Abstract
FSH activates the phosphatidylinositol-3 kinase (PI3K)/acute transforming retrovirus thymoma protein kinase pathway and thereby enhances granulosa cell differentiation in culture. To identify the physiological role of the PI3K pathway in vivo we disrupted the PI3K suppressor, Pten, in developing ovarian follicles. To selectively disrupt Pten expression in granulosa cells, Ptenfl/fl mice were mated with transgenic mice expressing cAMP response element recombinase driven by Cyp19 promoter (Cyp19-Cre). The resultant Pten mutant mice were fertile, ovulated more oocytes, and produced moderately more pups than control mice. These physiological differences in the Pten mutant mice were associated with hyperactivation of the PI3K/acute transforming retrovirus thymoma protein kinase pathway, decreased susceptibility to apoptosis, and increased proliferation of mutant granulosa cells. Strikingly, corpora lutea of the Pten mutant mice persisted longer than those of control mice. Although the follicular and luteal cell steroidogenesis in Ptenfl/fl;Cyp19-Cre mice was similar to controls, viable nonsteroidogenic luteal cells escaped structural luteolysis. These findings provide the novel evidence that Pten impacts the survival/life span of granulosa/luteal cells and that its loss not only results in the facilitated ovulation but also in the persistence of nonsteroidogenic luteal structures in the adult mouse ovary.
IN MAMMALS, OVARIAN follicular growth, ovulation, and luteinization are tightly regulated by FSH and LH. FSH is obligatory for supporting the development of follicles to the preovulatory stage. The LH surge rapidly initiates terminal differentiation of granulosa/cumulus cells, leading to meiotic maturation of oocytes and expansion of cumulus oophorus. Granulosa cells (GCs) luteinize to form the corpus lutea (CL) after ovulation (1). FSH and LH mediate these effects by inducing a complex pattern of gene expression in the GCs that is regulated by the coordinate input from different signaling cascades such as the cAMP/protein kinase A, ERK1/2, and phosphatidylinositol-3 kinase (PI3K) cascades (2).
FSH promotes rapid activation of the PI3K pathway in GCs, resulting in the phosphorylation of the downstream branch-point kinase AKT (acute transforming retrovirus thymoma protein kinase) (3,4). This pathway is known to regulate many aspects of cell function including cell cycle progression/arrest, DNA repair, and apoptosis (5,6). Targets of AKT include FOXO1 (Forkhead winged helix box O1) and FOXO3 (Forkhead winged helix box O3), transcription factors that are expressed abundantly in GCs and are presumed to regulate GC responsiveness to FSH (2,7,8). The critical roles of the PI3K pathway in gonadotropin-mediated GC differentiation, cumulus expansion, and oocyte maturation have been demonstrated in culture (3,4,7,8,9,10,11). Moreover, depletion of Foxo3a or Pten in mouse oocytes resulted in premature ovarian failure due to the global exit of follicles from the primordial pool (12,13). However, the impact of PI3K pathway components in regulating functions of ovarian somatic cells during follicle development, ovulation, and/or luteinization has not been determined in vivo.
The PI3K pathway is negatively regulated by phosphatase and tensin homolog (PTEN) that dephosphorylates PIP3 (phosphatidylinositol 3,4,5-triphosphate), the lipid product of PI3K. Pten was originally cloned as a tumor suppressor, and its importance is further noted because Pten-null mice are embryonic lethal. Pten mutations in human and mice present tumors in selected tissues suggesting that PTEN acts in a tissue-specific manner to regulate PI3K pathway (14,15).
In view of the potential importance of the PI3K pathway in the differentiation of GCs and luteal cells, we sought to identify the physiological role of Pten in developing follicles and its regulation of the PI3K pathway. Ptenflfl mice have been generated (16) and were used in these studies to obtain a conditional knockout mouse strain in which Pten is specifically depleted in GCs of antral follicles and luteal cells (LCs). Strikingly, mice with Pten deletion in GCs do not develop ovarian tumors but demonstrated increases in ovary volume due to the accumulation of CLs. The Pten mutant mice ovulate more oocytes in response to a superovulatory regimen of equine (e) chorionic gonadotropin (CG)/human (h) CG than do control mice. GCs of the mutant mice demonstrated increased proliferation whereas GCs and LCs exhibited decreased apoptosis. These findings provide in vivo evidence that activation of the PI3K pathway promotes granulosa/luteal cell survival, in part by regulating FOXO1 and other mediators of cell cycle arrest and cell survival (7).
RESULTS
Granulosa/Luteal Cell-Specific Disruption of the Pten Gene
The Ptenfl/fl mouse strain (16) was crossed with transgenic mice in which the Cyp19 promoter drives expression of Cre recombinase (Cyp19-Cre). To monitor the cAMP response element (CRE)-mediated recombination in ovaries, the Cyp19-Cre mice were crossed to the ROSA26 reporter mouse strain that expresses β-galactosidase only in cells that have CRE activity (17). As shown in Fig. 1, A and B, CRE activity was present in GCs of all antral follicles and most LCs, but was low/undetectable in GCs of primordial/primary follicles, theca cells, and oocytes. PCR analysis of ovarian and tail DNA templates confirmed that CRE-mediated recombination occurred exclusively in ovarian tissue of Ptenfl/fl;Cyp19-Cre (Pten CKO; conditional knockout) progeny (Fig. 1C).
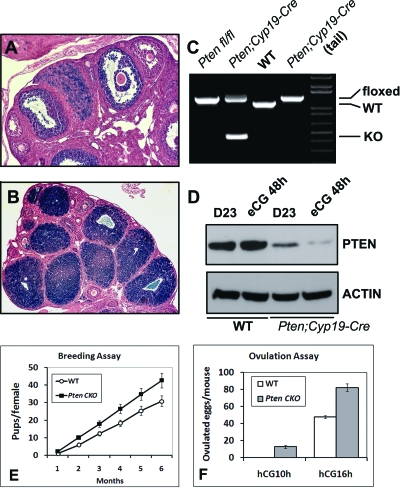
Figure 1.
Generation and Evaluation of Reproductive Functions of Ptenfl/fl;Cyp19-Cre Mice
Shown in ROSA26 reporter mouse strain, CRE activity was present in GCs of most antral follicles (A) and LCs (B). PCR analysis showed that recombination occurred exclusively in ovaries of Ptenfl/fl;Cyp19-Cre progeny (C). PTEN protein levels were reduced in the GCs of the Ptenfl/fl;Cyp19-Cre mice compared with wild-type mice (D). The Ptenfl/fl;Cyp19-Cre females gave birth to more pups than wild type (E). In superovulation assay, Ptenfl/fl;Cyp19-Cre mice ovulated more oocytes than do wild type at 16 h after hCG. At 10 h after hCG, 10–15 oocytes were ovulated by Ptenfl/fl;Cyp19-Cre mice, but not the control littermates (F). KO, Knockout; WT, wild type.
To determine the levels of PTEN protein in the mutant cells, GCs were isolated from immature mice at d 23 with or without treatment with eCG to stimulate follicle growth. Western blot showed that the levels of the PTEN protein were reduced dramatically in the GC extracts prepared from the Pten CKO mice compared with Ptenfl/fl control mice (Fig. 1D). More than three animals were analyzed in each experiment with similar results.
The low levels of PTEN protein that were detected in the GCs collected from Pten CKO mice likely come from GCs in which recombination is not complete as well as from non-GC ovarian cell components (theca, oocytes, endothelial cells, and interstitial cells) where PTEN expression is higher than that in GCs (see Fig. 4K). More efficient PTEN depletion in GCs after eCG treatment may contribute to the decrease in PTEN in these cells compared with untreated mice because Cyp19-Cre expression is increased by eCG treatment (35).
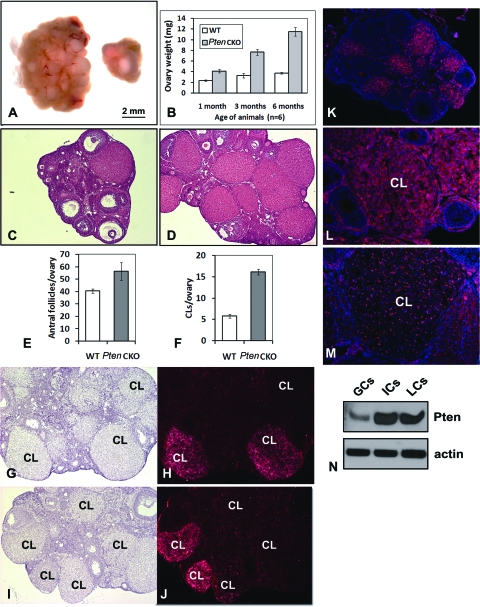
Figure 4.
Progressive Accumulation of CL in Pten CKO Mice
Ovaries of the Pten CKO mice are larger and heavier than wild-type mice (A and B). Hematoxylin and eosin staining (C and D) followed by quantification showed that the Pten CKO ovaries contained more antral follicles and CL than controls (E and F). In situ hybridization showed that whereas the fully developed, healthy CL in wild-type ovaries expressed Lhcgr (G and H), only some of the CL in Pten CKO ovaries expressed Lhcgr (I and J). Immunofluorescence showed that PTEN protein is highly expressed in wild-type CL (K and L), was markedly reduced in Pten mutant LCs, but remained in the endothelial cells (M). The protein levels of PTEN were higher in LCs and RT than in GCs (N). ICs, Interstitial cells; WT, wild type.
Increased Follicle Growth and Ovulation in Pten Conditional Knockout Mice
Although Pten mutations often lead to cell transformation and tumor development in different tissues, the conditional knockout of Pten in GCs does not lead to ovarian tumor formation even at 12 months of age (data not shown). Moreover, the Pten CKO mice are fertile and give birth to approximately 20% more pups than Ptenfl/fl control mice during a 6-month breeding period (Fig. 1E) (n = 6 per genotype). The increased number of pups born appears to be related to increased ovulation potential because immature Pten CKO mice primed with a superovulatory regimen of eCG (an FSH equivalent) followed by hCG (an LH equivalent) ovulate more oocytes than do control mice as determined by oviductal inspection at 16 h after hCG (Fig. 1F) (n = 8 per genotype). Even at 10 h after hCG, on average 10–15 oocytes were recovered from the oviducts of Pten CKO mice, whereas no oocytes had been ovulated in controls (Fig. 1F) (n = 6 per genotype). Thus, ovulation rate is advanced and increased in the mutant mice.
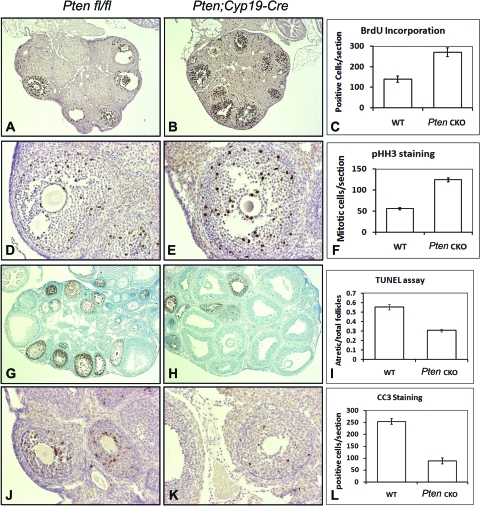
To characterize the observed changes of Pten knockout ovaries at the cellular level, we first examined the proliferative rate of GCs in the developing follicles. GCs of Pten CKO mice demonstrated increased proliferation based on bromodeoxyuridine (BrdU) incorporation (Fig. 2, A–C) and immunostaining for the mitosis marker phosphohistone H3 (pHH3) (Fig. 2, D–F). Apoptosis is a common feature of growing follicles in the mammalian ovary and serves to eliminate those follicles that do not become competent to ovulate. Because the Ptenfl/fl;Cyp19-Cre mice ovulate more oocytes than wild type, reduced apoptosis could permit more follicles to develop to the preovulatory stage. To test this, the TUNEL (terminal deoxynucleotide transferase-mediated deoxyuridine triphosphate nick end labeling) assay and immunostaining for cleaved caspase 3 (CC3) were performed. The numbers of both apoptotic follicles (Fig. 2, G–I) and CC3-positive GCs (Fig. 2, J–L) were decreased in the eCG-primed Pten mutant ovaries. Ovaries from more than four animals were analyzed in each experiment. These results suggest that Pten negatively impacts GC proliferation in vivo and promotes conditions favoring atresia/apoptosis of growing follicles, therefore constraining the number of oocytes to be ovulated in normal ovaries. Thus, follicle growth and ovulation were enhanced by disrupting of Pten in GCs.
Figure 2.
GC Proliferation and Apoptosis with or without Pten Depletion
Pten-depleted GCs demonstrated increased levels of proliferation, based on BrdU incorporation (A, control ovary; B, Pten CKO ovary; C, quantification of BrdU-positive cells) and immune staining for pHH3 (D, control ovary; E, Pten CKO ovary; F, quantification of pHH3-positive cells). According to TUNEL assay (G, control ovary; H, Pten CKO ovary; I, quantification of atretic follicles) and immunostaining of CC3 (J, control ovary; K, Pten CKO ovary; L, quantification of CC3-positive cells), the numbers of apoptotic follicles were decreased in the eCG-primed Pten knockout ovaries. WT, Wild type.
The PI3K/Protein Kinase B Pathway Is Hyperactivated in Pten-Depleted GCs
Based on these results and the well-established role of PTEN in regulating the PI3K/AKT pathway, we hypothesized that activation of this pathway by FSH, in the absence of PTEN, would increase AKT activation leading to increased follicular growth. To test whether this pathway was activated in vivo in response to eCG, wild-type mice were injected with eCG, and the phosphorylation of AKT was evaluated by immunofluorescence at 1, 8, and 48 h after eCG treatment. As shown in Fig. 3, A–D, phosphorylation of AKT was increased in GCs as early as 1 h after eCG treatment, and persisted in the mural GCs of fast growing antral follicles thereafter. These results were confirmed by Western blot analyses (Fig. 3E). At each time point, protein samples were collected by homogenizing the ovaries from three different animals; protein concentrations were determined by Bradford assay.
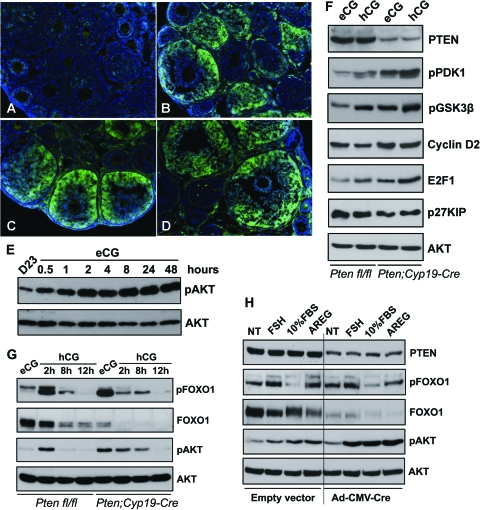
Figure 3.
Activation of PI3K Pathway in Ovaries with or without Pten Depletion
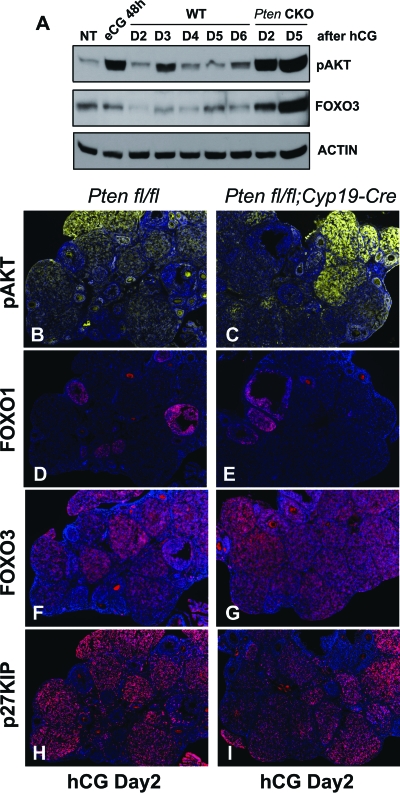
Phospho-AKT was absent in untreated immature ovaries (A) but was stimulated in GCs of growing follicles (B–D: 1, 8, and 24 h after eCG, respectively). These results were confirmed by Western blots (E). The levels of phospho-PDK1, -AKT, -GSK3β, and -FOXO1 were increased in Pten mutant GCs. Cyclin D2 and E2F1 levels were up-regulated whereas FOXO1 and p27KIP levels were down-regulated, in Pten CKO cells (F and G). In cultured Ptenfl/fl GCs, PTEN levels were decreased after infection with an adenoviral vector expressing Cre (Ad-CMV-Cre). FSH, FBS, and AREG induced more robust AKT phosphorylation in PTEN-depleted cells than in the PTEN-intact cells. FOXO1 levels decreased markedly in Pten-depleted cells (H). NT, No treatment.
We next compared the phosphorylation status of key components within the PI3K pathway and levels of AKT targets in GCs isolated from hormone-primed control and Pten CKO ovaries. As shown in Fig. 3, F and G, the levels of PTEN were reduced in the Pten mutant cells whereas levels of phospho-phosphoinositide-dependent protein kinase (PDK)1, -AKT, -glycogen synthase kinase 3 (GSK3)β were all increased in eCG- and hCG-primed Pten mutant GCs compared with controls. Moreover, levels of phospho-FOXO1 were increased whereas total FOXO1 protein was decreased in eCG-primed Pten mutant cells (Fig. 3G) providing additional evidence that hyperactivation of the PI3K pathway occurred in response to the loss of endogenous PTEN. Known targets of the PI3K pathway were also altered: cyclin D2 and E2F1 levels were up-regulated whereas p27KIP levels were down-regulated in Pten CKO cells compared with controls. Levels of total AKT showed no change. These results are consistent with the pattern of increased proliferation observed in the Pten-depleted GCs (Fig. 2).
We also tested the basal activity of the PI3K pathway in ovaries of 23-d-old females without hormonal stimulation. In these ovaries, there was little difference between wild-type and Pten CKO mice (data not shown), primarily because endogenous hormone levels appear too low to activate the PI3K pathway at this time. In addition, because Cyp19-Cre is preferentially expressed in antral follicles after eCG treatment (Fan et al., Development, in press), recombination is enhanced in antral, preovulatory follicles. Similar results were observed in cultured cells (Fig. 3H).
To study the synchronized and acute responses of GCs to Pten depletion, we isolated GCs from 21-d-old Ptenfl/fl mice and infected these cells with an adenoviral vector expressing CRE recombinase (Ad-CMV-Cre) or control vector. As shown in Fig. 3H, PTEN protein levels decreased dramatically in Ad-CMV-Cre-infected Ptenfl/fl cells. Although the basal level (NT, no treatment) of phospho-AKT in these cells increased only slightly when compared with cells infected with the control vector (similar to in vivo results; Fig. 3, F and G), addition of FSH, fetal bovine serum (FBS), and amphiregulin (AREG, a known intrafollicular effect of LH) to the culture medium induced more robust AKT phosphorylation in PTEN-depleted cells than in the PTEN-intact cells. Moreover, the levels of FOXO1 decreased markedly in PTEN-depleted cells causing the relative ratio of phospho-FOXO1 to total FOXO1 to increase in these cells. These in vitro experiments indicate that activation of the PI3K pathway by ligands is enhanced in GCs in which endogenous PTEN levels are reduced.
Structural But Not Functional (Steroidogenic) Luteolysis Is Repressed in Pten-Deficient CLs
One of the most dramatic differences in the Pten CKO mouse ovaries compared with control ovaries is the extraordinary abundance of corpora lutea (CL) present in the ovaries of the Pten mutant mice. Specifically, after 3 months of age the ovaries of Pten CKO mice were larger and visually appeared to contain more CL than control mice (Fig. 4, A and B). Histological analyses of ovaries from 6-month-old Pten mutant mice confirmed the abundance of CL (Fig. 4, C and D). Total numbers of antral follicles and CL in ovaries of control and Pten CKO mice were counted in serial ovarian sections. Whereas the Pten CKO ovaries contained slightly more antral follicles than controls (Fig. 4E), they had 3 times more CL than normal cycling ovaries (Fig. 4F). In situ hybridization results showed that the fully developed CL in wild-type ovaries expressed Lhcgr (Fig. 4, G and H), which encodes the LH receptor and is a marker of newly formed, healthy CL. But only some of the CL in Pten CKO ovaries expressed Lhcgr. However, other persistent CL did not express Lhcgr (Fig. 4, I and J). These results suggested that the numerous CL observed in Pten CKO ovaries were at different stages of differentiation, and that some of them might be nonfunctional [a fact that has been conformed (see Fig. 6)]. Furthermore, immunofluorescent staining of wild-type ovaries revealed that PTEN protein was highly expressed in CL of control mice (Fig. 4, K and L) but was markedly reduced in the endocrine LCs of Pten CKO ovaries (Fig. 4M). In contrast, PTEN remained highly expressed in the vascular endothelial cells present in corpora lutea (Fig. 4M). The relative abundance of PTEN protein levels in GCs, LCs, residual tissue (RT, stromal/interstitial cells, and remaining small follicles), were compared by Western blot analysis. GCs were collected from preovulatory follicles by needle puncture of ovaries isolated from eCG-primed control mice whereas LCs were collected by needle disruption of ovaries isolated from eCG-primed mice 48 h after hCG. RT was collected as that remaining after removal of LCs. As shown in Fig. 4N, the levels of PTEN were higher in LCs and RT than in GCs.
Figure 6.
Expression of PI3K Pathway Components in Fully Developed CLs
Western blots showed that AKT activity and FOXO3 were up-regulated in Pten CKO ovaries after CL formation (A). Immunofluorsecent staining showed higher levels of phospho-AKT in CL of the Pten mutant mice (C) than control mice (B) on d 2. Among the known targets of AKT, FOXO1 was undetectable in fully developed CLs, in both control (D) and Pten CKO (E) ovaries. In contrast, FOXO3 (F and G) and p27KIP (H and I) were highly expressed in the CLs, regardless of the genotypes. WT, Wild type; NT, no treatment.
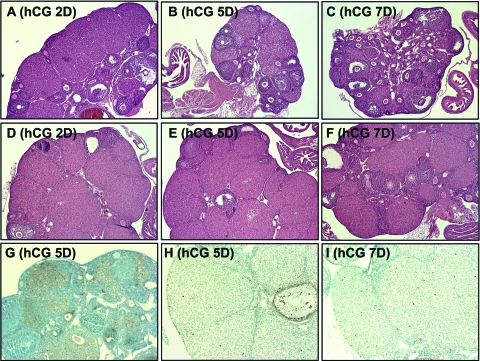
To determine the extent to which the life span of CL in the Pten mutant ovaries was extended, immature Pten CKO and control mice were injected with a superovulatory regimen of eCG/hCG, and the ovaries were examined on selected days thereafter. In control mice, CL that were present in ovaries on d 2 after hCG, began to regress by d 4 and disappeared by d 7 [Fig. 5A (hCG D2), B (hCG D5), C (hCG D7)]. In contrast, ovaries of the Pten mutant mice contained many large CL on d 5 and d 7 (four of eight mice) (Fig. 5, D–F) and even until d 15 (two of eight mice) (data not shown). TUNEL assays showed many apoptotic LCs in ovaries of control mice on d 4–5 after hCG (Fig. 5G), whereas no apoptotic cells were observed in LCs of the mutant mice at any time point examined after ovulation (Fig. 5, H and I), indicating that luteolysis was dramatically impaired and delayed.
Figure 5.
Luteal Regression after hCG Injection
In wild-type mice, CL began to regress by d 4 after hCG and disappeared by d 7 (A–C: hCG d 2, d 5, and d 7, respectively), whereas the Pten CKO ovaries retained many CL at d 2, d 5, and d 7 (D–F). Apoptosis of LCs culminated at d 4–5 after hCG in wild type (G), but not in Pten mutant CL at the hCG d 5 (H) or d 7 (I).
Because PTEN is highly expressed in LCs and the Pten-depleted CL exhibit a prolonged life span, additional analyses were done to determine the relative levels of PI3K pathway components in ovaries of Pten CKO mice compared with controls. Western blots of ovaries obtained from eCG/hCG-primed mice (n = 3 per genotype at each time point) showed that phospho-AKT and FOXO3 were dramatically up-regulated in Pten CKO ovaries compared with controls at d 2 and d 5 after hCG injection when many CL were present (Fig. 6A). Immunofluorescent staining documented elevated levels of phospho-AKT and FOXO3 in CL of the Pten mutant mice on d 2 (Fig. 6, panels B, C, F, and G). However, the known AKT targets, FOXO1 and p27KIP, were differentially regulated during luteinization. FOXO1 levels were dramatically down-regulated by LH/hCG (Fig. 3G). In contrast to the Western blot results, FOXO1 was undetectable in fully developed CL at d 2 after hCG, in both control (Fig. 6D) and Pten CKO (Fig. 6E) ovaries. In contrast, p27KIP (Fig. 6, H and I) was highly expressed in the CL, regardless of the genotype. Both the Western blots and immunostaining were performed at least twice with similar results.
Steroidogenically Inactive LCs Accumulate in Pten-Deficient CL
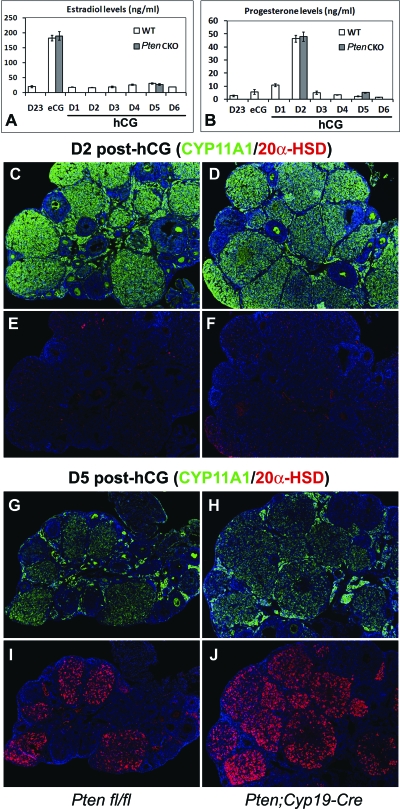
To evaluate the endocrine function of Pten-depleted GCs and LCs, we analyzed levels of serum estradiol and progesterone in control and Pten CKO mice. In mice of both genotypes (n = 3 per genotype at each time point), estradiol increased in association with the growth of preovulatory follicles in response to eCG and dropped precipitously in response to hCG (Fig. 7A). Progesterone peaked on d 2 after hCG, when CL have formed and declined precipitously thereafter (Fig. 7B). These observations indicated that steroid biosynthesis was similar in mice of each genotype even though CL structures persist longer in the Pten CKO mice. To assess the molecular basis for these endocrine events, we compared the expression patterns of key enzymes of progesterone metabolism in LCs of control and Pten CKO mice. At d 2 after hCG, cytochrome P450 (CYP)11A1, a key enzyme of progesterone biosynthesis and a marker of LC function, was expressed at high levels in CL of both control (Fig. 7C) and Pten CKO ovaries (Fig. 7D) but declined at d 5 after hCG regardless of genotype (Fig. 7, G and H). Moreover, 20α-hydroxysteroid dehydrogenase (20α-HSD), which catalyzes the inactivation of progesterone, and is a marker of functionally regressing LCs, was expressed in a reciprocal manner to CYP11A1, in both control (Fig. 7, E and I) and Pten CKO ovaries (Fig. 7, F and J). Thus, despite the extension of their life span, the CL in Pten CKO mice did not remain steroidogenically active. These observations clearly separate functional (endocrine) luteolysis from structural luteolysis.
Figure 7.
Endocrine Functions of CL in Pten CKO Mice
Levels of serum estradiol (A) and progesterone (B) were not different between control and Pten CKO mice after eCG/hCG treatments. At d 2 after hCG, CYP11A1 was expressed at high level in CL (C and D) but declined at d 5 regardless of genotype (E and F). Conversely, 20α-HSD was expressed in a reciprocal manner to CYP11A1, in both wild (G and I) and Pten CKO ovaries (H and J). WT, Wild type.
DISCUSSION
The pituitary gonadotropin FSH controls follicular development by regulating granulosa cell proliferation and differentiation (18,19). LH, in turn, initiates ovulation, terminates granulosa cell proliferation, and mediates the genetic transition of GCs to LCs (20). Both hormones bind cognate G-protein coupled receptors and activate adenylyl cyclase leading to increased levels of cAMP and protein kinase A (2,21). However, recent studies show that FSH and LH activate additional signaling cascades, independently of protein kinase A, that impact follicular development, ovulation, and oocyte maturation. Specifically, FSH and LH activate RAS and the downstream targets RAF1, MEK, and ERK1/2 (22) (35) as well as PI3K and the downstream targets AKT and the transcription factor FOXO1 (4,7). Phosphorylation of FOXO1 excludes its transport to the nucleus and leads to its degradation in the cytoplasm (23,24). FSH and LH also act to turn off expression of the Foxo1 gene in GCs in culture and preovulatory follicles, respectively, suggesting that FOXO1 may antagonize GC proliferation and/or differentiation (7,8,25).
One well-characterized negative regulator of the PI3K/AKT pathway is the tumor suppressor PTEN, which when mutated frequently leads to tumorigenesis (14,26). Based on the critical role of this pathway in cell proliferation and survival and because the physiological roles of the PI3K, AKT, and FOXO pathway during follicular development and luteinization have not been characterized, we hypothesized that selective disruption of the PI3K repressor, PTEN, in GCs using a novel, GC-specific Cyp19-Cre-mouse model would alter GC proliferation and differentiation and thereby impact follicular development, ovulation, and/or luteinization.
In agreement with our hypothesis, we show that disruption of the Pten gene in GCs altered both FSH regulation of follicular development and that this was related to increased phosphorylation of the PI3K components, PDK1, AKT, and FOXO1. Increased phosphorylation of FOXO1 was also associated with a dramatic reduction of FOXO1 protein in the mutant cells. These biochemical changes in GCs were associated with an enhanced number of follicles ovulating and the rate at which they ovulated. This enhancement of ovulation was mediated, in part, by increased proliferation of GCs that was associated with higher levels of the cell cycle activators cyclin D2 and E2F1 and reduced levels of the cell cycle inhibitor p27KIP. Enhanced follicular growth and the increased number of ovulating follicles were also related to a reduced number of follicles exhibiting apoptotic GCs. These results indicate that the increased phosphorylation and activation of AKT and the premature decrease in FOXO1 in vivo are associated with the promotion of GC survival and an increased number of follicles reaching the preovulatory stage.
The effects of Pten loss in GCs are much less dramatic than the loss of Pten in oocytes where disruption of this gene leads to premature follicle growth, depletion of the follicle reserve, and premature ovarian failure (13). The apparent lack of a more dramatic effect of Pten disruption in GCs on the number of follicles growing and ovulating may be due to the relatively low levels of PTEN protein present in GCs compared with other ovarian cell types, including oocytes. Therefore, we hypothesize that negative regulatory factors, in addition to PTEN, impact the PI3K pathway in GCs. The expression levels of PTEN in GCs may vary among different mammalian species. It is noteworthy that mouse is a polyovulatory species in which multiple oocytes are ovulated in each estrous cycle. In mono-ovulatory mammals PTEN might play more critical roles in restraining the activity of PI3K pathway and controlling the single follicle that reaches the ovulation stage. In support of this possibility, a recent study indicated that levels of PTEN increased in ovine GCs during terminal follicular growth, and that PTEN regulated the expression of p27KIP and E2F in these cells (27).
Because the Pten CKO mice were fertile and gave birth to live pups, the loss of Pten in GCs did not impair LH-induced ovulation, block the terminal differentiation of GCs to LCs, or disrupt the maintenance of functional CL during pregnancy. These results clearly indicate that responses to FSH and LH were not impaired. Moreover, the endocrine profiles of serum estradiol and progesterone and the expression of enzymes controlling progesterone biosynthesis were identical in hormonally primed Pten CKO mice and control mice. However, despite the normal pattern of endocrine functions in the LCs of the Pten CKO mice, the CL exhibited a prolonged existence that was not associated with prolonged steroidogenic activity. PTEN is expressed at low levels in GCs but is increased in LCs. These results support recent immunohistochemical localization of PTEN in human CL (28). Although the cell cycle repressor p27KIP is highly expressed in LCs is required for CL formation (29,30), p27KIP levels were not altered dramatically in the Pten-deficient cells and therefore do not appear to be the primary mediator of the Pten-null phenotype (Fig. 6).
Rather, the biochemical basis for the extended life span of the Pten-deficient LCs appears to be associated with enhanced phosphorylation and activation of AKT and the expression of a distinct set of PI3K pathway components, including FOXO3. Whereas levels of FOXO1 protein are rapidly decreased during luteinization, expression of FOXO3 continues in LCs (Fig. 6). These results support our previous in situ hybridization analyses and clearly indicate that each FOXO factor is expressed in a cell-specific manner and differentially regulated by specific hormones (7). Moreover, the levels of FOXO3 are elevated in the Pten-deficient LCs, indicating that activated AKT may exert a positive regulatory role on this factor. These results indicate that FOXO3 may also exert functions that are distinct from those of FOXO1, as has been observed in other cell types (31). Again it is important to note that PTEN, AKT, and FOXO3 are present at high levels in oocytes, and depletion of either Pten or Foxo3 causes premature ovarian failure (12,13,32). Thus, it is clear that the consequences of disrupting Pten in LCs are distinct from those in oocytes. Because apoptosis was decreased and delayed in the Pten mutant LCs, as determined by TUNEL assays and activated caspase 3 staining, it is possible that components of the apopotic pathway are targets of AKT and FOXO3. However, because caspase 3-null mice do not exhibit the marked accumulation of CL as observed in the Pten CKO mice (33,34), factors in addition to caspase 3 appear to be involved in regulating the extended life span of Pten-null LCs. The identification of these factors will provide important information on targets of the PI3K pathway that may be specific for LCs compared with GCs.
Collectively, these data provide evidence that PTEN is expressed in a cell-specific manner in the ovary and appears to exert cell-specific functions to regulate GC proliferation and apoptosis as well as LC longevity. That PTEN is expressed at higher levels in LCs than GCs was somewhat unexpected given the high levels of FOXO1 in GCs. Therefore, additional regulators of the PI3K pathway in GCs are likely to be operative. That the loss of PTEN leads to a dramatic extension of LC longevity and to the accumulation of CL in the adult Pten mutant ovaries indicates that this regulator of the PI3K pathway plays an important role in LC survival. Because FOXO3 is preferentially expressed in LCs suggests that this factor may play a specific role in these cells as well. Importantly, loss of PTEN and changes in activation of the PI3K pathway did not exert pronounced effects of GC differentiation or luteinization, indicating that factors controlling cell survival operate by mechanisms distinct from those controlling cell differentiation and steroidogenesis. Thus, repression of the AKT pathway by PTEN appears to be required to favor GC and LC apoptosis as well as structural regression of the CL but does not markedly impact the endocrine functions of these cells.
MATERIALS AND METHODS
Animals
Immature C57BL/6 mice were obtained from Harlan, Sprague Dawley, Inc. (Indianapolis, IN). Mice lacking Pten in GCs were generated by crossing Cyp19-Cre mice with previously reported Ptenfl/fl mice (16). The Cyp19-Cre transgenic mice were generated by oocyte microinjection of a DNA fragment in which the Cyp19 promoter (304 bp) is followed by iCre cDNA. On d 23 of age, female mice were injected ip with 4 IU of eCG (Pregnyl; Organon, West Orange, NJ) followed 48 h later with 5 IU hCG (Gestyl; Diosynth, Oss, The Netherlands) to promote synchronized follicle growth and ovulation. Animals were housed under a 16-h light, 8-h dark schedule, provided food and water ad libitum, and treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Serum Analysis
Mice were anesthetized and blood was collected by cardiac puncture. FSH, LH, progesterone, and estradiol measurements were made by the University of Virginia Ligand Core Facility (Specialized Cooperative Center Program in Reproduction and Infertility Research: NIH U54 HD28934).
Immunohistochemistry
Tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Immunohistochemistry was performed on 5-μm sections using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA). Rabbit antiphosphohistone H3 (Upstate Laboratories, Lake Placid, NY), and rabbit anticleaved caspase-3 (Cell Signaling Technology, Beverly, MA) were used to evaluate cell proliferation and apoptosis in follicles. For direct comparison, wild-type and Pten CKO ovary sections from four individual females were processed together. Phospho-HH3 or CC3-positive cell numbers were quantified in 10 high-power fields per section, and four individual sections were quantified per specimen.
TUNEL Assay
Analysis of apoptosis in ovarian follicles was carried out by TUNEL assay using the ApopTag Plus in situ apoptosis detection kit (Chemicon International, Temecula, CA). At least four different specimens from Pten CKO and Ptenfl/fl mice were analyzed in parallel. Total and TUNEL-positive follicles were quantified per section, and three individual sections were quantified per specimen.
BrdU Incorporation Assay
Mice received an ip injection of 50 mg/kg of BrdU and were killed 2 h after treatment. Incorporated BrdU was detected by immunohistochemistry using BrdU antibody (Sigma Chemical Co., St. Louis, MO) according to manufacturer’s instruction.
GC Culture
GCs were harvested from 23-d-old Ptenfl/fl mice and cultured at a density of 1 × 106 cells in serum-free medium (DMEM/F12) in 12-well culture dishes. After 4 h culture, cells were infected with adenoviral vectors expressing Cre recombinase (Vector Development Laboratory, Baylor College of Medicine) at a multiplicity of infection of 4:1. At 20 h after infection, GCs were stimulated with FSH (100 ng/ml), FBS (10%), or AREG (100 ng/ml).
Western Blot Analysis
Cell extracts containing 30 μg protein were resolved by SDS-PAGE and transferred to polyvinylidine difluoride membranes (Millipore Corp., Bedford, MA). The primary antibodies used were: p27KIP and E2F1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), cyclin D2 (Lab Vision Corp., Fremont, CA). FOXO1, phospho-FOXO1, AKT, phospho-AKT, phospho-ERK1/2, phospho-PDK1, phospho-GSK3β, PTEN (Cell Signaling Technology).
Acknowledgments
We thank Dr. Jan Gossen for providing the Cyp19-Cre mice, Dr. Geula Gibori for providing the 20α-HSD antibody, and Dr. Dale Hales for providing the CYP11A1 antibody. We thank Dr. Michael Mancini and members of the Microscopy Core for their time and assistance and Yuet Lo for her many contributions. We also thank Dr. Masayuki Shimada and Dr. Chao Tong for suggestions in the experiments and critical reading of the manuscript.
Footnotes
This work is supported by National Institutes of Health (NIH) Grants NIH-HD16229, NIH-HD16272, NIH-HD07495 (Specialized Cooperative Centers Program in Reproduction and Infertility Research), Project II (to J.S.R.) and NIH Postdoctoral Training Grant NIH-HD07165 (to H.Y.F.).
Disclosure Statement: The authors have nothing to declare.
First Published Online July 17, 2008
Abbreviations: AKT, Acute transforming retrovirus thymoma protein kinase; AREG, amphiregulin; BrdU, bromodeoxyuridine; CC3, cleaved caspase 3; CG, chorionic gonadotropin; CL, corpora lutea; CRE, cAMP response element; CYP, cytochrome P450; FBS, fetal bovine serum; GC, granulosa cell; GSK3, glycogen synthase kinase 3; 20α-HSD, 20α-hydroxysteroid dehydrogenase; LC, luteal cell; PDK, phosphoinositide-dependent protein kinase; pHH3, phosphohistone H3; PI3K, phosphatidylinositol-3 kinase; PTEN, phosphatase and tensin homolog; RT, residual tissue; TUNEL, terminal deoxynucleotide transferase-mediated deoxyuridine triphosphate nick end labeling.
References
- Stocco C, Telleria C, Gibori G 2007 The molecular control of corpus luteum formation, function, and regression. Endocr Rev 28:117–149 [DOI] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Maizels ET 2006 FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal 18:1351–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Robayna IJ, Falender AE, Ochsner S, Firestone GL, Richards JS 2000 Follicle-Stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid- induced kinase (Sgk): evidence for A kinase-independent signaling by FSH in granulosa cells. Mol Endocrinol 14:1283–1300 [DOI] [PubMed] [Google Scholar]
- Alam H, Maizels ET, Park Y, Ghaey S, Feiger ZJ, Chandel NS, Hunzicker-Dunn M 2004 Follicle-stimulating hormone activation of hypoxia-inducible factor-1 by the phosphatidylinositol 3-kinase/AKT/Ras homolog enriched in brain (Rheb)/mammalian target of rapamycin (mTOR) pathway is necessary for induction of select protein markers of follicular differentiation. J Biol Chem 279:19431–19440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Marte BM, Warne PH, Downward J 1996 Phosphatidylinositol 3′ kinase: one of the effectors of Ras. Philos Trans R Soc Lond B Biol Sci 351:225–231; discussion 231–222 [DOI] [PubMed] [Google Scholar]
- Gupta S, Ramjaun AR, Haiko P, Wang Y, Warne PH, Nicke B, Nye E, Stamp G, Alitalo K, Downward J 2007 Binding of Ras to phosphoinositide 3-kinase p110α is required for Ras-driven tumorigenesis in mice. Cell 129:957–968 [DOI] [PubMed] [Google Scholar]
- Richards JS, Sharma SC, Falender AE, Lo YH 2002 Expression of FKHR, FKHRL1, and AFX genes in the rodent ovary: evidence for regulation by IGF-I, estrogen, and the gonadotropins. Mol Endocrinol 16:580–599 [DOI] [PubMed] [Google Scholar]
- Park Y, Maizels ET, Feiger ZJ, Alam H, Peters CA, Woodruff TK, Unterman TG, Lee EJ, Jameson JL, Hunzicker-Dunn M 2005 Induction of cyclin D2 in rat granulosa cells requires FSH-dependent relief from FOXO1 repression coupled with positive signals from Smad. J Biol Chem 280:9135–9148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Ito J, Yamashita Y, Okazaki T, Isobe N 2003 Phosphatidylinositol 3-kinase in cumulus cells is responsible for both suppression of spontaneous maturation and induction of gonadotropin-stimulated maturation of porcine oocytes. J Endocrinol 179:25–34 [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ, Saxena D, Little-Ihrig L 2003 Protein kinase B is obligatory for follicle-stimulating hormone-induced granulosa cell differentiation. Endocrinology 144:3985–3994 [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Yokoo M, Yoshida N, Sasada H, Matsumoto H, Sato E 2004 Phosphatidylinositol 3-kinase and Akt participate in the FSH-induced meiotic maturation of mouse oocytes. Mol Reprod Dev 69:77–86 [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA 2003 Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 301:215–218 [DOI] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hamalainen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K 2008 Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 319:611–613 [DOI] [PubMed] [Google Scholar]
- Cantley LC, Neel BG 1999 New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA 96:4240–4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansal I, Sellers WR 2004 The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol 22:2954–2963 [DOI] [PubMed] [Google Scholar]
- Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, Liu X, Wu H 2002 Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis 32:148–149 [DOI] [PubMed] [Google Scholar]
- Soriano P 1999 Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21:70–71 [DOI] [PubMed] [Google Scholar]
- Rao MC, Midgley Jr AR, Richards JS 1978 Hormonal regulation of ovarian cellular proliferation. Cell 14:71–78 [DOI] [PubMed] [Google Scholar]
- Richards JS, Fitzpatrick SL, Clemens JW, Morris JK, Alliston T, Sirois J 1995 Ovarian cell differentiation: a cascade of multiple hormones, cellular signals, and regulated genes. Recent Prog Horm Res 50:223–254 [DOI] [PubMed] [Google Scholar]
- Robker RL, Richards JS 1998 Hormonal control of the cell cycle in ovarian cells: proliferation versus differentiation. Biol Reprod 59:476–482 [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Hsieh M, Doyle KH, Falender AE, Lo YK, Sharma SC 2002 Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog Horm Res 57:195–220 [DOI] [PubMed] [Google Scholar]
- Wayne CM, Fan HY, Cheng X, Richards JS 2007 FSH-induces multiple signaling cascades: evidence that activation of SRC, RAS and the EGF receptor are critical for granulosa cell differentiation. Mol Endocrinol 21:1940–1957 [DOI] [PubMed] [Google Scholar]
- Arden KC 2007 FoxOs in tumor suppression and stem cell maintenance. Cell 128:235–237 [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME 1999 Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857–868 [DOI] [PubMed] [Google Scholar]
- Rudd MD, Gonzalez-Robayna I, Hernandez-Gonzalez I, Weigel NL, Bingman III WE, Richards JS 2007 Constitutively active FOXO1a and a DNA-binding domain mutant exhibit distinct co-regulatory functions to enhance progesterone receptor A activity. J Mol Endocrinol 38:673–690 [DOI] [PubMed] [Google Scholar]
- Li G, Robinson GW, Lesche R, Martinez-Diaz H, Jiang Z, Rozengurt N, Wagner KU, Wu DC, Lane TF, Liu X, Hennighausen L, Wu H 2002 Conditional loss of PTEN leads to precocious development and neoplasia in the mammary gland. Development 129:4159–4170 [DOI] [PubMed] [Google Scholar]
- Froment P, Bontoux M, Pisselet C, Monget P, Dupont J 2005 PTEN expression in ovine granulosa cells increases during terminal follicular growth. FEBS Lett 579:2376–2382 [DOI] [PubMed] [Google Scholar]
- Goto M, Iwase A, Ando H, Kurotsuchi S, Harata T, Kikkawa F 2007 PTEN and Akt expression during growth of human ovarian follicles. J Assist Reprod Genet 24:541–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A 1996 Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell 85:721–732 [DOI] [PubMed] [Google Scholar]
- Robker RL, Richards JS 1998 Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol Endocrinol 12:924–940 [DOI] [PubMed] [Google Scholar]
- Auer KL, Park JS, Seth P, Coffey RJ, Darlington G, Abo A, McMahon M, Depinho RA, Fisher PB, Dent P 1998 Prolonged activation of the mitogen-activated protein kinase pathway promotes DNA synthesis in primary hepatocytes from p21Cip-1/WAF1-null mice, but not in hepatocytes from p16INK4a-null mice. Biochem J 336:551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P, Shen L, Ren C, Boman K, Lundin E, Ottander U, Lindgren P, Liu YX, Sun QY, Liu K 2005 Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev Biol 281:160–170 [DOI] [PubMed] [Google Scholar]
- Carambula SF, Matikainen T, Lynch MP, Flavell RA, Goncalves PB, Tilly JL, Rueda BR 2002 Caspase-3 is a pivotal mediator of apoptosis during regression of the ovarian corpus luteum. Endocrinology 143:1495–1501 [DOI] [PubMed] [Google Scholar]
- Carambula SF, Pru JK, Lynch MP, Matikainen T, Goncalves PB, Flavell RA, Tilly JL, Rueda BR 2003 Prostaglandin F2α- and FAS-activating antibody- induced regression of the corpus luteum involves caspase-8 and is defective in caspase-3 deficient mice. Reprod Biol Endocrinol 1:15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H-Y, Shimada M, Liu Z, Cahill N, Noma N, Wu Y, Gossen J, Richards JS 2008 Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development 135:2127–2137;6.6p> [DOI] [PMC free article] [PubMed] [Google Scholar]