Abstract
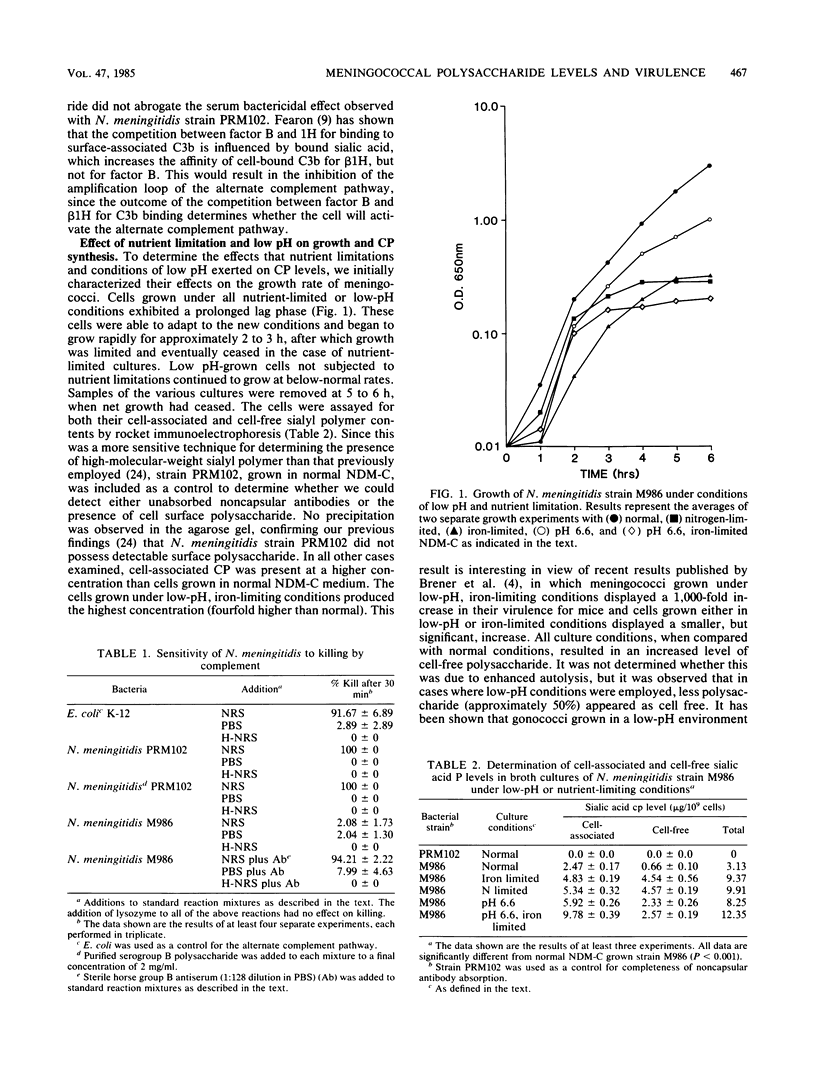
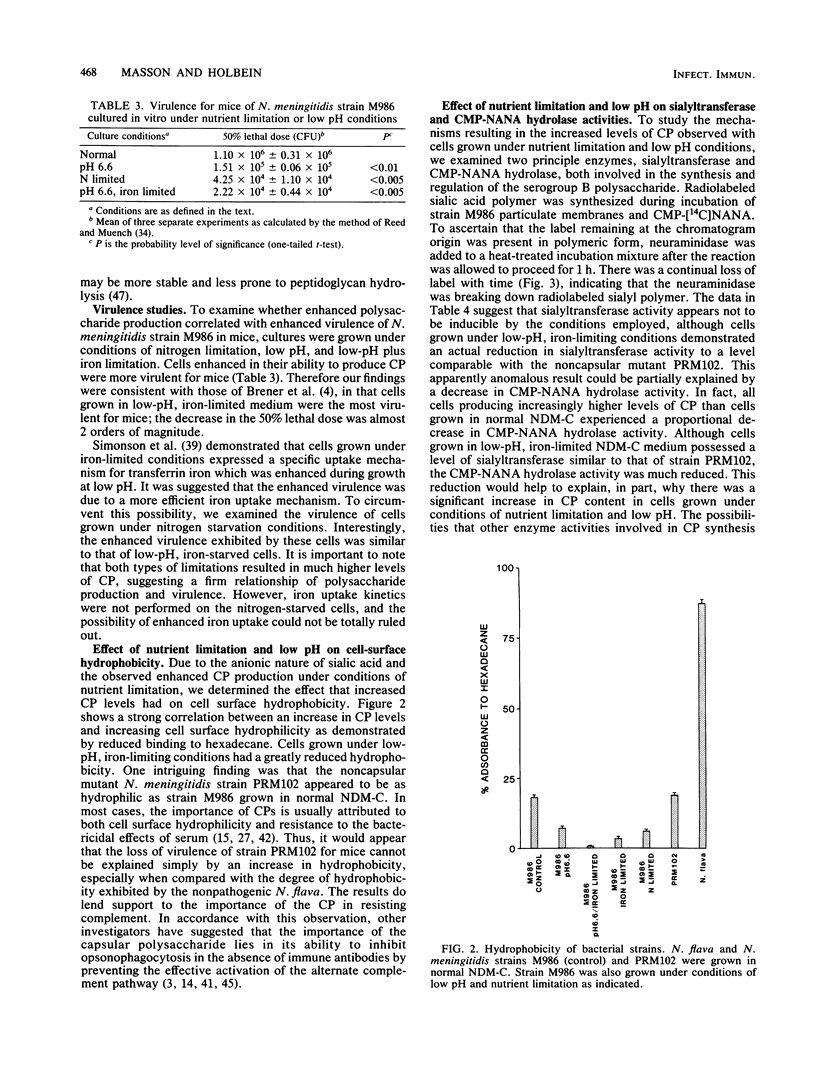
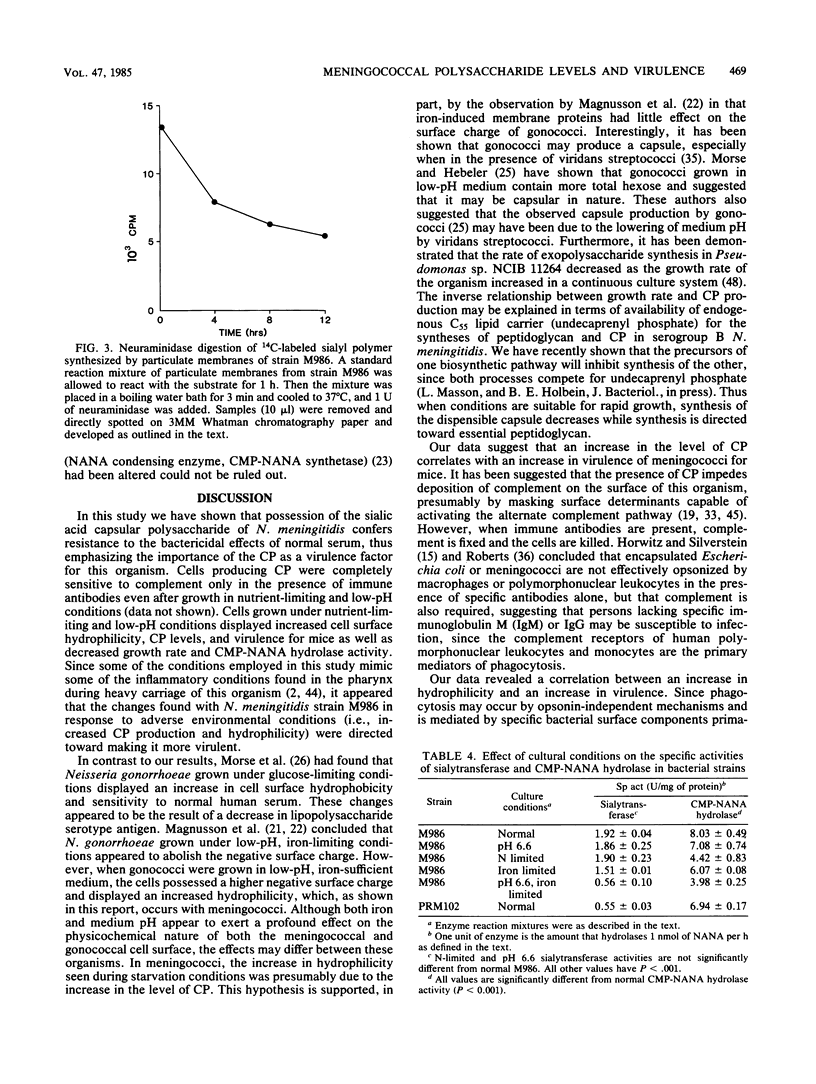
Neisseria meningitidis strain M986, which possesses a polyanionic sialic acid capsular polysaccharide, was resistant to the bactericidal effects of normal rabbit serum, but sensitive when immune serum and complement were present. An isogenic strain PRM102, deficient in the ability to produce capsular polysaccharide, was sensitive to normal serum. Strain M986, when grown under conditions of low pH or nutrient limitation, synthesized increased levels of capsular polysaccharide. This was accompanied by an increase in cell surface hydrophilicity and virulence for mice. Cells grown in low-pH, iron-limited medium synthesized the highest concentration of polysaccharide and exhibited the highest cell surface hydrophilicity and virulence among the cases examined. The increase in capsular polysaccharide was partly explained by a decrease in the specific activity of a membrane-bound cytidine monophosphate-N acetylneuraminic acid hydrolase. The results suggest that conditions of nutrient limitation and low pH exert profound effects on the physicochemical nature of the meningococcal cell surface which, in turn, cumulate in enhanced virulence of this organism for mice.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald F. S., DeVoe I. W. Iron in Neisseria meningitidis: minimum requirements, effects of limitation, and characteristics of uptake. J Bacteriol. 1978 Oct;136(1):35–48. doi: 10.1128/jb.136.1.35-48.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolussi R., Ferrieri P., Quie P. G. Influence of growth temperature of Escherichia coli on K1 capsular antigen production and resistance to opsonization. Infect Immun. 1983 Mar;39(3):1136–1141. doi: 10.1128/iai.39.3.1136-1141.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener D., DeVoe I. W., Holbein B. E. Increased virulence of Neisseria meningitidis after in vitro iron-limited growth at low pH. Infect Immun. 1981 Jul;33(1):59–66. doi: 10.1128/iai.33.1.59-66.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoe I. W. The meningococcus and mechanisms of pathogenicity. Microbiol Rev. 1982 Jun;46(2):162–190. doi: 10.1128/mr.46.2.162-190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine L. F., Hagerman C. R. Relationship of Serogroups of Neisseria meningitidis I. Microagglutination, Gel Diffusion, and Slide Agglutination Studies of Meningococcal Antisera Before and After Absorption with RAS-10 Strain of Meningococci. Infect Immun. 1970 Mar;1(3):226–231. doi: 10.1128/iai.1.3.226-231.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine L. F., Rhode S. L., 3rd, Hagerman C. R. Relationship of serogroups of Neisseria meningitidis. II. R variants of Neisseria meningitidis. Infect Immun. 1972 Jan;5(1):48–54. doi: 10.1128/iai.5.1.48-54.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969 Jun 1;129(6):1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbein B. E., Jericho K. W., Likes G. C. Neisseria meningitidis infection in mice: influence of iron, variations in virulence among strains, and pathology. Infect Immun. 1979 May;24(2):545–551. doi: 10.1128/iai.24.2.545-551.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest. 1980 Jan;65(1):82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan S. L., Mason E. O., Jr, Wiedermann B. L. Role of adherence in the pathogenesis of Haemophilus influenzae type b infection in infant rats. Infect Immun. 1983 Nov;42(2):612–617. doi: 10.1128/iai.42.2.612-617.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper D. L., Winkelhake J. L., Zollinger W. D., Brandt B. L., Artenstein M. S. Immunochemical similarity between polysaccharide antigens of Escherichia coli 07: K1(L):NM and group B Neisseria meningitidis. J Immunol. 1973 Jan;110(1):262–268. [PubMed] [Google Scholar]
- Klegerman M. E., Boyer K. M., Papierniak C. K., Levine L., Gotoff S. P. Type-specific capsular antigen is associated with virulence in late-onset group B Streptococcal type III disease. Infect Immun. 1984 Apr;44(1):124–129. doi: 10.1128/iai.44.1.124-129.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leist-Welsh P., Bjornson A. B. Requirements of immunoglobulin and the classical and alternative complement pathways for phagocytosis and intracellular killing of multiple strains of Gram-negative aerobic bacilli. Infect Immun. 1979 Oct;26(1):99–109. doi: 10.1128/iai.26.1.99-109.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson K. E., Kihlstrom E., Norqvist A., Davies J., Normark S. Effect of iron on surface charge and hydrophobicity of Neisseria gonorrhoeae. Infect Immun. 1979 Nov;26(2):402–407. doi: 10.1128/iai.26.2.402-407.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson K. E., Kihlström E., Norlander L., Norqvist A., Davies J., Normark S. Effect of colony type and pH on surface charge and hydrophobicity of Neisseria gonorrhoeae. Infect Immun. 1979 Nov;26(2):397–401. doi: 10.1128/iai.26.2.397-401.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson L., Holbein B. E. Physiology of sialic acid capsular polysaccharide synthesis in serogroup B Neisseria meningitidis. J Bacteriol. 1983 May;154(2):728–736. doi: 10.1128/jb.154.2.728-736.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Hebeler B. H. Effect of pH on the growth and glucose metabolism of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):87–95. doi: 10.1128/iai.21.1.87-95.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Mintz C. S., Sarafian S. K., Bartenstein L., Bertram M., Apicella M. A. Effect of dilution rate on lipopolysaccharide and serum resistance of Neisseria gonorrhoeae grown in continuous culture. Infect Immun. 1983 Jul;41(1):74–82. doi: 10.1128/iai.41.1.74-82.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A. M., Glynn A. A. Investigation of the effect of K antigen in Escherichia coli urinary tract infections by use of a mouse model. Br J Exp Pathol. 1975 Dec;56(6):549–553. [PMC free article] [PubMed] [Google Scholar]
- Nicholson A., Lepow I. H. Host defense against Neisseria meningitidis requires a complement-dependent bactericidal activity. Science. 1979 Jul 20;205(4403):298–299. doi: 10.1126/science.451601. [DOI] [PubMed] [Google Scholar]
- Ohman L., Hed J., Stendahl O. Interaction between human polymorphonuclear leukocytes and two different strains of type 1 fimbriae-bearing Escherichia coli. J Infect Dis. 1982 Dec;146(6):751–757. doi: 10.1093/infdis/146.6.751. [DOI] [PubMed] [Google Scholar]
- Peetz R. H., Kenny G. E. Prevention of autolysis in suspensions of Neisseria gonorrhoeae by mercuric ions. J Bacteriol. 1978 Jul;135(1):283–285. doi: 10.1128/jb.135.1.283-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B. H., Lee T. J., Snyderman R., Brooks G. F. Neisseria meningitidis and Neisseria gonorrhoeae bacteremia associated with C6, C7, or C8 deficiency. Ann Intern Med. 1979 Jun;90(6):917–920. doi: 10.7326/0003-4819-90-6-917. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Gekker G., Shapiro R., Freiberg M., Keane W. F. Polyamino acid enhancement of bacterial phagocytosis by human polymorphonuclear leukocytes and peritoneal macrophages. Infect Immun. 1984 Feb;43(2):561–566. doi: 10.1128/iai.43.2.561-566.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Mayden J., Achtman M., Levine R. P. Role of the capsule and the O antigen in resistance of O18:K1 Escherichia coli to complement-mediated killing. Infect Immun. 1983 Dec;42(3):907–913. doi: 10.1128/iai.42.3.907-913.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. P., Sadoff J. C. Production of a capsule of Neisseria gonorrhoeae. Infect Immun. 1977 Feb;15(2):663–664. doi: 10.1128/iai.15.2.663-664.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. B. The interaction in vitro between group B meningococci and rabbit polymorphonuclear leukocytes. Demonstration of type specific opsonins and bactericidins. J Exp Med. 1967 Nov 1;126(5):795–818. doi: 10.1084/jem.126.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Morton G. Adherence of Neisseria meningitidis to human epithelial cells. Infect Immun. 1981 Jan;31(1):430–435. doi: 10.1128/iai.31.1.430-435.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson C., Brener D., DeVoe I. W. Expression of a high-affinity mechanism for acquisition of transferrin iron by Neisseria meningitidis. Infect Immun. 1982 Apr;36(1):107–113. doi: 10.1128/iai.36.1.107-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. S., McGee Z. A. Association of virulence of Neisseria meningitidis with transparent colony type and low-molecular-weight outer membrane proteins. J Infect Dis. 1983 Feb;147(2):282–292. doi: 10.1093/infdis/147.2.282. [DOI] [PubMed] [Google Scholar]
- Sutton A., Schneerson R., Kendall-Morris S., Robbins J. B. Differential complement resistance mediates virulence of Haemophilus influenzae type b. Infect Immun. 1982 Jan;35(1):95–104. doi: 10.1128/iai.35.1.95-104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J. L., Masson P. L., Heremans J. F. The involvement of lactoferrin in the hyposideremia of acute inflammation. J Exp Med. 1974 Oct 1;140(4):1068–1084. doi: 10.1084/jem.140.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., van Dijk W. C., van Erne M. E., Peters R., Peterson P. K., Verhoef J. Quantitation of the third component of human complement attached to the surface of opsonized bacteria: opsonin-deficient sera and phagocytosis-resistant strains. Infect Immun. 1979 Dec;26(3):808–814. doi: 10.1128/iai.26.3.808-814.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeke B. Rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:37–46. doi: 10.1111/j.1365-3083.1973.tb03777.x. [DOI] [PubMed] [Google Scholar]
- Wegener W. S., Hebeler B. H., Morse S. A. Cell envelope of Neisseria gonorrhoeae: relationship between autolysis in buffer and the hydrolysis of peptidoglycan. Infect Immun. 1977 Oct;18(1):210–219. doi: 10.1128/iai.18.1.210-219.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. G., Wimpenny J. W. Extracellular polysaccharide biosynthesis by Pseudomonas NCIB 11264. Studies on precursor-forming enzymes and factors affecting exopolysaccharide production by washed suspensions. J Gen Microbiol. 1980 Jan;116(1):133–141. doi: 10.1099/00221287-116-1-133. [DOI] [PubMed] [Google Scholar]
- van Oss C. J. Phagocytosis as a surface phenomenon. Annu Rev Microbiol. 1978;32:19–39. doi: 10.1146/annurev.mi.32.100178.000315. [DOI] [PubMed] [Google Scholar]


