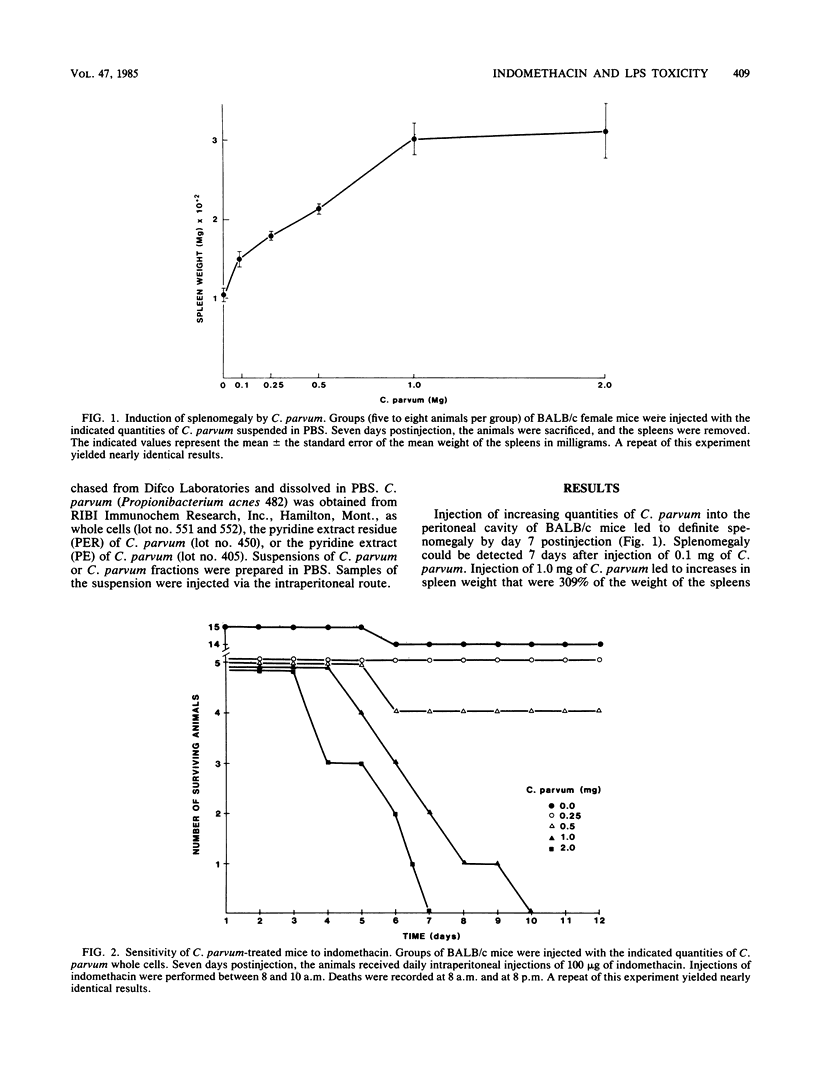
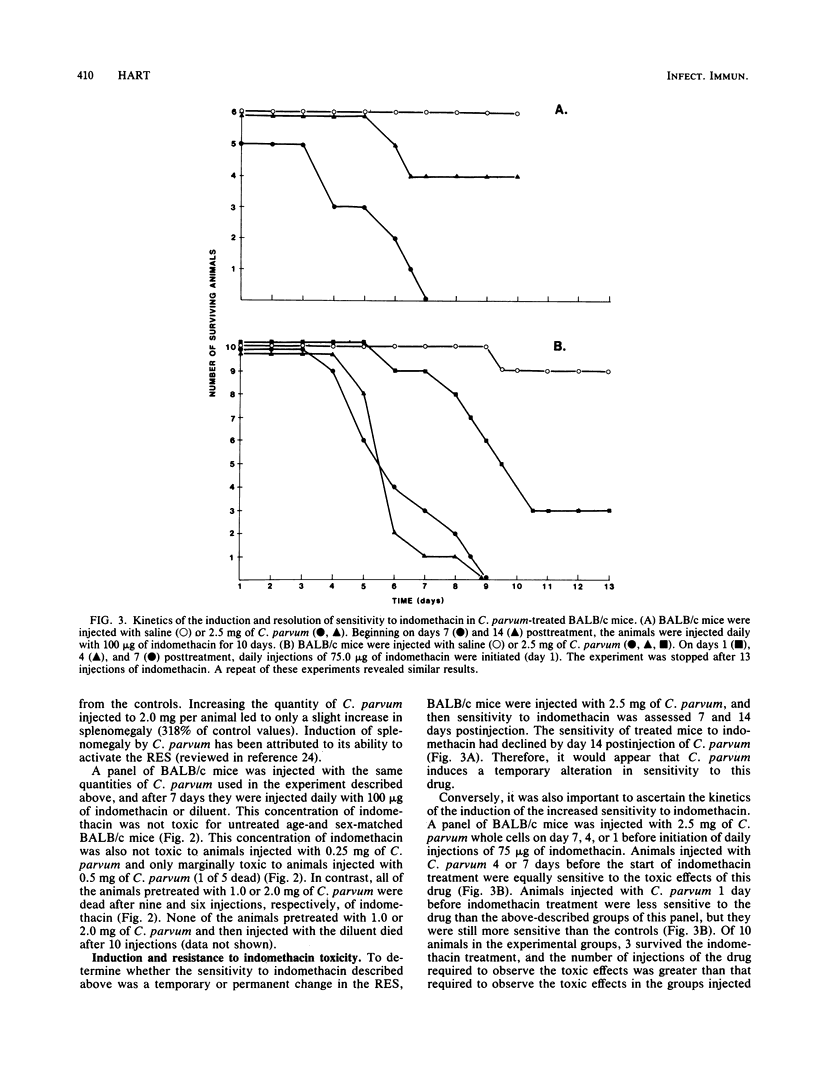
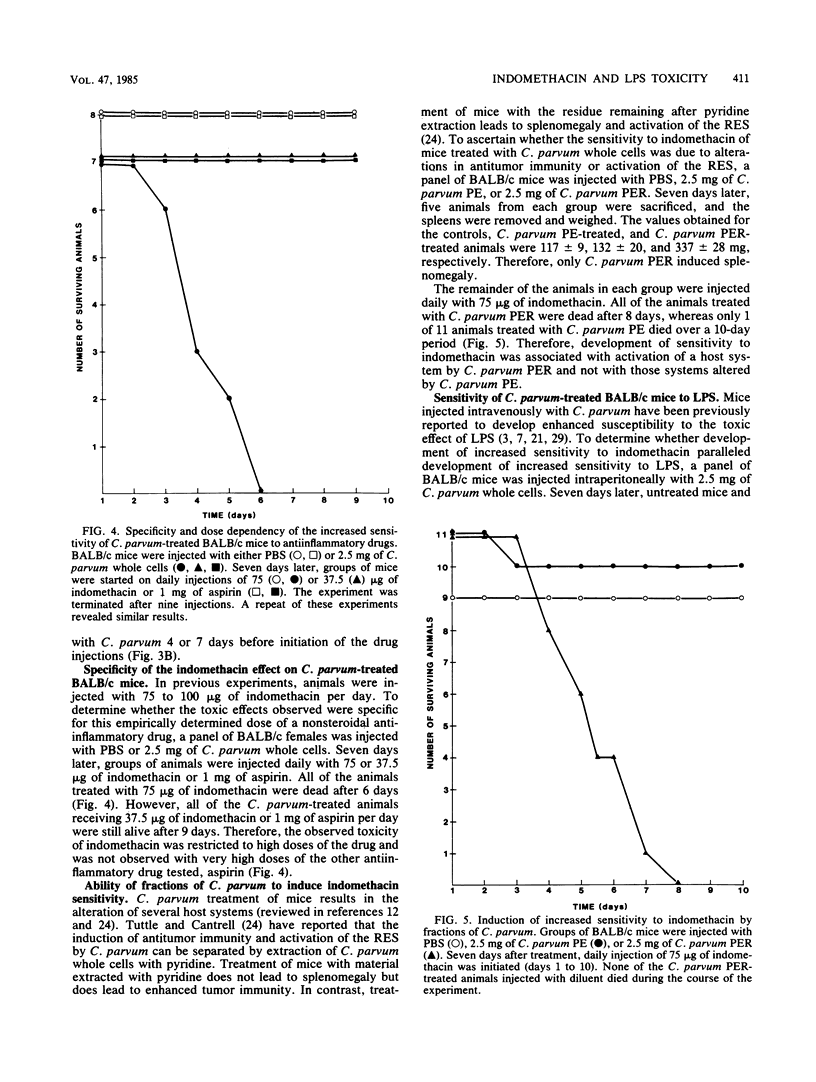
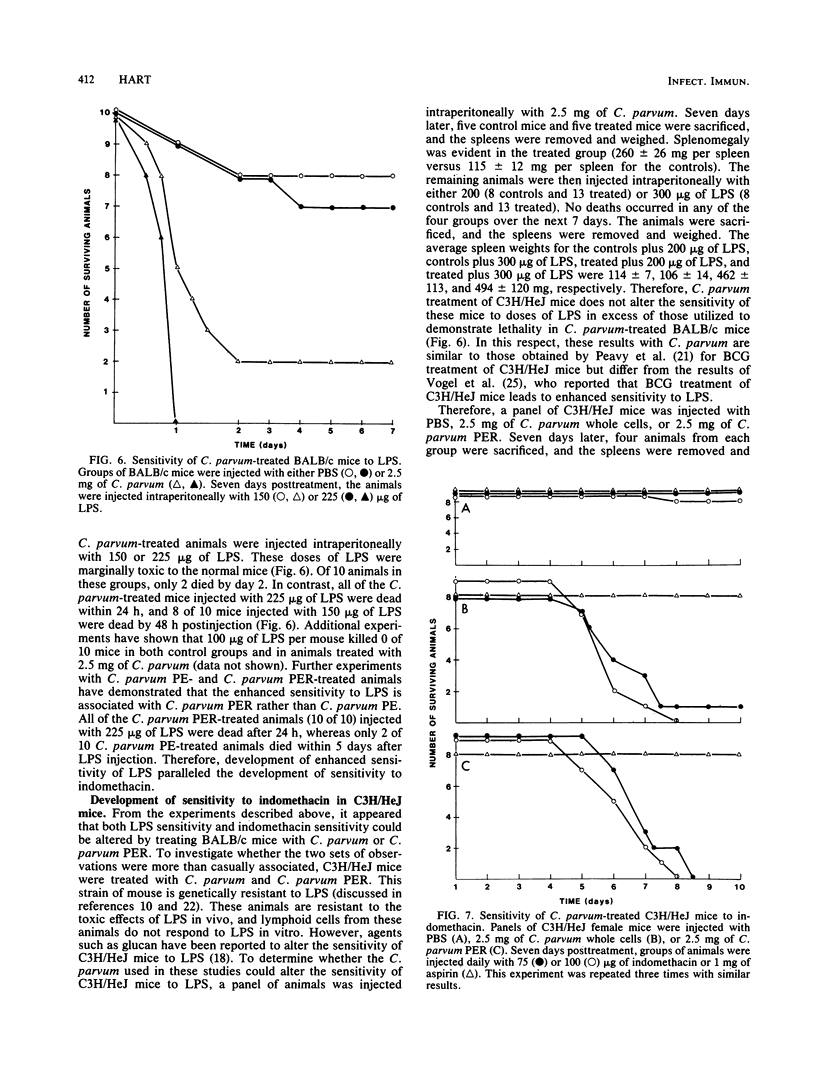
Abstract
Female BALB/c and C3H/HeJ mice develop increased sensitivity to the toxic effects of indomethacin after injection of nonviable Corynebacterium parvum. The increased sensitivity developed within 4 days of intraperitoneal injection of the organisms and started to resolve 14 days after injection. The development of increased sensitivity was dependent on the quantity of organisms injected and the concentration of indomethacin utilized. The effect was not observed when C. parvum-treated animals were injected with aspirin. C. parvum-treated BALB/c mice also developed increased sensitivity to E. coli lipopolysaccharide (LPS). Although increased sensitivity to LPS and indomethacin paralleled each other in BALB/c mice, the experiments with the LPS-resistant C3H/HeJ mice indicated that the two phenomena could be separated. The pyridine extract residue of C. parvum was as effective as C. parvum whole cells in inducing indomethacin and LPS sensitivity. Therefore, activation of the reticuloendothelial system is probably a critical element in the induction of sensitivity to these agents.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Sjöberg O., Möller G. Induction of immunoglobulin and antibody synthesis in vitro by lipopolysaccharides. Eur J Immunol. 1972 Aug;2(4):349–353. doi: 10.1002/eji.1830020410. [DOI] [PubMed] [Google Scholar]
- Bennett W. E., Cohn Z. A. The isolation and selected properties of blood monocytes. J Exp Med. 1966 Jan 1;123(1):145–160. doi: 10.1084/jem.123.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendt M. J., Newborg M. F., North R. J. Increased toxicity of endotoxin for tumor-bearing mice and mice responding to bacterial pathogens: macrophage activation as a common denominator. Infect Immun. 1980 May;28(2):645–647. doi: 10.1128/iai.28.2.645-647.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman G. A., Luster M. I., Dean J. H., Luebke R. W. Effect of indomethacin on the bone marrow and immune system of the mouse. J Clin Lab Immunol. 1982 Feb;7(2):119–126. [PubMed] [Google Scholar]
- Chapes S. K., Haskill S. Role of Corynebacterium parvum in the activation of peritoneal macrophages. 1. Association between intracellular C. parvum and cytotoxic macrophages. Cell Immunol. 1982 Jun;70(1):65–75. doi: 10.1016/0008-8749(82)90133-2. [DOI] [PubMed] [Google Scholar]
- Deml E., Oesterle D., Wolff T., Greim H. Age-, sex-, and strain-dependent differences in the induction of enzyme-altered islands in rat liver by diethylnitrosamine. J Cancer Res Clin Oncol. 1981;100(2):125–134. doi: 10.1007/BF00403362. [DOI] [PubMed] [Google Scholar]
- Ferluga J., Kaplun A., Allison A. C. Protection of mice against endotoxin-induced liver damage by anti-inflammatory drugs. Agents Actions. 1979 Dec;9(5-6):566–574. doi: 10.1007/BF01968129. [DOI] [PubMed] [Google Scholar]
- Flower R. J., Vane J. R. Inhibition of prostaglandin biosynthesis. Biochem Pharmacol. 1974 May 15;23(10):1439–1450. doi: 10.1016/0006-2952(74)90381-5. [DOI] [PubMed] [Google Scholar]
- Glode L. M., Scher I., Osborne B., Rosenstreich D. L. Cellular mechanism of endotoxin unresponsiveness in C3H/HeJ mice. J Immunol. 1976 Feb;116(2):454–461. [PubMed] [Google Scholar]
- Goodrum K. J., Moore R. N., Berry L. J. Effect of indomethacin on the response of mice to endotoxin. J Reticuloendothel Soc. 1978 Mar;23(3):213–221. [PubMed] [Google Scholar]
- Halpern B. N., Biozzi G., Stiffel C., Mouton D. Inhibition of tumour growth by administration of killed corynebacterium parvum. Nature. 1966 Nov 19;212(5064):853–854. doi: 10.1038/212853a0. [DOI] [PubMed] [Google Scholar]
- Hart D. A. Evidence that lithium ions can modulate lectin stimulation of lymphoid cells by multiple mechanisms. Cell Immunol. 1981 Mar 1;58(2):372–384. doi: 10.1016/0008-8749(81)90231-8. [DOI] [PubMed] [Google Scholar]
- Hurn B. A., Chantler S. M. Production of reagent antibodies. Methods Enzymol. 1980;70(A):104–142. doi: 10.1016/s0076-6879(80)70044-7. [DOI] [PubMed] [Google Scholar]
- Lazar G., Agarwal M. K. Reversal of nonresponsiveness to bacterial endotoxins in C3H/HeJ mice by glucan and streptozotocin. Biochem Med. 1982 Dec;28(3):310–318. doi: 10.1016/0006-2944(82)90085-0. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Rosenstreich D. L. Signals regulating in vitro activation of lymphocytes. Prog Allergy. 1976;20:65–194. doi: 10.1159/000398282. [DOI] [PubMed] [Google Scholar]
- Peavy D. L., Baughn R. E., Musher D. M., Musher D. M. Effects of BCG infection on the susceptibility of mouse macrophages to endotoxin. Infect Immun. 1979 Apr;24(1):59–64. doi: 10.1128/iai.24.1.59-64.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E., ULLMAN G. E., HOFFMAN R. G. Sensitivity of mice to endotoxin after vaccination with BCG (Bacillus Calmette-Guérin). Proc Soc Exp Biol Med. 1958 Oct;99(1):167–169. doi: 10.3181/00379727-99-24282. [DOI] [PubMed] [Google Scholar]
- Sultzer B. M. Genetic control of leucocyte responses to endotoxin. Nature. 1968 Sep 21;219(5160):1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- Vogel S. N., Moore R. N., Sipe J. D., Rosenstreich D. L. BCG-induced enhancement of endotoxin sensitivity in C3H/HeJ mice. I. In vivo studies. J Immunol. 1980 Apr;124(4):2004–2009. [PubMed] [Google Scholar]
- Wedner H. J., Parker C. W. Lymphocyte activation. Prog Allergy. 1976;20:195–300. [PubMed] [Google Scholar]
- White R. G. The adjuvant effect of microbial products on the immune response. Annu Rev Microbiol. 1976;30:579–600. doi: 10.1146/annurev.mi.30.100176.003051. [DOI] [PubMed] [Google Scholar]
- Wiener E., Levanon D. The in vitro interaction between bacterial lipopolysaccharide and differentiating monocytes. Lab Invest. 1968 Dec;19(6):584–590. [PubMed] [Google Scholar]
- Yoshikai Y., Miake S., Sano M., Nomoto K. Increased susceptibility to Escherichia coli infection in mice pretreated with Corynebacterium parvum. Microbiol Immunol. 1983;27(3):273–282. doi: 10.1111/j.1348-0421.1983.tb03589.x. [DOI] [PubMed] [Google Scholar]


