Abstract
Human immunodeficiency virus (HIV) infection begins with fusion between viral and host cell membranes and is catalyzed by the HIV gp41 fusion protein. The ~20 N-terminal apolar residues of gp41 are called the HIV fusion peptide (HFP), interact with the host cell membrane, and play a key role in fusion. In this study, the membrane location of peptides which contained the HFP sequence AVGIGALFLGFLGAAGSTMGARS was probed in samples containing either only phospholipids or phospholipids and cholesterol. Four HFPs were examined which each contained 13CO labeling at three sequential residues between G5 and G16. The 13CO chemical shifts indicated that HFP had predominant β strand conformation over the labeled residues in the samples. The internuclear distances between the HFP 13COs and the lipid 31Ps were measured using solid-state nuclear magnetic resonance rotational-echo double resonance experiments. The closest 13CO-31P distances of 5–6 Å were observed for HFP labeled between A14 and G16 and correlated with intimate association of β strand HFP and membranes. These results were confirmed with measurements using HFPs singly 13CO labeled at A6 or A14. To our knowledge, these data are the first measurements of distances between HIV fusion peptide nuclei and lipid P and qualitative models of membrane location of oligomeric β strand HFP are presented which are consistent with the experimental data. Observation of intimate contact between β strand HFP and membranes provides rationale for further investigation of the relationship between structure and fusion activity for this conformation.
Keywords: HIV, fusion peptide, membrane, carbonyl, phosphorus, REDOR, NMR
The infection of enveloped viruses such as human immunodeficiency virus (HIV) begins with fusion between the viral and host cell membranes (1–4). Fusion may be catalyzed by fusion proteins and several models of fusion protein catalysis have been proposed (2, 5–7). For HIV, fusion is catalyzed by a “gp160” glycoprotein complex which is incorporated in the virus membrane and is composed of two non-covalently associated subunits “gp120” and “gp41”. The gp120 subunit lies outside the virus and binds to receptors in the target cell membrane and the gp41 subunit contains a region inside HIV as well as a single-pass transmembrane domain (8, 9). The ~170- residue ectodomain of gp41 lies outside HIV and is subdivided into a more C-terminal “soluble ectodomain” and a ~20-residue N-terminal fusion peptide (HFP) which is apolar and fairly conserved. The HFP is believed to interact with the target cell membrane after gp120 binds to cellular receptors and fusion is greatly disrupted by mutation or deletion of the HFP (10–13).
Peptides with the HFP sequence catalyze vesicle fusion and there are good correlations between the mutation/fusion activity relationships of HFP-induced vesicle fusion and HIV/target cell fusion (14–17). Studies of membrane-associated HFP should therefore provide useful information about some aspects of biological fusion. Both the conformation and membrane location of the HFP have been hypothesized to be significant structural factors for fusion catalysis by the HFP (16, 18). The conformation of the HFP has been investigated in detergent micelles and membranes using a variety of biophysical techniques. For HFP associated with negatively charged sodium dodecyl sulfate micelles, one liquid-state nuclear magnetic resonance (NMR) study showed that there was uninterrupted α helical structure from I4 to M19 while another study showed a helix from I4 to A14 followed by a β turn (19, 20). For HFP associated with neutral dodecylphosphocholine micelles, helical structure was detected from I4 to L12 (21, C. M. Gabrys and D. P. Weliky, unpublished data). There is not yet a consensus for the micelle location of HFP and there are distinct models based on experiment and simulation of either predominant micelle surface location or micelle traversal by HFP (19–23). In one NMR study, residues I4 to A15 were found to be fully shielded from solvent and residues G3 and G16 were at the micelle/solvent interface (20).
The conformation of membrane-associated HFP has been investigated with different lipid components and different peptide:lipid ratios. A greater fraction of HFPs adopted helical structure at low peptide:lipid while non-helical structure became more favored at higher ratios (24). Helical structure was also promoted by negatively charged lipids while a higher fraction of β strand structure was adopted with neutral lipids or with bound Ca2+ (16, 24–26). Solid-state NMR provided residue-specific conformational information of HFP associated with membranes whose lipid headgroup and cholesterol composition was comparable to that of host cells of the virus. A β strand conformation was observed for residues A1 to G16 while A21 appeared to be unstructured (27, 28). Formation of β strand oligomers or aggregates was supported by detection of short distances between labeled 13COs on one HFP and labeled 15N on an adjacent HFP (29). Oligomerization/aggregation has also been detected by other biophysical methods (15, 30). There is evidence that at least the lipid mixing step of membrane fusion can occur with the HFP in either helical or β strand conformation although there is some controversy in the literature about this conclusion (14, 16, 18, 31–35).
HFP location in membranes has been primarily probed using a HFP-F8W mutant and by variation of the tryptophan fluorescence of this mutant with changes in environment (36, 37). Key results have included: (1) fluorescence was higher for membrane-associated HFP-F8W than for HFP-F8W in buffered saline solution; (2) greater fluorescence quenching by acrylamide was observed for a soluble tryptophan analog than for membrane-associated HFP-F8W; and (3) similar fluorescence quenching of membrane-associated HFP-F8W was observed in samples containing either 1-palmitoyl-2-stearoyl-phosphocholine brominated at the 6, 7 carbons of the stearoyl chain or the corresponding lipid brominated at the 11, 12 carbons of the chain. The first two results indicated that solvent exposure of the HFP-F8W tryptophan is reduced with membrane association and the third result indicated that the membrane location of the tryptophan indole group is centered near the carbon 9 position of the brominated lipid stearoyl chain; i.e. ~8.5 Å from the bilayer center and ~10 Å from the lipid phosphorus. Infrared (IR) and solid-state NMR spectra of membrane-associated HFP suggested that the HFP-F8W had predominant β strand conformation under the conditions of the fluorescence experiments (16, 27, 36, 37).
In a different set of experiments, electron spin resonance spectra showed that chromium oxalate in the aqueous phase quenched the signal of membrane-associated HFP which was spin-labeled at M19 but did not quench HFP spin-labeled at A1 (30). These data indicated a M19 location close to the aqueous interface of the membrane and an A1 location away from this interface.
Models for HFP location in membranes have also been developed from simulations of a single HFP molecule in membranes and have shown either partial insertion or traversal of the membrane. The HFP always adopted predominant α helical conformation and in one simulation was generally near the membrane surface with the F8 backbone and sidechain nuclei respectively 4 Å and 6 Å deeper than the phosphorus longitude (38). For a different simulation, HFP traversed the membrane and the backbone and sidechain F8 nuclei were at the bilayer center, i.e. ~19 Å from the phosphorus longitude (39).
This paper includes solid-state NMR measurements of distances between 13C labeled carbonyl (13CO) nuclei in HFP and lipid 31P nuclei. These studies provide information about the location of specific HFP residues relative to the phosphorus headgroups and are complementary to other solid-state NMR methods to probe membrane location of peptides and proteins (40–50). The 13CO-31P distance approach has previously been used to probe the locations of antimicrobial peptides, antibiotics, and sterols in membranes (51–53). Measurements were made both on HFP associated with membranes containing only phospholipids and on HFP associated with membranes which contained both phospholipids and cholesterol. The potential significance of cholesterol-containing membranes is suggested both by the cholesterol:phospholipid mol ratios of ~0.5 and 0.8 for HIV host cell and HIV membranes, respectively, and by the observation that β strand conformation of HFP is promoted by membrane cholesterol (27, 35, 54–57).
The 13CO-31P distances (r) were probed with the rotational-echo double resonance (REDOR) technique which is a solid-state NMR method to measure magnitudes of dipolar couplings (d) between spin ½ heteronuclei such as 13C and 31P (58). For a 13CO-31P spin pair, r = 23.05/d1/3 where r and d are in units of Å and Hz, respectively. The upper limit of REDOR detection of r is ~10 Å (d ≥ 10 Hz) (35).
MATERIALS AND METHODS
Materials
Resins and 9-fluorenylmethoxycarbonyl (FMOC) amino acids were obtained from Peptides International (Louisville, KY). 13CO-isotopically labeled amino acids were obtained from Cambridge Isotope Laboratories (Andover, MA). The lipids 1,2-di-Otetradecyl-sn-glycero-3-phosphocholine (DTPC), 1,2-di-O-tetradecyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (DTPG), and [1-13C]-1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC-13C) were obtained from Avanti Polar Lipids (Alabaster, AL). The 5 mM HEPES buffer at pH 7 contained 0.01% (w/v) NaN3 preservative.
Peptides
All peptides contained the sequence AVGIGALFLGFLGAAGSTMGARS which is the 23 N-terminal residues of HIV-1 gp41, LAV1a strain. A set of peptides were synthesized to probe peptide/lipid headgroup distances between G5 and G16. “HFP1-8FLG” had the sequence AVGIGALFLGFLGAAGSTMGARS-NH2 and was 13CO labeled at F8, L9, and G10. “HFP2-5GAL”, “HFP2-11FLG”, and “HFP2-14AAG” had the sequence AVGIGALFLGFLGAAGSTMGARSKKK-NH2 and were 13CO labeled at G5, A6, L7; F11, L12, G13; or A14, A15, G16, respectively. The three non-native lysines increased aqueous solubility and resulted in monomeric peptide in the buffer solution prior to membrane binding (59). “HFP3-8FLG” had sequence AVGIGALFLGFLGAAGSTMGARSKKKAβ and was 13CO labeled at F8, L9, and G10 and “HFP4”, “HFP4-6A”, and “HFP4-14A” had sequence AVGIGALFLGFLGAAGSTMGARSWKKKKKKAβ and were unlabeled or 13CO labeled at A6 or A14, respectively. The β-alanine resin used for the HFP3 and HFP4 syntheses had a low substitution which helped to increase yield. All peptides were synthesized using an ABI 431A peptide synthesizer (Foster City, CA) and FMOC chemistry. Peptides were cleaved from the resin for 2–3 hours using either a mixture of trifluoroacetic acid (TFA):water:phenol:thioanisole:ethanedithiol:water in a 33:2:2:2:1 volume ratio or a mixture of TFA:thioanisole:ethanedithiol:anisole in a 90:5:3:2 volume ratio. TFA was removed from the cleavage filtrate with nitrogen gas and peptides were precipitated with cold t-butyl methyl ether. Peptides were purified by reversed-phased high performance liquid chromatography using a semi-preparative C18 column and a water-acetonitrile gradient containing 0.1% TFA. Mass spectroscopy was used for peptide identification.
NMR sample preparation
Samples were made with the ether-linked lipids DTPC and DTPG because these are commercially available lipids which do not contain carbonyl groups. The liquid-crystalline to gel phase transition temperatures of DTPC and DTPG are ~28 C and are close to the ~23 C phase transition temperatures of the corollary ester-linked lipids 1,2-dimyristoyl-sn-glycero-3-phosphocholine and 1,2-dimyristoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (60). If NMR samples had been made with the more typical ester-linked lipids, there would be large natural abundance lipid 13CO signals which would overlap with the peptide 13CO signals (35). For such samples, analysis of the peptide 13CO-lipid 31P distances would then be complicated by the close proximity of the lipid 13CO to lipid 31P. All samples contained a 4:1 DTPC:DTPG mol ratio which reflected the approximate ratio of neutral:negatively charged headgroups in membranes of host cells of HIV (54, 57). For each triply 13CO-labeled HFP, a sample was made with DTPC:DTPG = 4:1 ≡ “PC:PG” and a sample was made with DTPC:DTPG:cholesterol = 8:2:5 ≡ “PC:PG:CHOL”. The latter total lipid:cholesterol mol ratio reflected the ratio observed in membranes of host cells of HIV and the former PC:PG composition without cholesterol was similar to membrane compositions used in previous structural studies of fusion peptides (54, 57, 61–63). The samples containing singly 13CO-labeled HFP were prepared with PC:PG:CHOL.
Each sample preparation began with dissolution in chloroform of 20 µmol total PC:PG or 30 µmol total PC:PG:CHOL. The chloroform was removed under a stream of nitrogen followed by overnight vacuum pumping. The lipid film was suspended in 2 mL buffer and homogenized with ten freeze-thaw cycles. Large unilamellar vesicles were formed by extrusion through a 100 nm diameter polycarbonate filter (Avestin, Ottawa, ON). HFP (0.8 µmol by weight) was dissolved in 2 mL buffer and the HFP and vesicle solutions were then gently vortexed together. The mixture was refrigerated overnight and ultracentrifuged at ~150000g for five hours. The membrane pellet with associated bound HFP was transferred to a 4 mm diameter magic angle spinning (MAS) NMR rotor. The majority of the HFP binds to membranes under these conditions and the membranes remain bilayers for HFP:lipid ~ 0.04 (27, 56, 59, 64).
A sample was also prepared for calibration of the NMR experiments and contained HFP4 (0.8 µmol) and DPPC-13C (20 µmol). The 13CO signal of this sample was dominated by DPPC-13C.
Solid-state NMR
Experiments were done on a 9.4 T solid-state NMR spectrometer (Varian Infinity Plus, Palo Alto, CA) equipped with a triple resonance MAS probe. The detection channel was tuned to 13C at 100.8 MHz, the decoupling channel was tuned to 1H at 400.8 MHz, and the third channel was tuned to 31P at 162.2 MHz. 13C shifts were externally referenced to the methylene resonance of adamantane at 40.5 ppm, 31P shifts were referenced to 85% H3PO4 at 0 ppm, and the 13C and 31P transmitter chemical shifts were 156 and −16 ppm, respectively. The 13C referencing allowed direct comparison with 13C shift databases derived from liquid-state NMR assignments of proteins (65, 66). These databases are appropriate for solid-state NMR data as evidenced by similar 13C shifts observed for the same protein in either aqueous solution or the microcrystalline state (67–69). experiments were done at −50 C to enhance 13C signal and to prevent motional averaging of the 13C-31P dipolar coupling which was the parameter used to assess HFP location in membranes. The 13C shifts and presumably the HFP conformation were comparable at −50 C and ambient temperature (70). At −50 C, the lipids were likely in the gel phase for the PC:PG samples and in the liquid-ordered phase for the PC:PG:CHOL samples (60, 71). Analyses of slow-spinning spectra yielded a 13CO chemical shift anisotropy range of ~90 to 240 ppm and a 31P chemical shift anisotropy range of ~ −75 to 100 ppm (72). The REDOR experiment contained in sequence: (1) a 50 kHz 1H π/2 pulse; (2) 1 ms cross-polarization with 52 kHz 1H field and 58–69 kHz ramped 13C field; (3) a dephasing period of duration τ which contained ~50 kHz 13C π and in some cases ~60 kHz 31P π pulses with XY-8 phase cycling on each channel; and (4) 13C detection with a four scan phase cycle (29, 35, 73–75). Two-pulse phase modulation 1H decoupling of ~100 kHz was applied during the dephasing and detection periods, the recycle delay was 1 s, and the MAS frequency was 8000 ± 2 Hz (76).
For each sample and each τ, two spectra were acquired. The dephasing period during the “S1” acquisition contained a 13C π pulse at the end of each rotor cycle except for the last cycle and a 31P π pulse in the middle of each cycle. The 31P pulses were absent during the “S0” acquisition. MAS averaged the 13C-31P dipolar coupling to zero over each rotor cycle of the dephasing period of the S0 acquisition, while incorporation of two π pulses per rotor cycle during the S1 acquisition resulted in a non-zero average value of the dipolar coupling and concomitant reduction in signals of 13C nuclei close to 31P. Determination of d was based on the difference in 13C signal intensity of the two spectra.
The 1H and 13C rf fields were initially calibrated with adamantane and the 13C cross-polarization field was then adjusted to give the maximum 13CO signal of the sample containing unlabeled HFP4 and DPPC-13C. The 31P π pulse length was set by minimization of the S1 signal in this sample for τ = 8 ms and the 1H TPPM pulse length was set to give the maximum S0 signal.
REDOR data analysis
All spectra were processed with Gaussian line broadening and with baseline correction. For each sample and each value of τ, spectra were integrated over a defined chemical shift range and the integrals of the S0 and S1 spectra were denoted as “S0” and “S1” and were used to calculate a normalized experimental dephasing parameter (ΔS/S0)exp = 1 − (S1/S0). Uncertainties in (ΔS/S0)exp were calculated:
| (1) |
where σS0 and σS1 were the experimental root-mean-squared-deviations of integrated intensities in regions of the spectra without signal (77). relative to the other labeled HFP samples, the HFP2-14AAG and HFP4-14A samples had significantly larger values of (ΔS/S0)exp and data from these samples were used to determine an approximate distance between the 31Ps and the labeled 13COs. The distance determination was done with (ΔS/S0)lab calculated to remove the natural abundance (na) contribution from (ΔS/S0)exp:
| (2) |
The values of (S0 na/S0lab) were 0.084 and 0.32 for the HFP2-14AAG and HFP4-14A samples, respectively. The values of (ΔS/S0)na were calculated as the average of (ΔS/S0)exp for the HFP2-5GAL, HFP3-8FLG, and HFP2-11FLG samples and for τ = 2, 8, 16, and 24 ms were 0.000, 0.029, 0.094, and 0.134 for the PC:PG samples and 0.026, 0.028, 0.083, and 0.092 for the PC:PG:CHOL samples. The σlab were calculated with σlab = (1 + S0na/S0lab) × σexp which neglected the contribution from the far-right term in Eq. 2. Discussion of this approximation and derivation of Eq. 2 are given in the Supporting Information.
Simulations of the experimental data were based on a single 13CO-31P spin pair model:
| (3) |
with λ = d × τ and Jk as the kth order Bessel function of the first kind (78). The samples contained multiple 13CO-31P distances and couplings and these are approximated as a single r and a single d in Eq. 3 (29, 51, 79).
For the HFP4/DPPC-13C sample, χ2(d) were calculated for an array of values of d:
| (4) |
where T was the number of experimental τ values. The best-fit d corresponded to minimum χ2(d).
At larger τ, the (ΔS/S0)lab for the HFP2-14AAG and HFP4-14A samples reached plateau values which were significantly smaller than 1 while the (ΔS/S0)sim had plateau values of ~1. This inconsistency was resolved using a model of two populations of membrane-associated HFPs. The “f ” fraction represented 13COs close to the lipid 31Ps with corresponding d ≠ 0 while the “1−f” fraction represented 13COs far from the lipid 31Ps with corresponding d = 0. Fitting was done with an array of values of d and f:
| (5) |
The uncertainty of d was calculated with the χ2 = χ2min + 1 criterion (77).
RESULTS
Overall strategy
Our long-term goal is a detailed structure of the membrane location of the HFP in helical and β strand conformations. Prior to beginning this study, there was relatively little information about the membrane location of HFP, particularly for the β strand conformation. It was likely that a large number of 13CO sites had 13CO-31P distances beyond the REDOR detection limit. In addition, HFP 13C linewidths are fairly broad which leads to overlap of 13CO resonances from different residues and the need for specific 13CO labeling. In an effort to reduce the numbers of specifically labeled peptides needed to develop a membrane location model, samples were first made with four peptides each of which had 13CO labels at three sequential residues between G5 and G16. The G5–G16 region was therefore rapidly scanned for 13CO-31P proximity. Although the (ΔS/S0)exp data for each of the samples had contributions from three distinct 13CO sites, the individual (ΔS/S0) would only be appreciably greater than zero for 13CO-31P distances ≤ 8 Å. The regions of HFP close to 31P would be defined from the REDOR data on the triply labeled samples and these regions would then provide a basis for choosing sites for single 13CO labeled peptides. 13CO-31P distances are more straightforwardly derived from (ΔS/S0) of singly labeled HFPs and REDOR data for two such HFPs are presented to refine the basic HFP membrane location model developed from the triply labeled HFP data.
REDOR calibration experiments
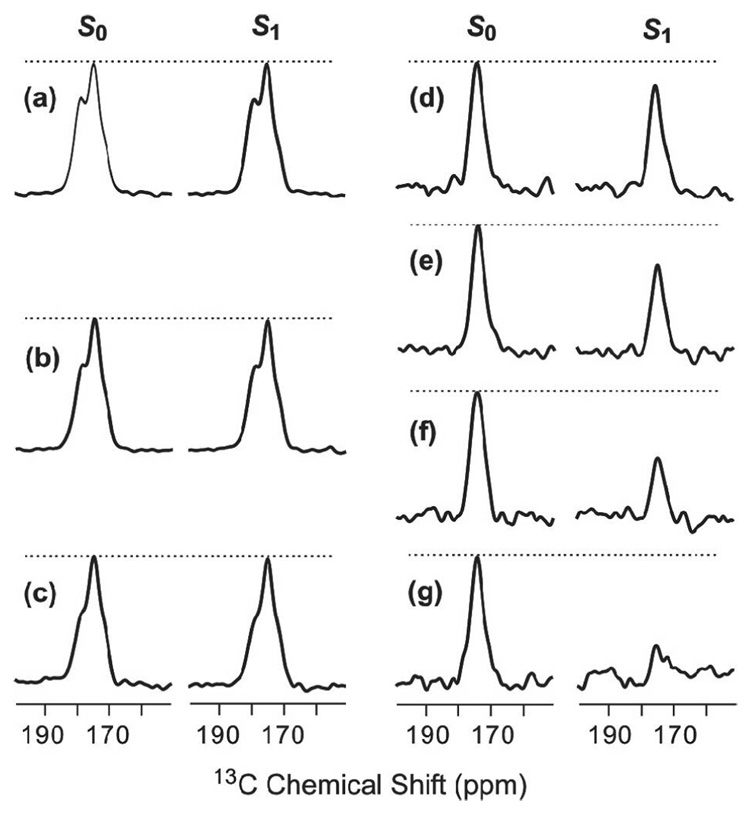
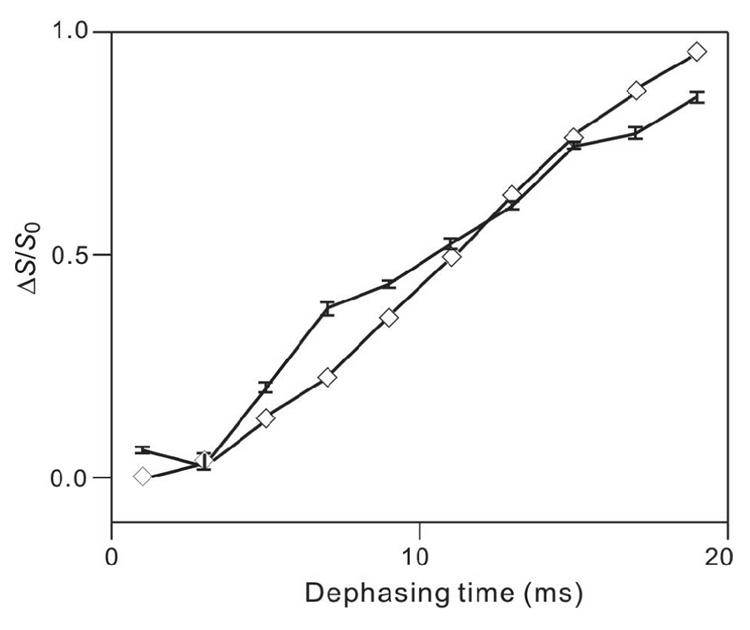
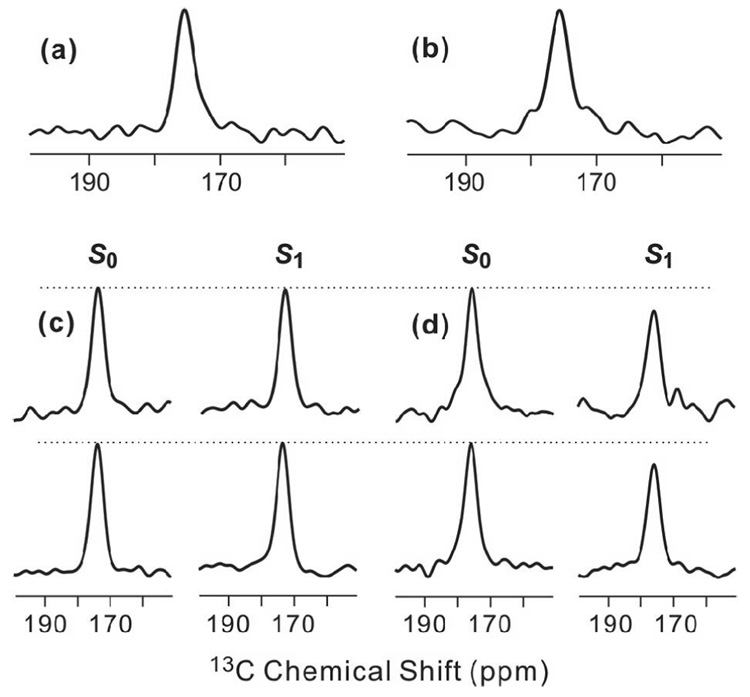
As an initial control experiment, 13CO-31P REDOR spectra were obtained for HFP2-11FLG lyophilized from water and resulted in (ΔS/S0)exp ~ 0 for values of τ between 1 and 19 ms, Fig. 1a–c. Non-zero values of (ΔS/S0)exp for 13COs in membrane-associated HFP samples can therefore be definitively ascribed to 13CO-31P proximity. As displayed in Fig. 1d–g, REDOR spectra were also obtained for the HFP4/DPPC-13C sample. Because HFP4 is unlabeled and DPPC-13C is labeled, the signals were primarily due to the DPPC 13COs and have non-zero (ΔS/S0)exp because of the proximity of the headgroup 31P. Fig. 2 displays (ΔS/S0)exp and best-fit (ΔS/S0)sim for this sample and yielded d = 68 Hz and r = 5.6 Å. The best-fit NMR value of r is comparable to the 5–6 Å values of r observed in the crystal structures of the related lipids, 1,2-dimyristoyl-sn-glycero-3-phosphocholine and 1,2-dipalmitoyl-sn-glycero-[phospho-rac-(1-glycerol)] (which had both been dehydrated) and in molecular dynamics simulations of gel-phase DPPC (80–82). The differences between (ΔS/S0)exp and (ΔS/S0)sim are likely due to: (1) contributions to (ΔS/S0)exp from intra- and intermolecular 31P with comparable values of r which contrasts with the single 13CO-31P spin pair model used to calculate (ΔS/S0)sim; (2) two structurally distinct 13COs in each headgroup with different intra- and intermolecular r values; and (3) structural disorder within the headgroups (79). Overall, the DPPC-13C fitting yielded good agreement between the NMR r value and the expected range of r values in the lipid.
Figure 1.
13CO-31P REDOR spectra of (a–c) lyophilized HFP2-11FLG and (d–g) the HFP4:DPPC-13C sample. The left and right spectra in each pair of 13C-detected spectra are S0 and S1 spectra, respectively. The dotted lines are drawn for visual comparison of S0 and S1 peak intensities. Each spectrum was processed with 200 Hz Gaussian line broadening and baseline correction. The τ values and numbers of S0 or S1 scans in each pair of spectra were: a, 1 ms, 120; b, 11 ms, 126; c, 19 ms, 138; d, 1 ms, 8; e, 7 ms, 56; f, 13 ms, 104; g, 19 ms, 152.
Figure 2.
(ΔS/S0)exp (vertical lines with error bars) and best-fit (ΔS/S0)sim (diamonds) vs dephasing time (τ) for the HFP4/DPPC-1-13C sample. Lines are drawn between points with adjacent values of τ. Each (ΔS/S0)exp valuewas obtained from a 1 ppm integration region centered at 173 ppm. The total (S0 + S1) numbers of scans for τ = 1, 3, 5, 7, 9, 11, 13, 15, 17 and 19 ms were 16, 48, 80, 112, 144, 176, 208, 240, 272 and 304, respectively. The displayed best-fit (ΔS/S0)sim values corresponded to d = 68 Hz and r = 5.6 Å.
For the HFP4/DPPC-13C sample, experiments were also done with a “one-channel” version of the REDOR sequence for which the S1 acquisitions contained a single 13C π pulse at the center of the dephasing period and 31P π pulses in the middle and end of each rotor cycle except for the center and end of the dephasing period. The S0 acquisition did not have 31P π pulses. relative to the “two-channel” version of REDOR described in Materials and Methods, one-channel REDOR has a reduced number of 13C π pulses which could result in reduced 13C-13C dipolar coupling and larger overall signals (83, 84). In fact, the experimental S0 intensities were comparable for the two versions of REDOR while (ΔS/S0)exp for one-channel REDOR was ~2/3 that of two-channel REDOR (29). All subsequent experiments were done with two-channel REDOR.
Triply labeled HFP
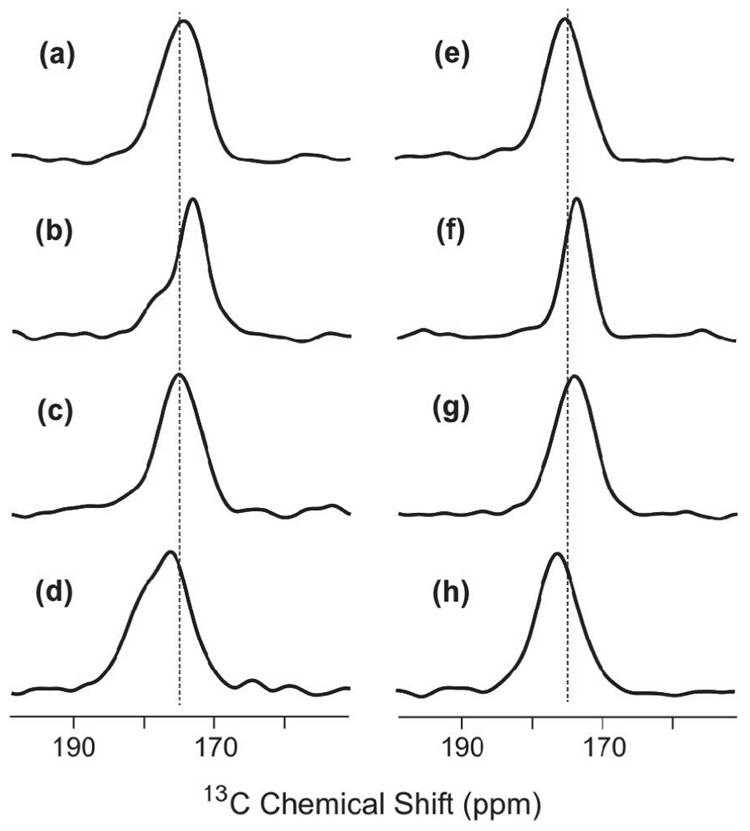
Local peptide conformation was examined by analysis of the 13CO chemical shift distributions in S0 spectra of HFPs obtained with τ = 2 ms, cf. Fig. 3. The data supported the following models: (1) the major fraction of peptides in PC:PG and PC:PG:CHOL adopted a β strand conformation from G5 to G16; and (2) there is a minor fraction of peptides in PC:PG with helical conformation. The detailed experimental support for the models is based on the known correlation between larger 13CO chemical shifts and local helical conformation and smaller 13CO chemical shifts and local β strand conformation. For example, average database values in ppm units of 13CO chemical shifts of helix (strand) conformations are: Gly, 175.5 (172.6); Ala, 179.4 (176.1); Leu, 178.5 (175.7); and Phe, 177.1 (174.2) (66). For the HFP2-5GAL, HFP3-8FLG, HFP2-11FLG, and HFP2-14AAG samples, the peak chemical shifts were ~175, 174, 175, and 176 ppm, respectively, and correlated with β strand conformation for the Ala, Leu, and Phe residues. For the HFP3-8FLG and HFP2-14AAG samples associated with PC:PG, there were shoulders at ~178 and 179 ppm, respectively, which correlated with helical conformation of Ala, Leu, and Phe residues. These results were consistent with previous studies of the conformation of membrane-associated HFP with peptide:lipid ~ 0.04 and with previous observations of greater preference for β strand conformation in cholesterol-containing membranes (27, 28, 35, 55, 56, 59, 70).
Figure 3.
S0 spectra for membrane-associated HFP with peptide:lipid ~0.04. The dotted lines are at 175 ppm. All spectra were obtained with τ = 2 ms and were processed with 200 Hz Gaussian line broadening and baseline correction. The membrane composition for samples a–d was PC:PG and the membrane composition for samples e–h was PC:PG:CHOL. The peptides were: a, e, HFP2-5GAL; b, f, HFP3-8FLG; c, g, HFP2-11FLG; and d, h HFP2-14AAG. The numbers of scans summed to obtain spectra a-h were 4823, 3867, 4823, 8500, 3259, 1001, 4320 and 6992, respectively.
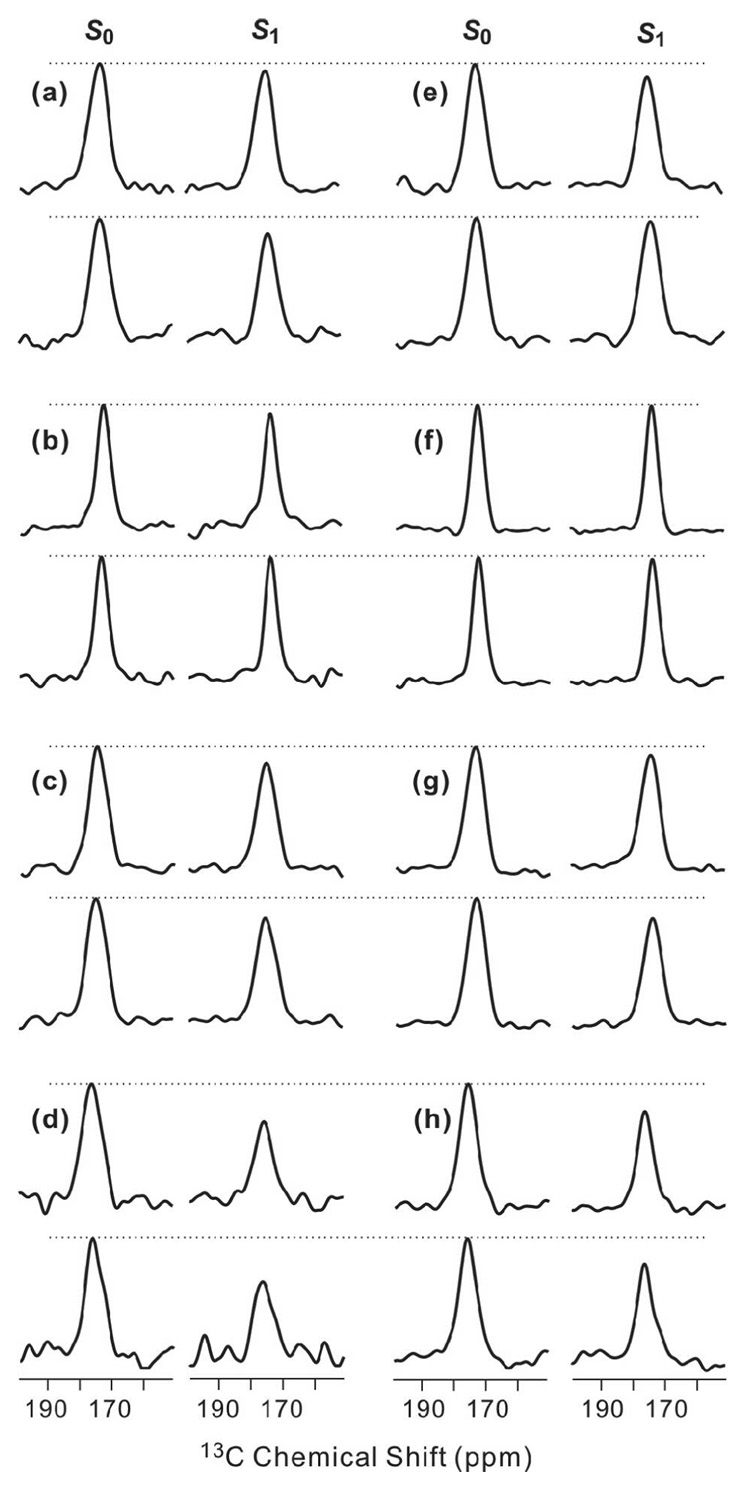
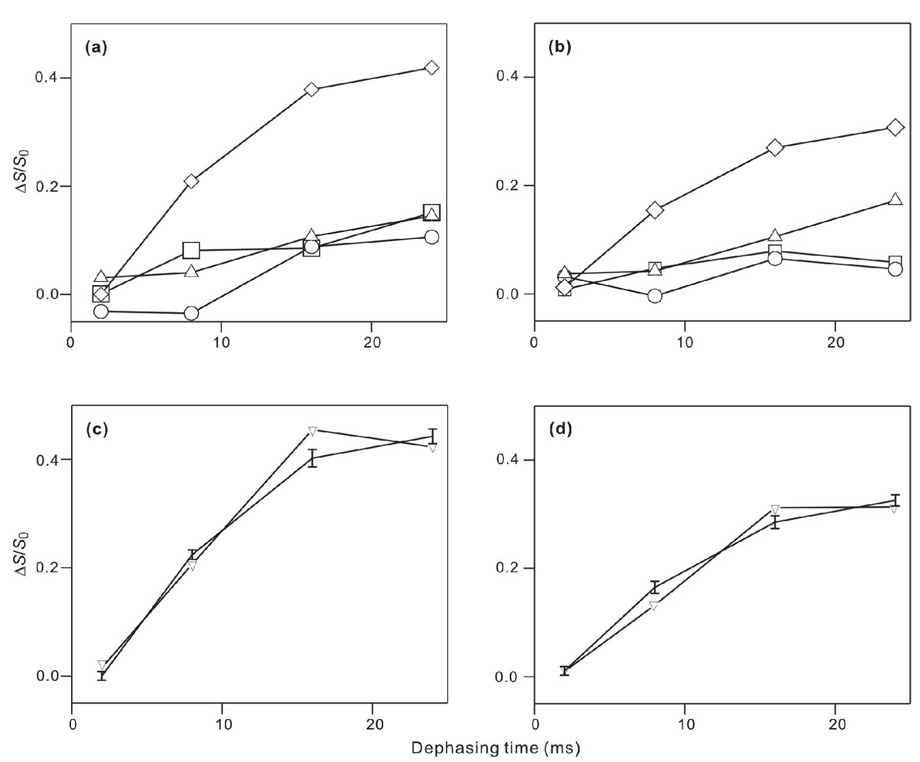
Fig. 4 displays the τ = 16 and 24 ms REDOR spectra of triply-labeled membrane-associated HFP samples and Fig. 5a,b displays comparative plots of (ΔS/S0)exp for the different samples. The data demonstrated that samples containing HFP2-14AAG have qualitatively larger (ΔS/S0)exp than do samples containing HFP labeled at other residues. Using the conformational results from Fig. 3, it appears: (1) a significant fraction of β strand HFP are in close contact with membranes; and (2) the 14AAG (A14 to G16) region is closer to the lipid 31P than is the 5GALFLGFLG (G5 to G13) region. Fig. 5 c,d displays plots of (ΔS/S0)lab and best-fit (ΔS/S0)sim for HFP2-14AAG in PC:PG and PC:PG:CHOL. The best-fit r was ~5.2 Å in both membrane compositions and the best-fit f in PC:PG and PC:PG:CHOL were 0.45 and 0.32, respectively. It was not possible to fit the HFP2-14AAG data well without inclusion of the f parameter. Although the (ΔS/S0)lab had contributions from three 13CO sites which would each have a distinct r, the number of data points and signal-to-noise dictated fitting to a single r value. The best-fit r should therefore be considered as both approximate and as likely representing the population of 13CO sites with greatest d and smallest r. Fitting was not done for data from the other samples because of the small (ΔS/S0)exp and because the (ΔS/S0)exp do not always reach asymptotic values at large τ.
Figure 4.
13CO-31P REDOR spectra of membrane-associated HFP with peptide:lipid ~0.04. Each letter corresponds to a single sample which contained (a–d) PC:PG or (e–h) PC:PG:CHOL and (a, e) HFP2-5GAL, (b, f) HFP3-8FLG, (c, g) HFP2-11FLG, or (d, h) HFP2-14AAG. For each letter/sample, S0 (left), S1 (right), τ= 16 ms (top), and τ= 24 ms (bottom) spectra are displayed. The dotted lines are drawn for visual comparison of S0 and S1 peak intensities. Each spectrum was processed with 300 Hz Gaussian line broadening and baseline correction. The numbers of S0 or S1 scans summed to obtain the top and bottom spectra were respectively: a, 30000, 56000; b, 27509, 29463; c, 20000, 40000; d, 44129, 48296; e, 8448, 52384; f, 5488, 21664; g, 28032, 52384; and h, 22576, 50240.
Figure 5.
(ΔS/S0) vs dephasing time for membrane-associated HFP in (a,c) PC:PG or (b,d) PC:PG:CHOL. For panels a and b, the points correspond to (ΔS/S0)exp and the symbol legend is: squares, HFP2-5GAL; circles, HFP3-8FLG; up triangles, HFP2-11FLG; and diamonds, HFP2-14AAG. The vertical dimensions of each symbol approximately correspond to the ±1 σ uncertainty limits. Lines are drawn between (ΔS/S0)exp values with adjacent values of τ. Each (ΔS/S0)exp value was determined by integration of 10 ppm regions of the S0 and S1 spectra. Panels c and d respectively correspond to the HFP2-14AAG/PC:PG and the HFP2-14AAG/PC:PG:CHOL samples and the points correspond to (ΔS/S0)lab (vertical lines with error bars) and best-fit (ΔS/S0)sim (down triangles). Lines are drawn between points with adjacent τ values. For plot c, the best-fit d = 91 ± 8 Hz with corresponding r = 5.12 ± 0.16 Å, f = 0.45 ± 0.02, and χ2min = 5.0. For plot d, the best-fit d = 85 ± 6 Hz with corresponding r = 5.24 ± 0.13 Å, f = 0.32 ± 0.02, and χ2min = 3.8.
Spectra were also obtained for samples made with HFP1-8FLG, the peptide which did not contain C-terminal lysines. For τ = 2, 8, 16, and 24 ms, (ΔS/S0)exp = −0.02, 0.06, 0.11, and 0.08 for the HFP1-8FLG/PC:PG sample and 0.01, 0.03, 0.01, and −0.01 for the HFP1-8FLG/PC:PG:CHOL sample. These values correlated with the (ΔS/S0)exp of the respective HFP3-8FLG/PC:PG and HFP3-8FLG/PC:PG:CHOL samples (circles in Fig. 5) and suggested that the additional C-terminal lysines of HFP2 and HFP3 do not greatly affect the REDOR results.
Singly labeled HFP
The triply labeled HFP results motivated experiments on singly labeled HFP4-6A and HFP4-14A associated with PC:PG:CHOL. As discussed in the Overall strategy subsection, analysis of singly labeled HFP data should result in more quantitative assessment of r. In addition, we were interested in studying HFP in a single conformation and the Fig. 3 data suggested that this was best achieved with PC:PG:CHOL and the resulting predominant β strand conformation. Finally, the Fig. 5 data suggested that (ΔS/S0)lab would be about zero for a HFP4-6A/PC:PG:CHOL sample and could be nonzero for a HFP4-14A/PC:PG:CHOL sample. REDOR data from these two sites could therefore further test the qualitative membrane location model derived from the triply labeled HFP results.
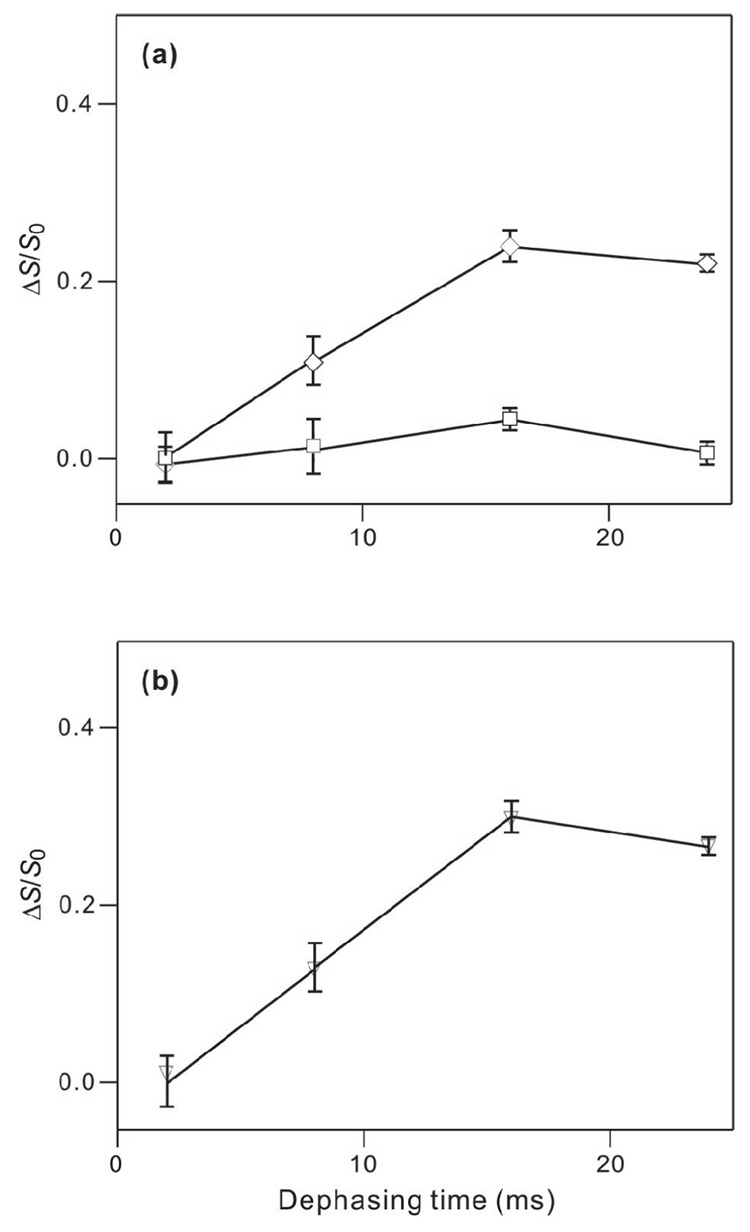
Fig. 6a,b displays the respective S0 spectra at τ = 8 ms for HFP4-6A and HFP4-14A associated with PC:PG:CHOL. Single peaks were observed with peak shifts of ~175 ppm which correlated with β strand conformation at these residues. The spectrum of HFP4-14A is similar to a difference spectrum representing the Ala-14 13CO signal for HFP (with no lysines) associated with an ester-linked lipid and cholesterol composition close to that of host cells of HIV (27). Fig. 6c,d displays the τ = 16 and 24 ms REDOR spectra of the singly labeled HFP4 samples and Fig. 7 shows (ΔS/S0)exp plots and data fitting for the HFP4-14A data. At large τ, (ΔS/S0)exp ≈ 0 for the HFP4-6A sample and (ΔS/S0)exp were significantly greater than zero for the HFP4-14A sample. Fitting of the HFP4-14A data with a single 13CO-31P spin pair model yielded best-fit r and f of 5.1 Å and 0.29, respectively. Thus, there was general consistency between the REDOR data of the HFP4-6A/PC:PG:CHOL and the HFP2-5GAL/PC:PG:CHOL samples and the REDOR data and fitting of the HFP4-14A/PC:PG:CHOL and the HFP2-14AAG/PC:PG:CHOL samples.
Figure 6.
13CO-31P REDOR spectra of (a,c) HFP4-6A/PC:PG:CHOL and (b,d) HFP4-14A/PC:PG:CHOL samples with peptide:lipid ~0.04. Panels a and b are S0 spectra obtained with τ = 8 ms and were processed with 200 Hz Guassian line broadening and baseline correction. Panels c and d display S0 (left), S1 (right), τ= 16 ms (top), and τ= 24 ms (bottom) spectra. The dotted lines are drawn for visual comparison of S0 and S1 peak intensities. Each spectrum was processed with 300 Hz Gaussian line broadening and baseline correction. The numbers of S0 or S1 scans summed were: a, 2304; b, 3680; c, 5504 (top), 28288 (bottom); and d, 5120 (top), 28736 (bottom).
Figure 7.
(ΔS/S0) vs dephasing time for HFP4-6A/PC:PG:CHOL and HFP-14A/PC:PG:CHOL samples. In panel a, (ΔS/S0)exp points with error bars are displayed with legend: HFP4-6A, squares; and HFP4-14A, diamonds. Each (ΔS/S0)exp value was determined from integrals of the entire S0 and S1 peaks. Panel b represents analysis of the HFP4-14A data with legend: (ΔS/S0)lab, vertical lines with error bars; and best-fit (ΔS/S0)sim, down triangles. Lines are drawn between points with adjacent τ values. The best-fit parameters were d = 93 ± 10 Hz with corresponding r = 5.08 ± 0.19 Å, f = 0.29 ± 0.02, and χ2min = 0.1.
DISCUSSION
Insertion models
The position of the HFP in the membrane has been postulated to be a significant structural factor in its fusion activity and to our knowledge, this study is the first example of direct distance measurements between the HFP and the lipid headgroups. Values of r ~5–6 Å were detected between the 13COs of residues from A14 to G16 and the lipid 31Ps. These r values support intimate association of the HFP and membranes containing either only phospholipids or phospholipids and cholesterol. The average r for 5GALFLGFLG 13COs was likely greater than 8 Å (d ≤ 25 Hz) as evidenced by the significantly smaller (ΔS/S0)exp, cf. Fig. 5, Fig 7. Thus, relative to the 5GALFLGFLG residues, the 14AAG residues are much closer to the lipid 31P.
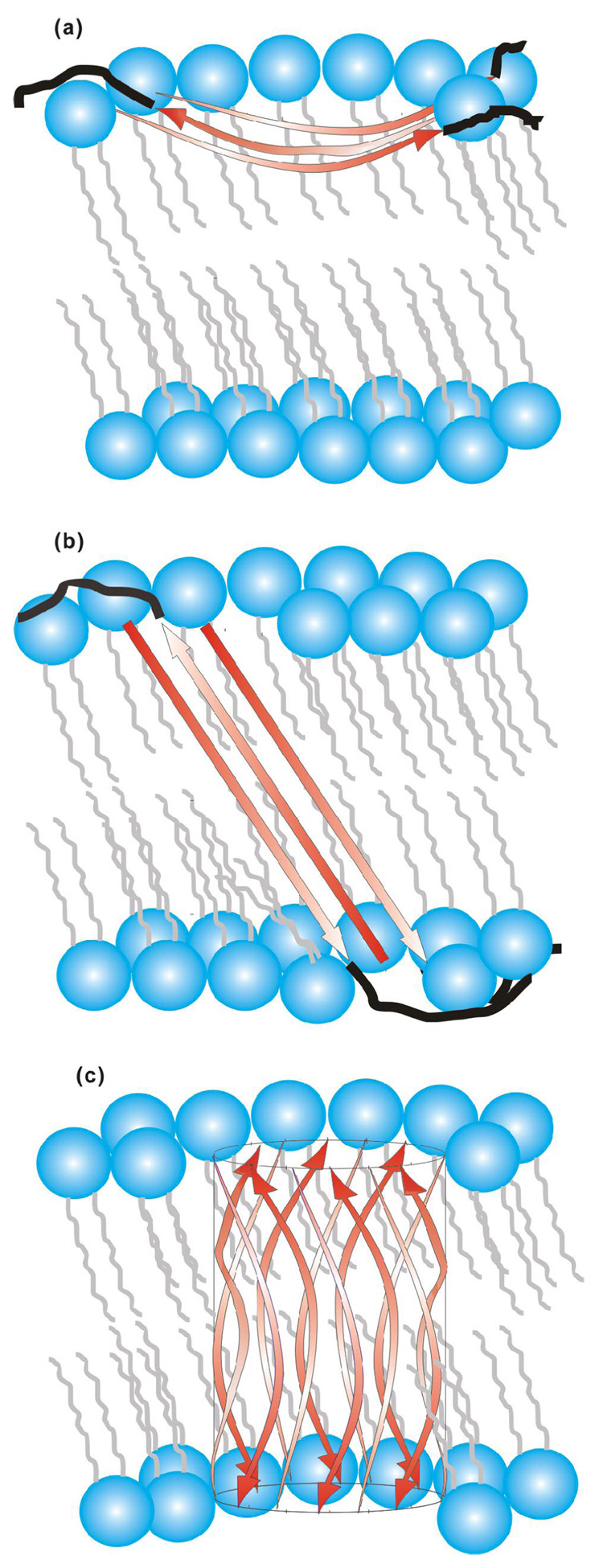
The G5 to G16 13CO chemical shift distributions of this study were consistent with a major population of HFP with β strand conformation for these residues. This result correlated with previous studies which supported the following structural features: (1) β strand HFP was fully extended between A1 and G16; (2) β strand HFP formed hydrogen bonded oligomers or aggregates; and (3) a significant fraction of the oligomers have an antiparallel arrangement with adjacent strand crossing between F8 and L9 (25, 27–29, 35, 37, 85, 86). Some of these studies also supported conformational disorder at A21 (27, 28). Although there are some data supporting a population of parallel strand arrangement, “partial membrane insertion (PMI)” and “full membrane insertion (FMI)” models are only presented for the antiparallel arrangement, cf. Fig. 8 (29, 35, 87). There have been high-resolution structures for the ~130-residue “soluble ectodomain” region of gp41 which begins about ten residues C-terminal of the HFP and ends about twenty residues N-terminal of the gp41 transmembrane domain (88–92). These structures showed trimeric gp41 with the residues closest to the HFPs in a parallel in-register coiled-coil. Antiparallel HFP strand arrangement in the context of gp41 would then require at least two gp41 trimers. Strands from trimer A (A1, A2, A3) would be parallel to one another and strands from trimer B (B1, B2, B3) would be parallel to one another and an antiparallel interleaved strand arrangement could be formed as A1B3A2B2A3B1. There is solid-state NMR evidence for the antiparallel arrangement of membrane-associated HFPs which were cross-linked at their C-termini (35).
Figure 8.
(a) Partial membrane insertion (PMI) and (b, c) full membrane insertion (FMI) models for antiparallel β strand HFP. The red arrows represent the A1 to G16 residues in strand conformation and the black lines represent the S17 to S23 residues in random coil conformations. For clarity, black lines are not displayed in c. Lipids are represented in blue and grey and cholesterol is not displayed. Three antiparallel strands are displayed in a, b and twelve strands are displayed in c but the actual number of strands in the oligomer/aggregate is not known. The curvature and angle of the strands with respect to the bilayer normal are not known but the models consider that A1-G16 has ~55 Å length and that the transbilayer distance is ~48 Å (100). The experiments do not provide information about the membrane locations of residues S17 to S23. relative to FMI model (b), the FMI β barrel variant (c) could have reduced energy because all of the residues in the membrane interior have backbone hydrogen bonds.
For antiparallel strands between A1 and G16, the 14AAG residues in both the PMI and FMI models are at the ends of the hydrogen-bonded oligomer and are closer to the lipid headgroups than residues 5GALFLGFLG. The F8 and L9 residues are at the center of the hydrogen-bonded oligomer and are most deeply membrane-inserted in all models. This result is consistent with the smallest (ΔS/S0)exp values observed for the HFP1-8FLG and HFP3-8FLG samples and with the large number of apolar sidechains in the central 7LFLGFL (L7 to L12) region. relative to the HFP1-8FLG and HFP3-8FLG samples, the models also predict smaller r and larger (ΔS/S0)exp for the HFP2-5GAL and HFP2-11FLG samples which generally correlates with the experimental data, cf. Fig. 5a–b. The models suggest small r and significant (ΔS/S0)expression for HFPs labeled at the N-terminal residues and future studies could examine samples labeled in this manner.
The PMI model in Fig. 8a would likely perturb the membrane and has some similarity with: (1) the PMI of extended conformation internal fusion peptides postulated from structures of dengue, Semliki forest, herpes, and vesicular stomatitis viral fusion proteins; (2) the PMI of helical influenza fusion peptide determined from electron spin resonance experiments; and (3) a PMI model based on the HFP-F8W fluorescence measurements (37, 61, 62, 93–96). However, the locations of lipids in the perturbed leaflet in the PMI model are not clear. For the FMI model of Fig. 8b, the positions of the lipids are more clear but there are non-hydrogen bonded CO and NH groups at the sheet edges with large Born energies. These energies would be reduced for a FMI β barrel structure, Fig. 8c. There is correlation between the FMI model and the deep insertion of the Trp sidechain suggested from fluorescence studies of the HFP-F8W mutant (36, 37). In the context of gp41, individual HFP trimers would be on the same side of the membrane in the PMI model but would be on different sides of the membrane in the FMI model. It is not clear how this FMI trimer topology would relate to the positions of the viral membrane-anchored gp41 trimers and the host cell membranes. The free energy difference between the A1 to G16 FMI state and a non-inserted state is ~3.9 kJ/mol as calculated from the sum of individual residue free energy values derived from transmembrane helices (97). The calculated difference for the I4 to G13 sequence is −2.3 kJ/mol and leads to the general conclusion that the free energy calculations do not strongly distinguish between the PMI and FMI models. Future studies could discriminate between the PMI and FMI models using REDOR distance measurements between peptide nuclei and lipid acyl chain nuclei (51).
There are similarities between these PMI and FMI models of oligomeric β strand HFP and PMI and FMI models which have been developed for a single HFP in a helical conformation (38, 39, 98). Much of the experimental data for helical HFP insertion has been based on detergent rather than membrane samples and there has been support for both micelle surface location and micelle traversal by HFP (19–23). Our results on oligomeric β strand HFP were consistent with the previous observations that the A15 and G16 residues of monomeric helical HFP were close to the water-micelle interface and that the F8 to G10 residues were furthest from this interface. Thus, there may be common features shared by the micelle and membrane locations of helical and β strand HFP.
Origin of f and effects of cholesterol
The HFP2-14AAG and HFP4-14A data could only be fit well with addition of the f parameter which approximately reflected the fractional population of peptides whose labeled residues were close to the 31P. Analysis of 13CO-31P REDOR data of a membrane-associated antimicrobial peptide also required an f parameter and the best-fit f and r values were similar to our results (51).
The membranes of host cells of HIV have cholesterol:lipid ~ 0.45 and the membranes of HIV have cholesterol:lipid ~ 0.8 (54, 57). These data suggest that it is interesting to probe the effect of cholesterol on HFP location in the membrane. Similar values of (ΔS/S0)exp were obtained for the 5GALFLGFLG residues in PC:PG and PC:PG:CHOL samples and the best-fit r for the 14AAG residues were comparable in both membrane compositions. These results suggest: (1) the inserted HFP population has similar location in membranes with or without cholesterol; and (2) the membrane location of the A14 to G16 residues may be similar in both helical and β strand conformations because there appeared to be some helical conformation in PC:PG and negligible helical conformation in PC:PG:CHOL. There was a difference in best-fit f for HFP2-14AAG in PC:PG and PC:PG:CHOL with values of 0.45 and 0.32, respectively. There are several potential explanations for this variation. First, the HFP/PC:PG samples likely had a small population of helical conformation which was absent in the HFP/PC:PG:CHOL samples and this helical population could have contributed to the larger f in PC:PG. Second, the presumably gel phase PC:PG and the liquid-ordered phase PC:PG:CHOL had lateral molecular densities of ~0.213 Å−2 and ~0.256 Å−2 as calculated from gel-phase PC and PG areas of 47 Å2, liquid-ordered PC and PG areas of 40 Å2, and cholesterol area of 37 Å2 (60, 71, 99, 100). The denser packing in PC:PG:CHOL could have shifted an inserted HFP:surface HFP equilibrium to surface HFP and led to reduced f. Finally, relative to the PC:PG sample, there was likely a reduced number of phospholipids close to the 14AAG residues in the PC:PG:CHOL sample because of statistical substitution of nearby phospholipids with cholesterol. This lipid dilution may have also reduced f in the PC:PG:CHOL sample.
In summary, 13CO-31P distance measurements have demonstrated close proximity of the 14AAG residues to the lipid 31P for a significant fraction of membrane-associated HFP. This proximity was observed both for membranes with and without cholesterol. The chemical shifts of this study as well as results from previous studies correlated with a predominant population of HFP with β strand conformation. Models of partial and full insertion of β strands are proposed which are consistent with the experimental data. Although there have been numerous previous observations of β strand HFP, the role of this conformation in fusion has been controversial (14, 16, 18, 31, 45). The present study demonstrates that β strand HFP is in intimate contact with membranes and merits serious consideration as a fusogenic conformation.
Interesting future work could include studies of cross-linked HFPs which are thought to mimic the HFP oligomeric topology in the gp41 protein and which have increased fusion rates relative to the non-cross-linked HFPs of the present study (34). There may be a distinct membrane location of the cross-linked HFPs which correlates with their fast fusion rate. It is also known that the cross-linked HFP trimer will form helical or β strand conformation in membranes without or with cholesterol, respectively, so that studies of the trimer in different membrane compositions can provide information about the conformational dependence of peptide location in membranes (35).
Supplementary Material
Eq. 2 and the σlab expression are derived and discussed. This material is available free of charge via the Internet at http://pubs.acs.org.
ACKNOWLEDGEMENT
Dr. Richard Venable provided the coordinates of crystalline DMPC and gel-phase DPPC. We acknowledge Dr. Michael Feig for useful discussions about the insertion models.
ABBREVIATIONS
- 14AAG
residues A14, A15, and G16
- 13CO
13C labeled carbonyl
- d
magnitude of 13C-31P dipolar coupling
- DPPC-13C
1,2-dipalmitoyl-sn-glycero-3-phosphocholine with 13C labeling at the both carbonyl sites
- DTPC
1,2-di-O-tetradecyl-sn-glycero-3-phosphocholine
- DTPG
1,2-di-O-tetradecyl-sn-glycero-3-[phospho-rac-(1-glycerol)]
- f
fraction of 13COs close to 31Ps
- FMI
full membrane insertion
- 5GALFLGFLG
residues G5, A6, L7, F8, L9, G10, F11, L12, and G13
- HEPES
N-(2-hydroxy-ethyl)piperazine-N’-2-ethanesulfonic acid
- HFP
HIV fusion peptide
- HFP1
AVGIGALFLGFLGAAGSTMGARS-NH2
- HFP1-8FLG
HFP1 13CO labeled at F8, L9, and G10
- HFP2
AVGIGALFLGFLGAAGSTMGARSKKK-NH2
- HFP2-5GAL
HFP2 13CO labeled at G5, A6, and L7
- HFP2-11FLG
HFP2 13CO labeled at F11, L12, and G13
- HFP2-14AAG
HFP2 13CO labeled at A14, A15, and G16
- HFP3
AVGIGALFLGFLGAAGSTMGARSKKKAβ
- HFP3-8FLG
HFP3 13CO labeled at F8, L9, and G10
- HFP4
AVGIGALFLGFLGAAGSTMGARSWKKKKKKAβ
- HFP4-6A
HFP4 13CO labeled at A6
- HFP4-14A
HFP4 13CO labeled at A14
- HIV
human immunodeficiency virus
- IR
infrared
- 7LFLGFL
residues L7, F8, L9, G10, F11, and L12
- MAS
magic angle spinning
- NMR
nuclear magnetic resonance
- PC:PG
4:1 DTPC:DTPG
- PC:PG:CHOL
8:2:5 DTPC:DTPG:cholesterol
- PMI
partial membrane insertion
- r
13C-31P internuclear distance
- REDOR
rotational-echo double resonance
- τ
duration of dephasing period
- TFA
trifluoroacetic acid
Footnotes
This work was supported by NIH award AI47153 to D. P. W.
REFERENCES
- 1.Hernandez LD, Hoffman LR, Wolfsberg TG, White JM. Virus-cell and cell-cell fusion. Annu. Rev. Cell. Dev. Biol. 1996;12:627–661. doi: 10.1146/annurev.cellbio.12.1.627. [DOI] [PubMed] [Google Scholar]
- 2.Eckert DM, Kim PS. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- 3.Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 4.Blumenthal R, Clague MJ, Durell SR, Epand RM. Membrane fusion. Chem. Rev. 2003;103:53–69. doi: 10.1021/cr000036+. [DOI] [PubMed] [Google Scholar]
- 5.Bentz J. Membrane fusion mediated by coiled coils: a hypothesis. Biophys. J. 2000;78:886–900. doi: 10.1016/S0006-3495(00)76646-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- 7.Cohen FS, Melikyan GB. The energetics of membrane fusion from binding, through hemifusion, pore formation, and pore enlargement. J. Membrane Biol. 2004;199:1–14. doi: 10.1007/s00232-004-0669-8. [DOI] [PubMed] [Google Scholar]
- 8.Turner BG, Summers MF. Structural biology of HIV. J. Mol. Biol. 1999;285:1–32. doi: 10.1006/jmbi.1998.2354. [DOI] [PubMed] [Google Scholar]
- 9.Colman PM, Lawrence MC. The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell Biol. 2003;4:309–319. doi: 10.1038/nrm1076. [DOI] [PubMed] [Google Scholar]
- 10.Freed EO, Myers DJ, Risser R. Characterization of the fusion domain of the human immunodeficiency virus type 1 envelope glycoprotein gp41. Proc. Natl. Acad. Sci. U.S.A. 1990;87:4650–4654. doi: 10.1073/pnas.87.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freed EO, Delwart EL, Buchschacher GL, Jr, Panganiban AT. A mutation in the human immunodeficiency virus type 1 transmembrane glycoprotein gp41 dominantly interferes with fusion and infectivity. Proc. Natl. Acad. Sci. U.S.A. 1992;89:70–74. doi: 10.1073/pnas.89.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaal H, Klein M, Gehrmann P, Adams O, Scheid A. Requirement of N-terminal amino acid residues of gp41 for human immunodeficiency virus type 1-mediated cell fusion. J. Virol. 1995;69:3308–3314. doi: 10.1128/jvi.69.6.3308-3314.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delahunty MD, Rhee I, Freed EO, Bonifacino JS. Mutational analysis of the fusion peptide of the human immunodeficiency virus type 1: identification of critical glycine residues. Virology. 1996;218:94–102. doi: 10.1006/viro.1996.0169. [DOI] [PubMed] [Google Scholar]
- 14.Durell SR, Martin I, Ruysschaert JM, Shai Y, Blumenthal R. What studies of fusion peptides tell us about viral envelope glycoprotein-mediated membrane fusion. Mol. Membr. Biol. 1997;14:97–112. doi: 10.3109/09687689709048170. [DOI] [PubMed] [Google Scholar]
- 15.Kliger Y, Aharoni A, Rapaport D, Jones P, Blumenthal R, Shai Y. Fusion peptides derived from the HIV type 1 glycoprotein 41 associate within phospholipid membranes and inhibit cell-cell fusion. Structure-function study. J. Biol. Chem. 1997;272:13496–13505. doi: 10.1074/jbc.272.21.13496. [DOI] [PubMed] [Google Scholar]
- 16.Pereira FB, Goni FM, Muga A, Nieva JL. Permeabilization and fusion of uncharged lipid vesicles induced by the HIV-1 fusion peptide adopting an extended conformation: dose and sequence effects. Biophys. J. 1997;73:1977–1986. doi: 10.1016/S0006-3495(97)78228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pritsker M, Rucker J, Hoffman TL, Doms RW, Shai Y. Effect of nonpolar substitutions of the conserved Phe11 in the fusion peptide of HIV-1 gp41 on its function, structure, and organization in membranes. Biochemistry. 1999;38:11359–11371. doi: 10.1021/bi990232e. [DOI] [PubMed] [Google Scholar]
- 18.Martin I, Schaal H, Scheid A, Ruysschaert JM. Lipid membrane fusion induced by the human immunodeficiency virus type 1 gp41 N-terminal extremity is determined by its orientation in the lipid bilayer. J. Virol. 1996;70:298–304. doi: 10.1128/jvi.70.1.298-304.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang DK, Cheng SF, Chien WJ. The amino-terminal fusion domain peptide of human immunodeficiency virus type 1 gp41 inserts into the sodium dodecyl sulfate micelle primarily as a helix with a conserved glycine at the micelle-water interface. J. Virol. 1997;71:6593–6602. doi: 10.1128/jvi.71.9.6593-6602.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaroniec CP, Kaufman JD, Stahl SJ, Viard M, Blumenthal R, Wingfield PT, Bax A. Structure and dynamics of micelle-associated human immunodeficiency virus gp41 fusion domain. Biochemistry. 2005;44:16167–16180. doi: 10.1021/bi051672a. [DOI] [PubMed] [Google Scholar]
- 21.Morris KF, Gao XF, Wong TC. The interactions of the HIV gp41 fusion peptides with zwitterionic membrane mimics determined by NMR spectroscopy. Biochim. Biophys. Acta-Biomembranes. 2004;1667:67–81. doi: 10.1016/j.bbamem.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Chang DK, Cheng SF. Determination of the equilibrium micelle-inserting position of the fusion peptide of gp41 of human immunodeficiency virus type 1 at amino acid resolution by exchange broadening of amide proton resonances. J. Biomol. NMR. 1998;12:549–552. doi: 10.1023/a:1008399304450. [DOI] [PubMed] [Google Scholar]
- 23.Langham A, Kaznessis Y. Simulation of the N-terminus of HIV-1 glycoprotein 41000 fusion peptide in micelles. J. Pept. Sci. 2005;11:215–224. doi: 10.1002/psc.623. [DOI] [PubMed] [Google Scholar]
- 24.Rafalski M, Lear JD, DeGrado WF. Phospholipid interactions of synthetic peptides representing the N-terminus of HIV gp41. Biochemistry. 1990;29:7917–7922. doi: 10.1021/bi00486a020. [DOI] [PubMed] [Google Scholar]
- 25.Nieva JL, Nir S, Muga A, Goni FM, Wilschut J. Interaction of the HIV-1 fusion peptide with phospholipid vesicles: different structural requirements for fusion and leakage. Biochemistry. 1994;33:3201–3209. doi: 10.1021/bi00177a009. [DOI] [PubMed] [Google Scholar]
- 26.Peisajovich SG, Epand RF, Pritsker M, Shai Y, Epand RM. The polar region consecutive to the HIV fusion peptide participates in membrane fusion. Biochemistry. 2000;39:1826–1833. doi: 10.1021/bi991887i. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Gabrys CM, Weliky DP. Solid-state nuclear magnetic resonance evidence for an extended beta strand conformation of the membrane-bound HIV-1 fusion peptide. Biochemistry. 2001;40:8126–8137. doi: 10.1021/bi0100283. [DOI] [PubMed] [Google Scholar]
- 28.Bodner ML. Ph. D. thesis. East Lansing: Michigan State University; 2006. Solid state nuclear magnetic resonance of the HIV-1 and influenza fusion peptides associated with membranes. [Google Scholar]
- 29.Yang J, Weliky DP. Solid state nuclear magnetic resonance evidence for parallel and antiparallel strand arrangements in the membrane-associated HIV-1 fusion peptide. Biochemistry. 2003;42:11879–11890. doi: 10.1021/bi0348157. [DOI] [PubMed] [Google Scholar]
- 30.Gordon LM, Curtain CC, Zhong YC, Kirkpatrick A, Mobley PW, Waring AJ. The amino-terminal peptide of HIV-1 glycoprotein 41 interacts with human erythrocyte membranes: peptide conformation, orientation and aggregation. Biochim. Biophys. Acta. 1992;1139:257–274. doi: 10.1016/0925-4439(92)90099-9. [DOI] [PubMed] [Google Scholar]
- 31.Epand RM. Fusion peptides and the mechanism of viral fusion. Biochim. Biophys. Acta-Biomembranes. 2003;1614:116–121. doi: 10.1016/s0005-2736(03)00169-x. [DOI] [PubMed] [Google Scholar]
- 32.Afonin S, Glaser RW, Berditchevskaia M, Wadhwani P, Guhrs KH, Mollmann U, Perner A, Ulrich AS. 4-fluorophenylglycine as a label for 19F NMR structure analysis of membrane-associated peptides. Chembiochem. 2003;4:1151–1163. doi: 10.1002/cbic.200300568. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann MW, Weise K, Ollesch J, Agrawal P, Stalz H, Stelzer W, Hulsbergen F, de Groot H, Gerwert K, Reed J, Langosch D. De novo design of conformationally flexible transmembrane peptides driving membrane fusion. Proc. Natl. Acad. Sci. U.S.A. 2004;101:14776–14781. doi: 10.1073/pnas.0405175101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang R, Prorok M, Castellino FJ, Weliky DP. A trimeric HIV-1 fusion peptide construct which does not self-associate in aqueous solution and which has 15-fold higher membrane fusion rate. J. Am. Chem. Soc. 2004;126:14722–14723. doi: 10.1021/ja045612o. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Z, Yang R, Bodner ML, Weliky DP. Conformational flexibility and strand arrantements of the membrane-associated HIV fusion peptide trimer probed by solid-state NMR spectroscopy. Biochemistry. 2006;45:12960–12975. doi: 10.1021/bi0615902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agirre A, Flach C, Goni FM, Mendelsohn R, Valpuesta JM, Wu FJ, Nieva JL. Interactions of the HIV-1 fusion peptide with large unilamellar vesicles and monolayers. A cryo-TEM and spectroscopic study. Biochim. Biophys. Acta-Biomembranes. 2000;1467:153–164. doi: 10.1016/s0005-2736(00)00214-5. [DOI] [PubMed] [Google Scholar]
- 37.Haque ME, Koppaka V, Axelsen PH, Lentz BR. Properties and structures of the influenza and HIV fusion peptides on lipid membranes: Implications for a role in fusion. Biophys. J. 2005;89:3183–3194. doi: 10.1529/biophysj.105.063032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamath S, Wong TC. Membrane structure of the human immunodeficiency virus gp41 fusion domain by molecular dynamics simulation. Biophys. J. 2002;83:135–143. doi: 10.1016/S0006-3495(02)75155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maddox MW, Longo ML. Conformational partitioning of the fusion peptide of HIV-1 gp41 and its structural analogs in bilayer membranes. Biophys. J. 2002;83:3088–3096. doi: 10.1016/S0006-3495(02)75313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grobner G, Glaubitz C, Watts A. Probing membrane surfaces and the location of membrane-embedded peptides by 13C MAS NMR using lanthanide ions. J. Mag. Reson. 1999;141:335–339. doi: 10.1006/jmre.1999.1894. [DOI] [PubMed] [Google Scholar]
- 41.Buffy JJ, Hong T, Yamaguchi S, Waring AJ, Lehrer RI, Hong M. Solid-state NMR investigation of the depth of insertion of protegrin-1 in lipid bilayers using paramagnetic Mn2+ Biophys. J. 2003;85:2363–2373. doi: 10.1016/s0006-3495(03)74660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogel A, Scheidt HA, Huster D. The distribution of lipid attached spin probes in bilayers: Application to membrane protein topology. Biophys. J. 2003;85:1691–1701. doi: 10.1016/S0006-3495(03)74599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian CL, Gao PF, Pinto LH, Lamb RA, Cross TA. Initial structural and dynamic characterization of the M2 protein transmembrane and amphipathic helices in lipid bilayers. Prot. Sci. 2003;12:2597–2605. doi: 10.1110/ps.03168503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gallagher GJ, Hong M, Thompson LK. Solid-state NMR spin diffusion for measurement of membrane-bound peptide structure: Gramicidin A. Biochemistry. 2004;43:7899–7906. doi: 10.1021/bi0356101. [DOI] [PubMed] [Google Scholar]
- 45.Grage SL, Afonin S, Grune M, Ulrich AS. Interaction of the fusogenic peptide B18 in its amyloid-state with lipid membranes studied by solid state NMR. Chem. Phys. Lipids. 2004;132:65–77. doi: 10.1016/j.chemphyslip.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Henzler-Wildman KA, Martinez GV, Brown MF, Ramamoorthy A. Perturbation of the hydrophobic core of lipid bilayers by the human antimicrobial peptide LL-37. Biochemistry. 2004;43:8459–8469. doi: 10.1021/bi036284s. [DOI] [PubMed] [Google Scholar]
- 47.Abu-Baker S, Qi XY, Newstadt J, Lorigan GA. Structural changes in a binary mixed phospholipid bilayer of DOPG and DOPS upon saposin C interaction at acidic pH utilizing 31P and 2H solid-state NMR spectroscopy. Biochim. Biophys. Acta-Biomembranes. 2005;1717:58–66. doi: 10.1016/j.bbamem.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 48.Zhang WY, Smith SO. Mechanism of penetration of Antp(43–58) into membrane bilayers. Biochemistry. 2005;44:10110–10118. doi: 10.1021/bi050341v. [DOI] [PubMed] [Google Scholar]
- 49.Sharpe S, Yau WM, Tycko R. Structure and dynamics of the HIV-1 Vpu transmembrane domain revealed by solid-state NMR with magic-angle spinning. Biochemistry. 2006;45:918–933. doi: 10.1021/bi051766k. [DOI] [PubMed] [Google Scholar]
- 50.Harada E, Todokoro Y, Akutsu H, Fujiwara T. Detection of peptide-phospholipid interaction sites in bilayer membranes by 13C NMR spectroscopy: Observation of 2H/31P-selective 1H-depolarization under magic-angle spinning. J. Am. Chem. Soc. 2006;128:10654–10655. doi: 10.1021/ja062811u. [DOI] [PubMed] [Google Scholar]
- 51.Toke O, Maloy WL, Kim SJ, Blazyk J, Schaefer J. Secondary structure and lipid contact of a peptide antibiotic in phospholipid Bilayers by REDOR. Biophys. J. 2004;87:662–674. doi: 10.1529/biophysj.103.032706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cegelski L, Rice CV, O'Connor RD, Caruano AL, Tochtrop GP, Cai ZY, Covey DF, Schaefer J. Mapping the locations of estradiol and potent neuroprotective analogues in phospholipid bilayers by REDOR. Drug Develop. Res. 2005;66:93–102. [Google Scholar]
- 53.Matsuoka S, Ikeuchi H, Matsumori N, Murata M. Dominant formation of a single-length channel by amphotericin B in dimyristoylphosphatidylcholine membrane evidenced by 13C-13P rotational echo double resonance. Biochemistry. 2005;44:704–710. doi: 10.1021/bi049001k. [DOI] [PubMed] [Google Scholar]
- 54.Aloia RC, Tian H, Jensen FC. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc. Natl. Acad. Sci. U.S.A. 1993;90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang J, Parkanzky PD, Bodner ML, Duskin CG, Weliky DP. Application of REDOR subtraction for filtered MAS observation of labeled backbone carbons of membrane-bound fusion peptides. J. Magn. Reson. 2002;159:101–110. doi: 10.1016/s1090-7807(02)00033-2. [DOI] [PubMed] [Google Scholar]
- 56.Wasniewski CM, Parkanzky PD, Bodner ML, Weliky DP. Solid-state nuclear magnetic resonance studies of HIV and influenza fusion peptide orientations in membrane bilayers using stacked glass plate samples. Chem. Phys. Lipids. 2004;132:89–100. doi: 10.1016/j.chemphyslip.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 57.Brugger B, Glass B, Haberkant P, Leibrecht I, Wieland FT, Krasslich HG. The HIV lipidome: A raft with an unusual composition. Proc. Natl. Acad. Sci. U.S.A. 2006;103:2641–2646. doi: 10.1073/pnas.0511136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gullion T, Schaefer J. Rotational-echo double-resonance NMR. J. Magn. Reson. 1989;81:196–200. doi: 10.1016/j.jmr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Yang J, Prorok M, Castellino FJ, Weliky DP. Oligomeric β-structure of the membrane-bound HIV-1 fusion peptide formed from soluble monomers. Biophys. J. 2004;87:1951–1963. doi: 10.1529/biophysj.103.028530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cevc G. How membrane chain melting properties are regulated by the polar surface of the lipid bilayer. Biochemistry. 1987;26:6305–6310. doi: 10.1021/bi00394a002. [DOI] [PubMed] [Google Scholar]
- 61.Macosko JC, Kim CH, Shin YK. The membrane topology of the fusion peptide region of influenza hemagglutinin determined by spin-labeling EPR. J. Mol. Biol. 1997;267:1139–1148. doi: 10.1006/jmbi.1997.0931. [DOI] [PubMed] [Google Scholar]
- 62.Han X, Bushweller JH, Cafiso DS, Tamm LK. Membrane structure and fusion-triggering conformational change of the fusion domain from influenza hemagglutinin. Nat. Struct. Biol. 2001;8:715–720. doi: 10.1038/90434. [DOI] [PubMed] [Google Scholar]
- 63.Afonin S, Dur UHN, Glaser RW, Ulrich AS. 'Boomerang'-like insertion of a fusogenic peptide in a lipid membrane revealed by solid-state 19F NMR. Magn. Reson. Chem. 2004;42:195–203. doi: 10.1002/mrc.1340. [DOI] [PubMed] [Google Scholar]
- 64.Yang J, Parkanzky PD, Khunte BA, Canlas CG, Yang R, Gabrys CM, Weliky DP. Solid state NMR measurements of conformation and conformational distributions in the membrane-bound HIV-1 fusion peptide. J. Mol. Graph. Model. 2001;19:129–135. doi: 10.1016/s1093-3263(00)00128-5. [DOI] [PubMed] [Google Scholar]
- 65.Morcombe CR, Zilm KW. Chemical shift referencing in MAS solid state NMR. J. Magn. Reson. 2003;162:479–486. doi: 10.1016/s1090-7807(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 66.Zhang HY, Neal S, Wishart DS. RefDB: A database of uniformly referenced protein chemical shifts. J. Biomol. NMR. 2003;25:173–195. doi: 10.1023/a:1022836027055. [DOI] [PubMed] [Google Scholar]
- 67.Igumenova TI, Wand AJ, McDermott AE. Assignment of the backbone resonances for microcrystalline ubiquitin. J. Am. Chem. Soc. 2004;126:5323–5331. doi: 10.1021/ja030546w. [DOI] [PubMed] [Google Scholar]
- 68.Franks WT, Zhou DH, Wylie BJ, Money BG, Graesser DT, Frericks HL, Sahota G, Rienstra CM. Magic-angle spinning solid-state NMR spectroscopy of the β1 immunoglobulin binding domain of protein G (GB1): 15N and 13C chemical shift assignments and conformational analysis. J. Am. Chem. Soc. 2005;127:12291–12305. doi: 10.1021/ja044497e. [DOI] [PubMed] [Google Scholar]
- 69.Marulanda D, Tasayco ML, Cataldi M, Arriaran V, Polenova T. Resonance assignments and secondary structure analysis of E-coli thioredoxin by magic angle spinning solid-state NMR spectroscopy. J. Phys. Chem. B. 2005;109:18135–18145. doi: 10.1021/jp052774d. [DOI] [PubMed] [Google Scholar]
- 70.Bodner ML, Gabrys CM, Parkanzky PD, Yang J, Duskin CA, Weliky DP. Temperature dependence and resonance assignment of 13C NMR spectra of selectively and uniformly labeled fusion peptides associated with membranes. Magn. Reson. Chem. 2004;42:187–194. doi: 10.1002/mrc.1331. [DOI] [PubMed] [Google Scholar]
- 71.Bloom M, Evans E, Mouritsen OG. Physical properties of the fluid lipid-bilayer component of cell membranes: a perspective. Q. Rev. Biophys. 1991;24:293–397. doi: 10.1017/s0033583500003735. [DOI] [PubMed] [Google Scholar]
- 72.Gabrys CM, Yang J, Weliky DP. Analysis of local conformation of membrane-bound and polycrystalline peptides by two-dimensional slow-spinning rotor-synchronized MAS exchange spectroscopy. J. Biomol. NMR. 2003;26:49–68. doi: 10.1023/a:1023060102409. [DOI] [PubMed] [Google Scholar]
- 73.McDowell LM, Holl SM, Qian SJ, Li E, Schaefer J. Intertryptophan distances in rat cellular retinol-binding Protein Ii by solid-state NMR. Biochemistry. 1993;32:4560–4563. doi: 10.1021/bi00068a011. [DOI] [PubMed] [Google Scholar]
- 74.Anderson RC, Gullion T, Joers JM, Shapiro M, Villhauer EB, Weber HP. Conformation of [1-13C,15N]acetyl-L-carnitine. Rotational-echo, double-resonance nuclear magnetic resonance spectroscopy. J. Am. Chem. Soc. 1995;117:10546–10550. [Google Scholar]
- 75.Gullion T. Introduction to rotational-echo, double-resonance NMR. Concept Magnetic Res. 1998;10:277–289. doi: 10.1016/j.jmr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 76.Bennett AE, Rienstra CM, Auger M, Lakshmi KV, Griffin RG. Heteronuclear decoupling in rotating solids. J. Chem. Phys. 1995;103:6951–6958. [Google Scholar]
- 77.Bevington PR, Robinson DK. Data Reduction and Error Analysis for the Physical Sciences. 2nd ed. Boston: McGraw-Hill; 1992. [Google Scholar]
- 78.Mueller KT. Analytic solutions for the time evolution of dipolar-dephasing NMR signals. J. Magn. Reson. Ser. A. 1995;113:81–93. [Google Scholar]
- 79.Raghunathan V, Gibson JM, Goobes G, Popham JM, Louie EA, Stayton PS, Drobny GP. Homonuclear and heteronuclear NMR studies of a statherin fragment bound to hydroxyapatite crystals. J. Phys. Chem. B. 2006;110:9324–9332. doi: 10.1021/jp056644g. [DOI] [PubMed] [Google Scholar]
- 80.Pearson RH, Pascher I. Molecular structure of lecithin dihydrate. Nature. 1979;281:499–501. doi: 10.1038/281499a0. [DOI] [PubMed] [Google Scholar]
- 81.Pascher I, Sundell S, Harlos K, Eibl H. Conformation and packing properties of membrane lipids: The crystal structure of sodium dimyristoylphosphatidylglycerol. Biochim. Biophys. Acta. 1987;896:77–88. doi: 10.1016/0005-2736(87)90358-0. [DOI] [PubMed] [Google Scholar]
- 82.Venable RM, Brooks BR, Pastor RW. Molecular dynamics simulations of gel (LβI) phase lipid bilayers in constant pressure and constant surface area ensembles. J. Chem. Phys. 2000;112:4822–4832. [Google Scholar]
- 83.Bennett AE, Ok JH, Griffin RG, Vega S. Chemical-shift correlation spectroscopy in rotating solids - radio frequency-driven dipolar recoupling and longitudinal exchange. J. Chem. Phys. 1992;96:8624–8627. [Google Scholar]
- 84.Gullion T, Vega S. A simple magic angle spinning NMR experiment for the dephasing of rotational echoes of dipolar coupled homonuclear spin pairs. Chem. Phys. Lett. 1992;194:423–428. [Google Scholar]
- 85.Gordon LM, Mobley PW, Pilpa R, Sherman MA, Waring AJ. Conformational mapping of the N-terminal peptide of HIV-1 gp41 in membrane environments using 13C-enhanced Fourier transform infrared spectroscopy. Biochim. Biophys. Acta-Biomembranes. 2002;1559:96–120. doi: 10.1016/s0005-2736(01)00443-6. [DOI] [PubMed] [Google Scholar]
- 86.Castano S, Desbat B. Structure and orientation study of fusion peptide FP23 of gp41 from HIV-1 alone or inserted into various lipid membrane models (mono-, bi- and multibi-layers) by FT-IR spectroscopies and Brewster angle microscopy. Biochim. Biophys. Acta-Biomembranes. 2005;1715:81–95. doi: 10.1016/j.bbamem.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 87.Sackett K, Shai Y. The HIV fusion peptide adopts intermolecular parallel β-sheet structure in membranes when stabilized by the adjacent N-terminal heptad repeat: A 13C FTIR study. J. Mol. Biol. 2005;350:790–805. doi: 10.1016/j.jmb.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 88.Chan DC, Fass D, Berger JM, Kim PS. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 89.Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 90.Tan K, Liu J, Wang J, Shen S, Lu M. Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc. Natl. Acad. Sci. U.S.A. 1997;94:12303–12308. doi: 10.1073/pnas.94.23.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Caffrey M, Cai M, Kaufman J, Stahl SJ, Wingfield PT, Covell DG, Gronenborn AM, Clore GM. Three-dimensional solution structure of the 44 kDa ectodomain of SIV gp41. EMBO J. 1998;17:4572–4584. doi: 10.1093/emboj/17.16.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang ZN, Mueser TC, Kaufman J, Stahl SJ, Wingfield PT, Hyde CC. The crystal structure of the SIV gp41 ectodomain at 1.47 A resolution. J. Struct. Biol. 1999;126:131–144. doi: 10.1006/jsbi.1999.4116. [DOI] [PubMed] [Google Scholar]
- 93.Modis Y, Ogata S, Clements D, Harrison SC. Structure of the dengue virus envelope protein after membrane fusion. Nature. 2004;427:313–319. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 94.Gibbons DL, Vaney MC, Roussel A, Vigouroux A, Reilly B, Lepault J, Kielian M, Rey FA. Conformational change and protein protein interactions of the fusion protein of Semliki Forest virus. Nature. 2004;427:320–325. doi: 10.1038/nature02239. [DOI] [PubMed] [Google Scholar]
- 95.Roche S, Bressanelli S, Rey FA, Gaudin Y. Crystal structure of the low-pH form of the vesicular stomatitis virus glycoprotein G. Science. 2006;313:187–191. doi: 10.1126/science.1127683. [DOI] [PubMed] [Google Scholar]
- 96.Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. Crystal structure of glycoprotein B from herpes simplex virus 1. Science. 2006;313:217–220. doi: 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- 97.Hessa T, Kim H, Bihlmaier K, Lundin C, Boekel J, Andersson H, Nilsson I, White SH, von Heijne G. Recognition of transmembrane helices by the endoplasmic reticulum translocon. Nature. 2005;433:377–381. doi: 10.1038/nature03216. [DOI] [PubMed] [Google Scholar]
- 98.Charloteaux B, Lorin A, Crowet JM, Stroobant V, Lins L, Thomas A, Brasseur R. The N-terminal 12 residue long peptide of HIV gp41 is the minimal peptide sufficient to induce significant T-cell-like membrane destabilization in vitro. J. Mol. Biol. 2006;359:597–609. doi: 10.1016/j.jmb.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 99.Smaby JM, Brockman HL, Brown RE. Cholesterol's interfacial interactions with sphingomyelins and phosphatidylcholines: Hydrocarbon chain structure determines the magnitude of condensation. Biochemistry. 1994;33:9135–9142. doi: 10.1021/bi00197a016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tristram-Nagle S, Liu YF, Legleiter J, Nagle JF. Structure of gel phase DMPC determined by X-ray diffraction. Biophys. J. 2002;83:3324–3335. doi: 10.1016/S0006-3495(02)75333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Eq. 2 and the σlab expression are derived and discussed. This material is available free of charge via the Internet at http://pubs.acs.org.