Abstract
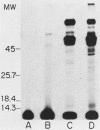
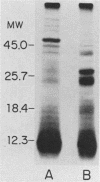
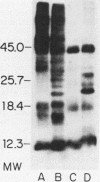
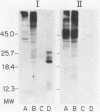
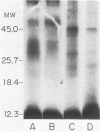
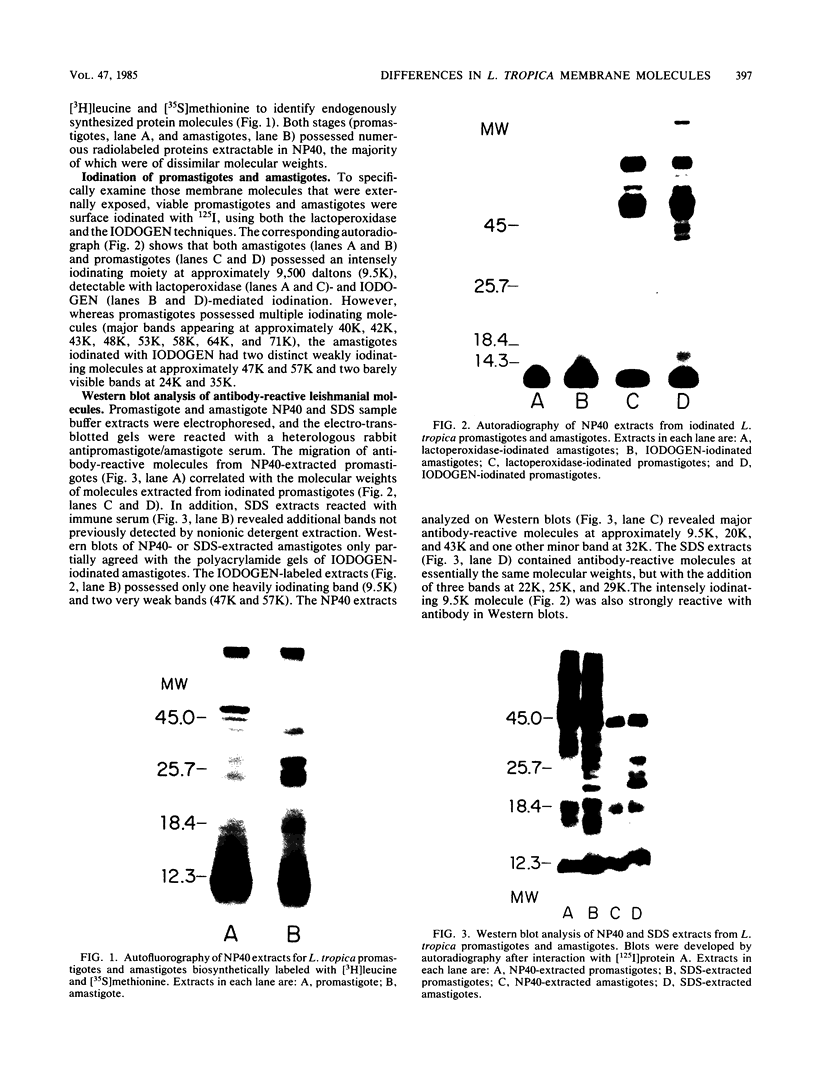
The accessibility of particular Leishmania tropica promastigote (extracellular) and amastigote (intracellular) membrane molecules might be related to the relative abilities of the two stages to induce host immune responses. To examine the exposure of membrane antigens on resident macrophage-susceptible promastigotes and resident macrophage-resistant amastigotes, both stages were analyzed by polyacrylamide gel electrophoresis and immunoblotting after specific labeling and extraction procedures. Protein compositional studies, using metabolic labeling of promastigotes and amastigotes, demonstrated that both forms possessed numerous endogenously synthesized proteins. In addition, a marked difference was revealed in the external exposure of promastigote and amastigote membrane constituents when analyzed by 125I surface labeling or Western blot analysis. Whereas nine promastigote proteins were intensely to moderately iodinated, only one amastigote membrane component was similarly labeled (9.5K band). Western blot analyses with serum from a rabbit immunized with a mixture of both L. tropica stages indicated that the majority of promastigote molecules accessible to 125I may also react with immune serum. However, Western blots of extracted amastigotes identified several bands not seen on radiographs and thus not accessible to 125I. The external exposure of these amastigote molecules was confirmed in that immune serum adsorbed with viable, intact amastigotes was no longer reactive with amastigote extracts. Further, by Western blot analyses of sodium dodecyl sulfate- but not Nonidet P-40-extracted amastigotes, three amastigote-specific membrane antigens not previously observed with nonionic detergent extraction methods were identified. The autofluorographic pattern of amastigotes intrinsically labeled with N-[3H]acetylglucosamine, an amino sugar which is incorporated into membrane carbohydrates, was in excellent agreement with the pattern of antigens reactive with antibody in Western blots. Thus, with these cell surface labeling and extraction methods, promastigote and amastigote membranes were shown to be significantly different. Amastigotes possessed several membrane-associated molecules, but few appeared to be either accessible or reactive with 125I. Moreover, the majority of molecules not reactive with 125I, but reactive with antibodies, may be glycosylated. These observations are discussed relative to the ability of amastigotes both to survive within the degradative milieu of macrophage phagolysosomes and to evade host immune reactivity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter W. G. The cooperative role of the transformation-sensitive glycoproteins, GP140 and fibronectin, in cell attachment and spreading. J Biol Chem. 1982 Mar 25;257(6):3249–3257. [PubMed] [Google Scholar]
- Chang K. P. Human cutaneous lieshmania in a mouse macrophage line: propagation and isolation of intracellular parasites. Science. 1980 Sep 12;209(4462):1240–1242. doi: 10.1126/science.7403880. [DOI] [PubMed] [Google Scholar]
- Dwyer D. M., Langreth S. G., Dwyer N. K. Evidence for a polysaccharide surface coat in the developmental stages of Leishmania donovani: a fine structure-cytochemical study. Z Parasitenkd. 1974 Jun 21;43(4):227–249. doi: 10.1007/BF00328879. [DOI] [PubMed] [Google Scholar]
- Dwyer D. M. Leishmania donovani: surface membrane carbohydrates of promastigotes. Exp Parasitol. 1977 Apr;41(2):341–358. doi: 10.1016/0014-4894(77)90107-2. [DOI] [PubMed] [Google Scholar]
- El-On J., Schnur L. F., Greenblatt C. L. Leishmania donovani: physicochemical, immunological, and biological characterization of excreted factor from promastigotes. Exp Parasitol. 1979 Apr;47(2):254–269. doi: 10.1016/0014-4894(79)90078-x. [DOI] [PubMed] [Google Scholar]
- Gardiner P. R., Dwyer D. M. Radioiodination and identification of externally disposed membrane components of Leishmania tropica. Mol Biochem Parasitol. 1983 Aug;8(4):283–295. doi: 10.1016/0166-6851(83)90075-0. [DOI] [PubMed] [Google Scholar]
- Gardiner P. R., Jaffe C. L., Dwyer D. M. Identification of cross-reactive promastigote cell surface antigens of some leishmanial stocks by 125I labeling and immunoprecipitation. Infect Immun. 1984 Feb;43(2):637–643. doi: 10.1128/iai.43.2.637-643.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Curtis J. M. Leishmania tropica: surface antigens of intracellular and flagellate forms. Exp Parasitol. 1982 Oct;54(2):243–249. doi: 10.1016/0014-4894(82)90133-3. [DOI] [PubMed] [Google Scholar]
- Handman E., Hocking R. E. Stage-specific, strain-specific, and cross-reactive antigens of Leishmania species identified by monoclonal antibodies. Infect Immun. 1982 Jul;37(1):28–33. doi: 10.1128/iai.37.1.28-33.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Jarvis H. M., Mitchell G. F. Leishmania major: identification of stage-specific antigens and antigens shared by promastigotes and amastigotes. Parasite Immunol. 1984 May;6(3):223–233. doi: 10.1111/j.1365-3024.1984.tb00795.x. [DOI] [PubMed] [Google Scholar]
- Handman E., Mitchell G. F., Goding J. W. Identification and characterization of protein antigens of Leishmania tropica isolates. J Immunol. 1981 Feb;126(2):508–512. [PubMed] [Google Scholar]
- Hodgkinson V. H., Patton C. L. Leishmania enriettii amastigotes: demonstration of fibrillar surface. J Infect Dis. 1982 Dec;146(6):758–762. doi: 10.1093/infdis/146.6.758. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Liew F. Y., Hale C., Nicklin S. Prophylactic immunization against experimental leishmaniasis. II. Further characterization of the protective immunity against fatal Leishmania tropica infection induced by irradiated promastigotes. J Immunol. 1984 Jan;132(1):450–455. [PubMed] [Google Scholar]
- Howard R. J., Kaushal D. C., Carter R. Radioiodination of parasite antigens with 1,3,4,6-tetrachloro-3 alpha, 6 alpha-diphenylglycoluril (IODOGEN): studies with zygotes of Plasmodium gallinaceum. J Protozool. 1982 Feb;29(1):114–117. doi: 10.1111/j.1550-7408.1982.tb02891.x. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe C. L., Bennett E., Grimaldi G., Jr, McMahon-Pratt D. Production and characterization of species-specific monoclonal antibodies against Leishmania donovani for immunodiagnosis. J Immunol. 1984 Jul;133(1):440–447. [PubMed] [Google Scholar]
- Jaffe C. L., McMahon-Pratt D. Monoclonal antibodies specific for Leishmania tropica. I. Characterization of antigens associated with stage- and species-specific determinants. J Immunol. 1983 Oct;131(4):1987–1993. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lepay D. A., Nogueira N., Cohn Z. Surface antigens of Leishmania donovani promastigotes. J Exp Med. 1983 May 1;157(5):1562–1572. doi: 10.1084/jem.157.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. H., Peters W. The resistance of intracellular Leishmania parasites to digestion by lysosomal enzymes. Ann Trop Med Parasitol. 1977 Sep;71(3):295–312. doi: 10.1080/00034983.1977.11687192. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Howard J. G., Hale C. Prophylactic immunization against experimental leishmaniasis. III. Protection against fatal Leishmania tropica infection induced by irradiated promastigotes involves Lyt-1+2- T cells that do not mediate cutaneous DTH. J Immunol. 1984 Jan;132(1):456–461. [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S., Leonard E. J., Wyler D. J. Intracellular replication and lymphokine-induced destruction of Leishmania tropica in C3H/HeN mouse macrophages. J Immunol. 1981 Dec;127(6):2381–2386. [PubMed] [Google Scholar]
- Nguyen T. V., Mauel J., Etges R. J., Bouvier J., Bordier C. Immunogenic proteins of Leishmania major during mouse infection. Parasite Immunol. 1984 May;6(3):265–273. doi: 10.1111/j.1365-3024.1984.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Preston P. M., Carter R. L., Leuchars E., Davies A. J., Dumonde D. C. Experimental cutaneous leishmaniasis. 3. Effects of thymectomy on the course of infection of CBA mice with Leishmania tropica. Clin Exp Immunol. 1972 Feb;10(2):337–357. [PMC free article] [PubMed] [Google Scholar]
- Scott P., Sacks D., Sher A. Resistance to macrophage-mediated killing as a factor influencing the pathogenesis of chronic cutaneous leishmaniasis. J Immunol. 1983 Aug;131(2):966–971. [PubMed] [Google Scholar]