266. doi: 10.1016/0921-8734(92)90030-s. [DOI] [PubMed] [Google Scholar]
- Cutler R. Antioxidants and aging. Am J Clin Nutr. 1991;53:373S–379S. doi: 10.1093/ajcn/53.1.373S. [DOI] [PubMed] [Google Scholar]
- Golden T, Hinerfield D, Melov S. Oxidative stress and aging: beyond correlation. Aging Cell. 2002;1:117–123. doi: 10.1046/j.1474-9728.2002.00015.x. [DOI] [PubMed] [Google Scholar]
- Lavrovsky Y, Chatterjee B, Clark R, Roy A. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Exp Gerontol. 2000;35:521–532. doi: 10.1016/s0531-5565(00)00118-2. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Theories of biological aging: genes, proteins, and free radicals. Free Radic Res. 2006;40:1230–1238. doi: 10.1080/10715760600911303. [DOI] [PubMed] [Google Scholar]
- Stadtman E. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Esmann J, Griffiths CEM, Voorhees JJ. Cellular, immunologic and biochemical characterization of topical retinoic acid-treated human skin. J Invest Dermatol. 1991;96:699–707. doi: 10.1111/1523-1747.ep12470632. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Shao Y, Lin L, Kang S, Voorhees J, Fisher G. Elevated cystein-rich 61 mediates aberrant collagen homeostasis in chronologically aged and photoaged human skin. Am J Pathol. 2006;169:482–490. doi: 10.2353/ajpath.2006.060128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J, Perone P, Griffiths CE, Inman DR, Fligiel SE, Voorhees JJ. All-trans retinoic acid (RA) stimulates events in organ-cultured human skin that underlie repair. Adult skin from sun-protected and sun-exposed sites responds in an identical manner to RA while neonatal foreskin responds differently. J Clin Invest. 1994;94:1747–1756. doi: 10.1172/JCI117522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J, Fay K, Perone P. MDI 301, a non-irritating retinoid, induces changes in human skin that underlie repair. Arch Dermatol Res. 2007;298:439–448. doi: 10.1007/s00403-006-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lateef H, Stevens MJ, Varani J. All-trans-retinoic acid suppresses matrix metalloproteinase activity and increases collagen synthesis in diabetic human skin in organ culture. Am J Pathol. 2004;165:167–174. doi: 10.1016/S0002-9440(10)63285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, He T, Kang S, Voorhees J, Fisher G. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-ß type II receptor/Smad signaling. Am J Pathol. 2004;165:741–751. doi: 10.1016/s0002-9440(10)63337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, He T, Kang S, Voorhees J, Fisher G. Connective tissue growth factor: expression in human skin in vivo and inhibition by ultraviolet irradiation. J Invest Dermatol. 2002;118:402–408. doi: 10.1046/j.0022-202x.2001.01678.x. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Voorhees J, Fisher G. Ultraviolet irradiation induces Smad7 via induction of transcription factor AP-1 in human skin fibroblasts. J Biol Chem. 2005;280:8079–8085. doi: 10.1074/jbc.M409647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Datta S, Wang Z, Li XY, Quan T, Chung JH, Kang S, Voorhees JJ. c-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J Clin Invest. 2000;106:663–670. doi: 10.1172/JCI9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Voorhees JJ. Molecular mechanisms of photoaging and its prevention by retinoic acid: ultraviolet irradiation induces MAP kinase signal transduction cascades that induce AP-1-regulated matrix metalloproteinases that degrade human skin in vivo. J Investig Dermatol Symp Proc. 1998;3:61–68. [PubMed] [Google Scholar]
- Shukla A, Jung M, Sern M, Fukagawa N, Taatfes D, Sawyer D, Van Houten B, Mossman B. Asbestos induces mitochondrial DNA damage and dysfunction linked to the development of apoptosis. Am J Physiol. 2003;285:L1018–L1025. doi: 10.1152/ajplung.00038.2003. [DOI] [PubMed] [Google Scholar]
- Brew K, Dinakarapandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure, and function. Biochim Biophys Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- Fisher G, Datta S, Wang Z, Li X, Quan T, Chung J, Kang S, Voorhees J. c-Jun dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoid acid. J Clin Invest. 2000;106:663–670. doi: 10.1172/JCI9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007;74:324–329. doi: 10.1016/j.jbspin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev. 2007;87:1175–1213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Markesbery WR. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer’s disease. Nucleic Acids Res. 2007;35:7497–7504. doi: 10.1093/nar/gkm821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzioglu M, Larsson NG. Mitochondrial dysfunction in mammalian ageing. Novartis Found Symp. 2007;287:197–208. doi: 10.1002/9780470725207.ch14. discussion 208–113. [DOI] [PubMed] [Google Scholar]
- Danson EJ, Paterson DJ. Reactive oxygen species and autonomic regulation of cardiac excitability. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S104–S112. doi: 10.1111/j.1540-8167.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- Boudreau N, Jones P. Extracellular matrix and integrin signalling: the shape of things to come. Biochem J. 1999;339:481–488. [PMC free article] [PubMed] [Google Scholar]
- Hay ED, editor. New York: Plenum Press,; Cell Biology of Extracellular Matrix. 1991:pp. 221–249. [Google Scholar]
- Bissell MJ, Kenny PA, Radisky DC. Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: the role of extracellular matrix and its degrading enzymes. Cold Spring Harb Symp Quant Biol. 2005;70:343–356. doi: 10.1101/sqb.2005.70.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitano S. From the extracellular matrix to cell and tissue function in the alveolar epithelium. Am J Physiol. 2001;280:L189–L190. doi: 10.1152/ajplung.2001.280.2.L189. [DOI] [PubMed] [Google Scholar]
- Madri JA, Basson MD. Extracellular matrix-cell interactions: dynamic modulators of cell, tissue and organism structure and function. Lab Invest. 1992;66:519–521. [PubMed] [Google Scholar]
- Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. J Cell Sci. 2004;117:667–675. doi: 10.1242/jcs.01005. [DOI] [PubMed] [Google Scholar]
- Ali-Bahar M, Bauer B, Tredget EE, Ghahary A. Dermal fibroblasts from different layers of human skin are heterogeneous in expression of collagenase and types I and III procollagen mRNA. Wound Repair Regener. 2004;12:175–182. doi: 10.1111/j.1067-1927.2004.012110.x. [DOI] [PubMed] [Google Scholar]
- Sorrell JM, Baber MA, Caplan AI. Clonal characterization of fibroblasts in the superficial layer of the adult human dermis. Cell Tissue Res. 2007;327:499–510. doi: 10.1007/s00441-006-0317-y. [DOI] [PubMed] [Google Scholar]
- Wenk J, Brenneisen P, Wlaschek M, Poswig A, Briviba K, Oberley TD, Scharffetter-Kochanek K. Stable overexpression of manganese superoxide dismutase in mitochondria identifies hydrogen peroxide as a major oxidant in the AP-1-mediated induction of matrix-degrading metalloprotease-1. J Biol Chem. 1999;274:25869–25876. doi: 10.1074/jbc.274.36.25869. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Uitto J. Connective tissue biochemistry of the aging dermis. Age-related alterations in collagen and elastin. Dermatol Clin. 1986;4:433–446. [PubMed] [Google Scholar]
- Monnier VM, Mustata GT, Biemel KL, Reihl O, Lederer MO, Zhenyu D, Sell DR. Cross-linking of the extracellular matrix by the maillard reaction in aging and diabetes: an update on “a puzzle nearing resolution.”. Ann NY Acad Sci. 2005;1043:533–544. doi: 10.1196/annals.1333.061. [DOI] [PubMed] [Google Scholar]
- Farout L, Friguet B. Proteasome function in aging and oxidative stress: implications in protein maintenance failure. Antioxid Redox Signal. 2006;8:205–216. doi: 10.1089/ars.2006.8.205. [DOI] [PubMed] [Google Scholar]
- Cortopassi G, Arnheim N. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res. 1990;18:6927–6933. doi: 10.1093/nar/18.23.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortopassi G, Shibata D, Soong N-W, Arnheim N. A pattern of accumulation of a somatic deletion of mitochondrial DNA in aging human tissues. Proc Natl Acad Sci USA. 1992;89:7370–7374. doi: 10.1073/pnas.89.16.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-C, Pang C-Y, Hsu H-S, Wei Y-H. Differential accumulations of 4,977bp deletion in mitochondrial DNA of various tissues in human ageing. Biochim Biophys Acta. 1994;1226:37–43. doi: 10.1016/0925-4439(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Linnane A, Marzuki S, Ozawa T, Tanaka M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet. 1989;1:642–645. doi: 10.1016/s0140-6736(89)92145-4. [DOI] [PubMed] [Google Scholar]
- Wei Y-H, Lee H-C. Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Exp Biol Med (Maywood) 2002;227:671–682. doi: 10.1177/153537020222700901. [DOI] [PubMed] [Google Scholar]
- Birch-Machin M, Tindall M, Turner R, Haldane F, Rees J. Mitochondrial DNA deletions in human skin reflect photo-rather than chronologic aging. J Invest Dermatol. 1998;110:149–152. doi: 10.1046/j.1523-1747.1998.00099.x. [DOI] [PubMed] [Google Scholar]
- Chen CS, Gee KR. Redox-dependent trafficking of 2,3,4,5, 6-pentafluorodihydrotetramethylrosamine, a novel fluorogenic indicator of cellular oxidative activity. Free Radic Biol Med. 2000;28:1266–1278. doi: 10.1016/s0891-5849(00)00265-3. [DOI] [PubMed] [Google Scholar]
- Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause KH. Aging: a revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp Gerontol. 2007;42:256–262. doi: 10.1016/j.exger.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Varani J, Dame M, Rittie L, Fligiel S, Kang S, Fisher G, Voorhees J. Decreased collagen production in chronologically aged skin. Am J Pathol. 2006;168:1861–1868. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alenghat FJ, Nauli SM, Kolb R, Zhou J, Ingber DE. Global cytoskeletal control of mechanotransduction in kidney epithelial cells. Exp Cell Res. 2004;301:23–30. doi: 10.1016/j.yexcr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- Silver FH, Siperko LM, Seehra GP. Mechanobiology of force transduction in dermal tissue. Skin Res Technol. 2003;9:3–23. doi: 10.1034/j.1600-0846.2003.00358.x. [DOI] [PubMed] [Google Scholar]
- Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- James AM, Cocheme HM, Smith RA, Murphy MP. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J Biol Chem. 2005;280:21295–21312. doi: 10.1074/jbc.M501527200. [DOI] [PubMed] [Google Scholar]
- James AM, Sharpley MS, Manas AR, Frerman FE, Hirst J, Smith RA, Murphy MP. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J Biol Chem. 2007;282:14708–14718. doi: 10.1074/jbc.M611463200. [DOI] [PubMed] [Google Scholar]
- Wenk J, Brenneisen P, Wlaschek M, Poswig A, Briviba K, Oberley T, Scharffetter-Kochanek K. Stable overexpression of manganese superoxide dismutase in mitochondria identifies hydrogen peroxide as a major oxidant in the AP-1-mediated induction of matrix-degrading metalloproteinase-1. J Biol Chem. 1999;274:25869–25876. doi: 10.1074/jbc.274.36.25869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.