Abstract
Periodontitis is a bacterium-induced chronic inflammation that destroys tissues that attach teeth to jaw bone. Pathologically excessive matrix metalloproteinase 8 (MMP-8) is among the key players in periodontal destruction by initiating type I collagen degradation. We studied MMP-8 in Porphyromonas gingivalis-induced periodontitis by using MMP-8-deficient (MMP8−/−) and wild-type (WT) mice. Alveolar bone loss, inflammatory mediator expression, serum immunoglobulin, and lipoprotein responses were investigated to clarify the role of MMP-8 in periodontitis and systemic inflammatory responses. P. gingivalis infection induced accelerated site-specific alveolar bone loss in both MMP8−/− and WT mice relative to uninfected mice. The most extensive bone degradation took place in the P. gingivalis-infected MMP8−/− group. Surprisingly, MMP-8 significantly attenuated (P < 0.05) P. gingivalis-induced site-specific alveolar bone loss. Increased alveolar bone loss in P. gingivalis-infected MMP8−/− and WT mice was associated with increase in gingival neutrophil elastase production. Serum lipoprotein analysis demonstrated changes in the distribution of high-density lipoprotein (HDL) and very-low-density lipoprotein (VLDL) particles; unlike the WT mice, the MMP8−/− mice underwent a shift toward a smaller HDL/VLDL particle sizes. P. gingivalis infection increased the HDL/VLDL particle size in the MMP8−/− mice, which is an indicator of lipoprotein responses during systemic inflammation. Serum total lipopolysaccharide activity and the immunoglobulin G-class antibody level in response to P. gingivalis were significantly elevated in both infected mice groups. Thus, MMP-8 appears to act in a protective manner inhibiting the development of bacterium-induced periodontal tissue destruction, possibly through the processing anti-inflammatory cytokines and chemokines. Bacterium-induced periodontitis, especially in MMP8−/− mice, is associated with systemic inflammatory and lipoprotein changes that are likely involved in early atherosclerosis.
Periodontitis is a chronic infection-induced inflammatory disease that causes tooth loss and is considered a modifying factor in systemic health (1, 6). Several pathogens are associated with periodontitis. Porphyromonas gingivalis is one of the major pathogens in chronic periodontitis (59). P. gingivalis has a number of virulence factors such as capsule, fimbriae, lipopolysaccharide (LPS), and potent proteolytic enzymes, gingipains (23). These factors can induce an inflammatory cascade involving proinflammatory cytokines, reactive oxygen species, and matrix metalloproteinases (MMP), thus leading to the destruction of supportive soft and hard tissues around the teeth.
Pathologically excessive MMP plays a significant role in periodontal destruction (48, 50). MMP-8 (collagenase 2) is a collagenolytic enzyme that can initiate the digestion of type I collagen, the most dominant interstitial collagen type in the periodontal tissues. Collagen degradation is regarded as one of the key factors in the uncontrolled tissue destruction in periodontitis (48). In addition to periodontitis (52), elevated MMP-8 levels are attributable to many diseases such as bronchiectasis, asthma (40, 41), atherosclerosis (28, 55), inflammatory bowel disease (39), oral cysts (61), and oral cancer (33). MMP-8 is predominantly synthesized in the bone marrow and stored within the secondary granules of neutrophils (polymorphonuclear leukocytes) (58). Even though MMP-8 in tissues is primarily derived from degranulating neutrophils, de novo expression of MMP-8 has been identified in non-neutrophil-lineage cells such as gingival fibroblasts, odontoblasts, epithelial cells, plasma cells, and monocytes/macrophages (25, 50). Recent studies suggest that in addition to surrogate tissue destructive properties (48, 50), MMP-8 can exert anti-inflammatory effects in the host defense by processing anti-inflammatory cytokines and chemokines (37). MMP-8 can also regulate apoptotic and immune responses and play a protective role in lung inflammation (18), cancer progression (2, 20, 27), and wound healing (19).
Although chronic periodontitis is localized to the tissues surrounding the teeth, it is linked to serious systemic conditions such as cardiovascular disease (4, 13), stroke (62), diabetes (10), and complications during pregnancy (12). Increased bacterial burden in inflamed periodontal pockets leads to the presence of oral bacteria and their components, such as LPS, in the systemic circulation (15, 22). Periodontitis is also accompanied by the systemic antibody response against periodontal pathogens and proatherogenic changes in lipoprotein metabolism (42-45).
Knockout mouse models are useful in studies of the roles of specific MMPs in physiological and pathological situations. We evaluated the role of MMP-8 in P. gingivalis-induced periodontitis by comparing alveolar bone destruction between MMP-8-deficient (MMP8−/−) and wild-type (WT) mice. Furthermore, serum antibody level and lipoprotein determinations were performed to clarify the systemic effects of MMP-8 during the inflammatory process of periodontitis.
MATERIALS AND METHODS
Animals.
Experimental groups comprised 14-week-old male mice bred and were maintained in the experimental animal facilities of the University of Oulu, Oulu, Finland. MMP8−/− mice of a mixed C57BL/6J/129 background (2) were used, and WT littermates served as controls (27). Prior to the animal experiments, statistical power analysis was performed to determine an appropriate sample size to achieve adequate power. The MMP8−/− mice (2) were kindly provided by Carlos Lopéz-Otín of Oviedo, Spain. All mice were maintained in a barrier facility (27), and the experiments were conducted in accordance with the guidelines of the Committee of Animal Experimentation of the University of Oulu, Oulu, Finland.
Induction of experimental periodontitis.
The mouse groups created for the experiments were WT (n = 10) infected (experimental) and WT uninfected (control, n = 8), MMP8−/− infected (experimental, n = 12) and MMP8−/− uninfected (control, n = 10) (total n = 40). A pilot experiment (n = 17) was carried out before the present study.
Experimental periodontitis was induced as described previously (11). The mice received 20 mg of kanamycin and 20 mg of ampicillin in 1 ml of sterile water twice daily for 3 days to eliminate the native flora and to promote the subsequent colonization of P. gingivalis in the oral cavity. The antibiotics were allowed to clear from the system for 4 days. The oral cavity of the mice were inoculated with P. gingivalis to induce marginal periodontitis (36, 64). A clinical strain ATCC 33277 (American Type Culture Collection) of P. gingivalis was revived from a frozen (−70°C) stock. The bacterial cells were cultured on brucella agar plates and incubated in anaerobic jars filled with mixed gas (5% CO2, 10% H2, 85% N2) at 37°C for 3 days. After the purity of the cultures was checked with a dissecting microscope, single bacterial colonies were transferred to new brucella agar plates and incubated anaerobically at 37°C for 2 days. Bacterial cells were harvested to sterile 3% (a carboxymethyl cellulose medium used to facilitate the retention of the bacterial suspension in the oral cavity), and the density of the culture was determined spectrophotometrically at 492 nm to achieve a concentration of ∼2 × 109 CFU/ml. To ensure the colonization of P. gingivalis in the oral cavity, 0.1 to 0.2 ml of 3% carboxymethyl cellulose suspension containing viable P. gingivalis cells was swabbed into the mouth twice daily for 3 days. The control mice receiving saline served as negative controls. At 30 days after the last inoculation, blood samples were collected under CO2 anesthesia by cardiac puncture from each mouse, and the serum was separated, frozen in liquid nitrogen, and stored at −70°C until analyses for serum lipid and inflammatory markers. The mice were then killed by cervical dislocalization. The skulls were dissected, hemisected, and collected for alveolar bone loss measurement and immunohistochemical analysis as described below.
Analysis of alveolar bone loss.
After collection, hemisected skulls were fixed in 10% formalin, decalcified in 12.5% EDTA, and embedded in paraffin. Serial sections best representing the longitudinal cutting of the first and second molars from the maxillae and mandible were selected and stained with routine hematoxylin and eosin (H&E) for the histological analysis of alveolar bone loss. The depth of the bony pocket was measured as the vertical distance from the cemento-enamel junction to the alveolar ridge by using AnalySIS-program under a Olympus BX61 light microscope (14, 31). Each site was measured three times at random. Double-blind histological analysis was performed by a single evaluator.
Immunohistochemistry.
Immunohistological stainings were performed using standard procedures and antibodies as described previously (38). Paraffin-embedded tissue specimens were deparaffinized, and immunostainings for MMP-9 (R&D Systems, Minneapolis, MN), MMP-13 (Chemicon, Temecula, CA), MMP-25 (Sigma-Aldrich Co., St. Louis, MO), COX-1 (Santa Cruz Biotechnology, Santa Cruz, CA), COX-2 (Santa Cruz Biotechnology), myeloperoxidase (MPO) (HyCult Biotechnology, b.v., Uden, The Netherlands), laminin-332 (32), neutrophil elastase (NE; Calbiochem-Novabiochem, San Diego, CA), interleukin-1β (IL-1β; R&D Systems), and tumor necrosis factor alpha (TNF-α; R&D Systems) were performed as described previously (30). The polyclonal rabbit anti-laminin-332 antibody was kindly provided by Sirpa Salo of the University of Oulu, Oulu, Finland: the specificity of the antibody has been previously verified (32). The stainings were performed with polyclonal Vectastain Elite rabbit or goat ABC kits (Vector Laboratories, Burlingame, CA). The sections were pretreated with 0.4% pepsin, and endogenous peroxidase activity was blocked by incubation in 0.6% H2O2 in methanol. Samples were blocked with goat or horse normal serum in 2% bovine serum albumin and incubated with the following polyclonal antibodies: goat MMP-9 (1:50), goat MMP-13 (1:100), rabbit MMP-25 (1:700), goat COX-1 (1:50), goat COX-2 (1:50), rabbit MPO (1:200), rabbit laminin-332 (1:200), rabbit NE (1:500), goat IL-1β (1:500), and goat TNF-α (1:500) overnight. The control sections were incubated with nonimmune rabbit or goat serum. Subsequently, samples were incubated with biotinylated anti-rabbit or anti-goat secondary antibody and thereafter with avidin-biotin enzyme complex. Sections were stained with 3-amino-9-ethylcarbazole as a chromogen, counterstained with Mayer's hematoxylin (Merck KGaA, Darmstadt, Germany), mounted in Dako's glycergel (Dako Corp., Carpinteria, CA), and evaluated by using the AnalySIS-program under an Olympus BX61 light microscope. Any intensity if present in immunohistochemical stainings was semiquantified and graded as 0, no staining; 1, very mild staining; 2, mild staining; 3, moderate staining; and 4, abundant positive staining (40).
Serum determinations.
Serum samples were analyzed for concentrations of total cholesterol, triglycerides (Roche, Basel, Switzerland), apolipoprotein A-I (apoA-I) (57), and LPS (LAL chromogenic endpoint assay; HyCult Biotechnology). Serum immunoglobulin A (IgA)- and IgG-class antibody levels against P. gingivalis were determined by using multiserotype enzyme-linked immunosorbent assay. Formalin-killed whole cells of three serotypes of P. gingivalis served as antigens (42). Two dilutions (1:100 and 1:200) of each serum (stored at −70°C) in duplicate were used for the measurements, and the results consisting of mean absorbances were calculated.
Lipoprotein profiles.
To obtain lipoprotein profiles, serum samples from each group were pooled (8 to 12 mice/pool). Aliquots of 200 μl were applied to a Superose 6HR size exclusion chromatography (Pharmacia Biotech, Uppsala, Sweden) column previously equilibrated with phosphate-buffered saline (PBS) at a flow rate of 0.5 ml/min in PBS, and 0.5-ml fractions were collected. In order to make a separation between the lipoprotein subclasses, the fractions were analyzed for cholesterol, triglyceride, and apoA-I concentrations.
Statistical analysis.
Using analysis of variance, we compared the alveolar bone loss and serum lipoprotein profiles between the four groups studied. In the case of significant differences, we used Duncan's test to perform post-hoc multiple comparisons. In immunohistochemical analysis, we performed post-hoc multiple group comparisons using the Mann-Whitney U test.
RESULTS
Alveolar bone loss.
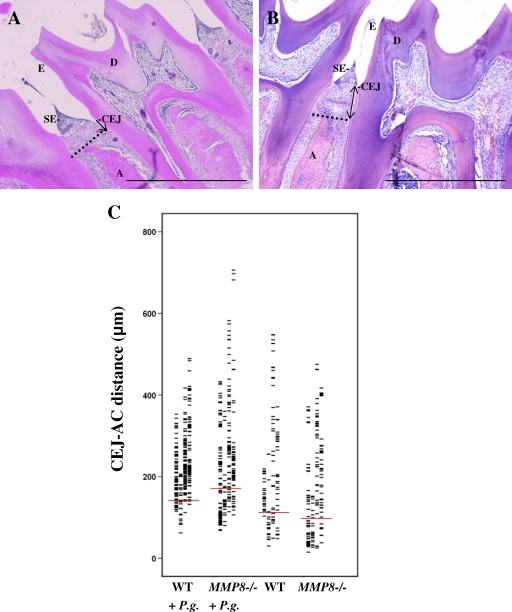
Quantitative analysis of site-specific alveolar bone loss revealed that both P. gingivalis-infected mouse groups exhibited more severe bone loss than did the noninfected control groups (P < 0.05) (Fig. 1). No statistical difference in alveolar bone loss was found between the two uninfected groups. In the P. gingivalis-infected MMP8−/− group, the bone loss was enhanced relative to the P. gingivalis-infected WT group. When we compared mandibular sites, the difference was statistically significant (P < 0.05) (Fig. 1C). Bone loss varied considerably between different periodontal sites. Maximally, the bone loss in the WT + P. gingivalis-infected group was 489 μm, and the bone loss in the MMP8−/− + P. gingivalis-infected group was 706 μm. Two separate sets of experiments (n = 17 and n = 40) on the development of site-specific alveolar bone loss yielded similar results.
FIG. 1.
Alveolar bone loss assessment from the cemento-enamel junction (CEJ) to the alveolar ridge. (A) Schematic diagram showing measurement of the CEJ-A distance on an H&E-stained section of healthy WT mouse periodontium. The dotted line indicates the top of the alveolar crest, and the CEJ-A distance is the vertical distance from the CEJ to the alveolar crest (single-headed arrow). A, alveolar bone crest; CEJ, cemento-enamel junction; D, dentin; E, enamel; SE, epithelium. Scale bar, 500 μm. (B) Schematic diagram showing measurement of the CEJ-AC distance on an H&E-stained section of P. gingivalis-infected MMP8−/− mouse periodontium. The dotted line shows the top of the alveolar crest, and the CEJ-A distance is the vertical distance from the CEJ to the alveolar crest (double-headed arrow). A, alveolar bone crest; CEJ, cemento-enamel junction; D, dentin; E, enamel; SE, epithelium. Scale bar, 500 μm. (C) Diagram showing measurements of the distance between the cemento-enamel junction and the alveolar bone crest. The red lines show the means of four separate measurements.
Immunohistochemistry.
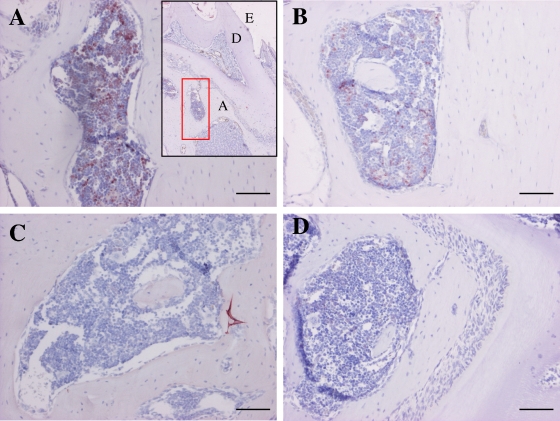
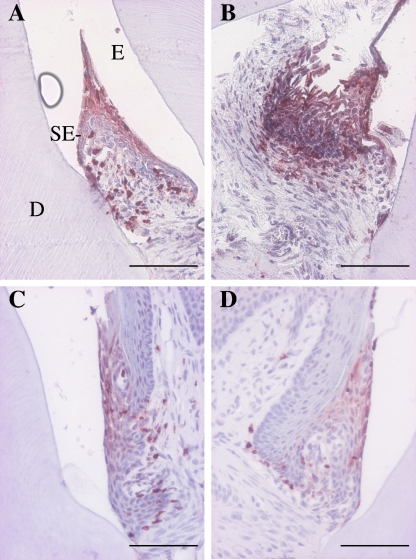
The main findings of the immunohistochemical analyses are shown in Table 1. The NE levels were significantly (P < 0.05) higher in both infected groups than in the uninfected control groups. (Table 1 and Fig. 2). The MMP8−/− + P. gingivalis group exhibited significantly (P < 0.05) higher MMP-9 expression than did the MMP8−/− uninfected group. MMP-9 expression was highest in the MMP8−/− + P. gingivalis group,but showed no difference from that in the WT + P. gingivalis group (Table 1 and Fig. 3). The mean COX-1 expression was highest in the MMP8−/− + P. gingivalis group, but the difference from the WT + P. gingivalis group was not significant (Table 1). TNF-α expression was not only highest in the MMP8−/− + P. gingivalis mice but was also significantly different from that of the MMP8−/− uninfected group (P < 0.05) (Table 1). Ln-332 expression was highest in the WT + P. gingivalis mice. It was also significantly different (P < 0.05) from that of the WT control group (Table 1).
TABLE 1.
Semiquantitative analysis of the immunohistological stainings
| Proteina | Mean protein level ± SDb
|
|||
|---|---|---|---|---|
| WT + P. gingivalis (n = 10) | MMP8−/− + P. gingivalis (n = 12) | WT control (n = 8) | MMP8−/− control (n = 10) | |
| NE | 2.60 ± 0.52* | 2.00 ± 0.77† | 0.63 ± 0.74 | 0.80 ± 0.63 |
| MPO | 2.90 ± 0.32 | 2.55 ± 0.69 | 2.50 ± 0.76 | 2.40 ± 0.52 |
| MMP-9 | 3.00 ± 0.67 | 3.18 ± 0.60† | 2.50 ± 0.53 | 2.30 ± 0.48 |
| MMP-13 | 0.50 ± 0.53 | 0.82 ± 0.40 | 0.88 ± 0.64 | 0.90 ± 0.32 |
| MMP-25 | 0.30 ± 0.48 | 0.00 ± 0.00 | 0.13 ± 0.35 | 0.00 ± 0.00 |
| TNF-α | 1.90 ± 0.57 | 2.27 ± 0.47† | 2.00 ± 0.00 | 1.60 ± 0.70 |
| IL-1β | 0.50 ± 0.53 | 0.64 ± 0.51 | 0.5 ± 0.54 | 0.5 ± 0.53 |
| COX-1 | 1.40 ± 0.52 | 1.64 ± 0.81 | 0.75 ± 0.71 | 1.50 ± 0.71 |
| COX-2 | 1.50 ± 0.85 | 1.73 ± 0.90 | 0.75 ± 0.71 | 1.20 ± 0.63 |
| Ln-332 | 3.30 ± 0.48* | 3.00 ± 0.63 | 2.50 ± 0.53 | 2.80 ± 0.63 |
Proteins with significant differences between the groups are indicated in boldface.
The protein levels were semiquantified as follows: 0, none; 1, very mild; 2, mild; 3, moderate; 4, abundant. *, Significantly different than WT control group (P < 0.05); †, significantly different than MMP8−/− control group (P < 0.05).
FIG. 2.
Expression of NE in P. gingivalis-infected mouse gingival tissue. Mouse gingival tissues were immunohistochemically stained with polyclonal anti-NE antibody as described in Materials and Methods. (A) WT + P. gingivalis group. The insert with the red square shows the area in the figure. A, alveolar bone; D, dentin; E, enamel. (B) MMP8−/− + P. gingivalis group. (C) WT control group. (D) MMP8−/− control group. Scale bars, 100 μm.
FIG. 3.
Expression of MMP-9 in P. gingivalis-infected mouse gingival tissue. Mouse gingival tissues were immunohistochemically stained with polyclonal anti-MMP-9 antibody as described in Materials and Methods. (A) WT + P. gingivalis group; (B) MMP8−/− + P. gingivalis group; (C) WT control group; (D) MMP8−/− control group. D, dentin; E, enamel; SE, sulcular epithelium. Scale bars, 200 μm.
Serum immunoglobulin and lipoprotein profiles.
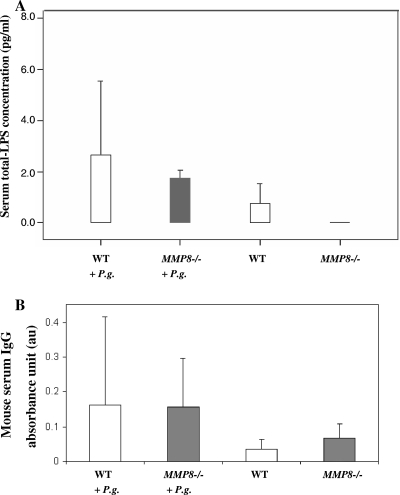
Serum LPS concentrations were significantly (P < 0.05) higher in the P. gingivalis-infected MMP8−/− mice than in the uninfected MMP8−/− mice. The corresponding tendency between infected and uninfected WT group was, however, not significant (Fig. 4A). Serum P. gingivalis IgG levels were higher in both WT and MMP8−/− bacterium-treated mice than in the controls (Fig. 4B); the difference between the infected and uninfected WT groups was significant (P = 0.05), whereas P. gingivalis IgA-class antibodies were undetectable (data not shown).
FIG. 4.
Infection markers in serum. Mouse serum total-LPS activity (A) and IgG-class antibody levels to P. gingivalis (B) were analyzed as described in Materials and Methods from WT mice without (n = 10) or with (n = 8) P. gingivalis infection and from MMP8−/− mice with (n = 12) or without (n = 10) P. gingivalis infection. Statistical comparisons were carried out between the infected mice and their corresponding controls (*, P < 0.05).
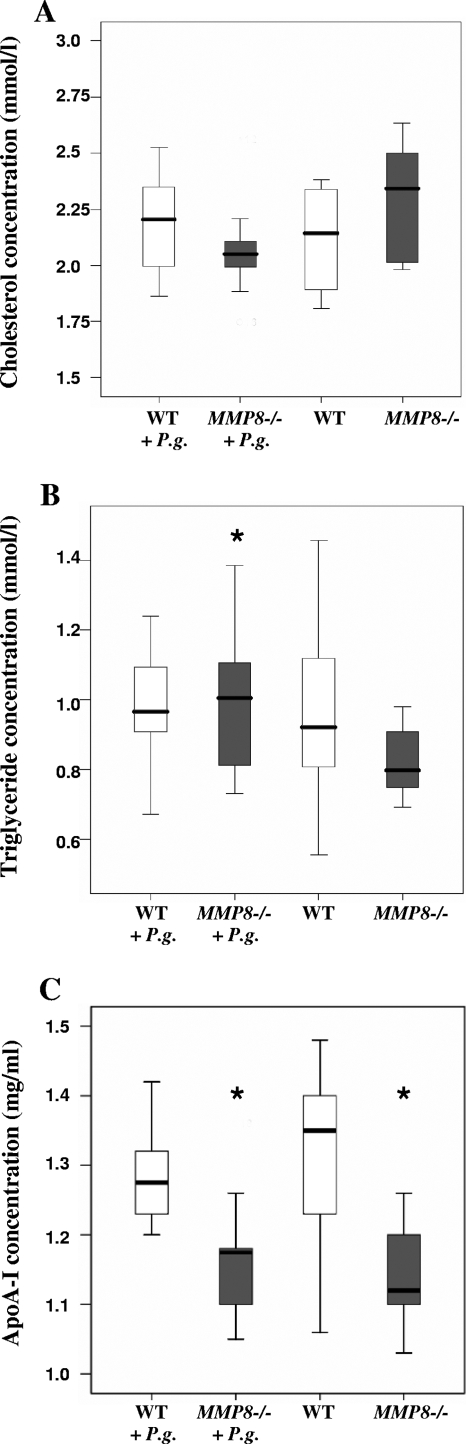
Serum lipid and lipoprotein profiles revealed that the total cholesterol concentration was clearly lower in the P. gingivalis-infected mice than in the uninfected MMP8−/− mice. The serum triglyceride concentration was higher among the infected MMP8−/− mice than among the control group (P < 0.05). apoA-I levels were lower in both MMP8−/− groups than in both WT groups (P < 0.05) (Fig. 5).
FIG. 5.
Serum lipid analysis. Mouse serum cholesterol (A), triglyceride (B), and apoA-I (C) concentrations were analyzed from WT mice without (n = 10) or with (n = 8) P. gingivalis infection and from MMP8−/− mice with (n = 12) or without (n = 10) P. gingivalis infection. The statistical comparisons were carried out between the infected animals and their corresponding controls (*, P < 0.05).
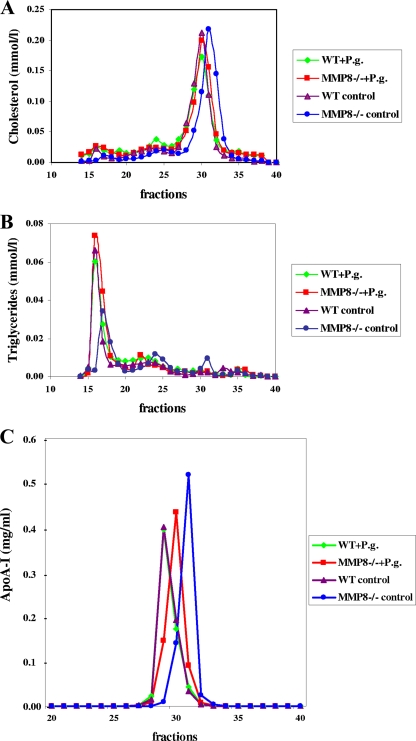
Compared to the infected MMP8−/− group and both WT groups, the cholesterol elution peak in uninfected MMP8−/− mice shifted toward a smaller high-density lipoprotein (HDL) particle size. In a similar fashion, apoA-I, a major apolipoprotein of HDL, profile in the uninfected MMP8−/− group shifted toward a smaller particle size in the HDL fraction. An obvious rearrangement in the distribution of HDL subclasses was demonstrated in MMP8−/− mice. Among the WT mice, P. gingivalis infection showed no influence on the elution position of HDL, thus suggesting no significant changes in HDL particle size. Moreover, the triglyceride elution peak shifted toward a smaller very-low-density lipoprotein (VLDL) particle size in uninfected MMP8−/− mice (Fig. 6). No significant changes were observed in the elution position of LDL particles (fractions 21 to 25) between the mouse groups.
FIG. 6.
Lipoprotein profiles in serum. Sera from WT mice without (n = 10) or with (n = 8) P. gingivalis infection and MMP8−/− mice with (n = 12) or without (n = 10) P. gingivalis infection were pooled within the groups and applied to a Superose 6HR gel filtration column in PBS at a flow rate of 0.5 ml/min. We collected 0.5-ml fractions, from which we determined cholesterol (A), triglyceride (B), and apoA-I (C) concentrations.
DISCUSSION
This study confirmed that oral inoculation with P. gingivalis in mice leads to alveolar bone loss and is a useful model for studying periodontitis in vivo. The results of our study are in line with and further extend those of previous studies. Lalla et al. (29) reported that oral inoculation with P. gingivalis in apo-E-deficient mice caused more extensive alveolar bone loss than in uninfected controls. In addition, oral inoculation with another periodontal pathogen, Actinobacillus actinomycetemcomitans, leads to the formation of periodontitis in mice (16). Previous studies have found MMP-8 as one of the key mediators of tissue destruction in periodontal inflammation (49, 50). We investigated the role of MMP-8 in tissue destruction by using mutant mice deficient in MMP-8 (2, 27). The present study demonstrates that oral infection of MMP8−/− mice with P. gingivalis results in more severe alveolar bone loss than in WT mice. The variation of the bone loss was big in all animal groups. Therefore, the final conclusion of the effect of the role of MMP-8 in periodontitis cannot be made. The results, however, suggest that MMP-8 plays, at least in part, a protective role in alveolar bone loss during periodontal infection.
Our findings are in line with the studies on the role of MMP-8 in lung inflammation, wound healing, and cancer development (2, 18, 27, 37). These studies performed with gene knockout animals have demonstrated that MMP-8 exerts anti-inflammatory properties on experimental LPS- and allergen-induced lung inflammation (18, 37). Balbin et al. (2) reported that the absence of MMP-8 increased the incidence of skin tumors in MMP8−/− male and ovariectomized female mice compared to WT mice. Moreover, MMP-8-deficient female mice developed tongue squamous cell carcinomas at a significantly higher rate than did WT mice (27). A significant delay in wound closure in MMP8−/− mice and an altered inflammatory response have been observed (19). Overall, these findings, together with our present study, indicate that MMP-8 may play a protective role in inflammation and cancer development and most probably contributes to the resolution of inflammation by processing certain anti-inflammatory cytokines and chemokines (18, 37). In our semiquantitative immunohistochemical analysis we observed a tendency for higher IL-1β, TNF-α, COX-1, and COX-2 expression in inflamed periodontal tissue of MMP8−/− mice than in the WT mice, but the difference was not significant.
A study of wound healing in MMP-8-deficient mice recently reported a significant increase in MMP-9 expression (19). Our study also demonstrated increased MMP-9 expression. This result suggests that the enhanced production of MMP-9 in MMP8−/− mice could indicate a compensatory MMP upregulation. NE expression was significantly elevated in infected mice in inflammatory cells within the gingival connective tissue surrounding the alveolar bone. No significant differences were found, however, between the infected WT and MMP8−/− groups. Elevated TNF-α mRNA expression has been reported in advanced periodontal lesions among A. actinomycetemcomitans-infected mice (16). These phenomena could be attributed to increased infiltration and activation of neutrophils in inflamed tissue in MMP8−/− mice (19). The association of NE, TNF-α, MMP-9, COX-1, and COX-2 in P. gingivalis-induced periodontitis lesions characterized by inflammatory cell infiltration and alveolar bone loss indicates similar pathogenic aspects of periodontitis in our mouse model compared to periodontitis in humans and rats (5, 7, 11, 34, 47, 63).
Recent studies have demonstrated that oral infection with a periodontal pathogen, such as A. actinomycetemcomitans, can induce proatherogenic changes in apolipoprotein-E-deficient mice (56). The novel finding that inoculation with P. gingivalis bacteria resulted in somewhat more severe alveolar bone loss in MMP8−/− mice than in WT mice led us to investigate systemic changes in the MMP-8-deficient mice. We found that P. gingivalis infection was accompanied with changes in inflammatory or infection-related parameters (IgG and LPS) and in lipid metabolism (cholesterol, triglycerides, and apoA-I lipoprotein profile). The serum total LPS activity and IgG-class antibody concentrations to the pathogen were significantly higher among both infected mouse groups than among the uninfected controls. This further confirms that systemic exposure of the host to the pathogen and corresponding host responses accompany oral infections (29, 35, 42, 45, 46). Our results therefore are in line with the concept that chronic periodontitis should be considered a risk factor for the progression of cardiovascular disease.
Infection with P. gingivalis decreased the serum total cholesterol levels in MMP8−/− mice but increased the total triglyceride concentrations. This result suggests that MMP-8 deficiency makes the animal more sensitive to responses in serum triglyceride and cholesterol pools.
apoA-I plays a key role in the formation, remodeling, and tissue uptake of HDL. Serum apoA-I concentrations were lower in both infected and uninfected MMP8−/− mice than in WT mice groups, which suggests that MMP-8 deficiency has a regulatory influence on apoA-I levels. apoA-I and poorly lipidated apoA-I also contributes to the reverse cholesterol transport process by interacting with ATP-binding cassette transporter A 1 (ABCA1) in macrophage foam cells and facilitates the efflux of cholesterol (24, 51). ABCA1 is also important in the liver and the intestine since hepatocytes and enterocytes secrete nascent apoA-I HDL into circulation via this transporter protein (9, 53). Certain MMPs are involved in the modification of ABCA1 (54). Our results suggest that MMP-8 deficiency could lead to the modification of ABCA1 perhaps via increased protease function (calpain, MMP-9, etc.), which leads to disturbed and attenuated secretion of HDL and decreased levels in serum.
As apparent in the cholesterol and apoA-I profiles, the HDL population shifted toward a smaller particle size in uninfected MMP8−/− mice. MMP-8 deficiency appears to affect the formation of small, possibly lipid-poor HDL particles, which are generally catabolized more rapidly from circulation via kidney function than are large particles (8, 21). This could explain the reduced HDL cholesterol concentrations observed in the MMP8−/− mice. Infection with P. gingivalis caused the formation of larger-sized HDL particles. In addition, the triglyceride profile suggested that the VLDL population shifted toward larger particle sizes in MMP8−/− mice after infection, although P. gingivalis infection failed to affect HDL or VLDL particle size when MMP-8 was present. An obvious change in the distribution of HDL and VLDL particles occurred in MMP8−/− mice. P. gingivalis infection increased the HDL/VLDL particle size among MMP8−/− mice, thus indicating that lipoprotein responses during systemic inflammation. Several factors, e.g., infections, may affect both VLDL and HDL particle size. For instance, elevated phospholipid transfer protein activity is known to generate large HDL particles (3, 43). Furthermore, plasma triglyceride concentrations increase with increased VLDL secretion as a result of adipose tissue lipolysis, increased de novo hepatic fatty acid synthesis, and the suppression of fatty acid oxidation. In a severe infection, VLDL clearance decreases secondary to decreased lipoprotein lipase and apolipoprotein E in VLDL. All of this could be implicated as the increased size of triglyceride-enriched VLDL particles (26).
The results of the present study demonstrate that the presence of MMP-8 causes at least a partially defensive local inflammatory response against the P. gingivalis-induced development of periodontal bone destruction. In humans, MMP-8 is the predominant collagenase present in periodontitis-affected gingival tissue, gingival crevicular fluid, and saliva (48), and the severity of periodontal disease is positively correlated with MMP-8 levels (50). The present study, together with others (18-20, 58, 60), points to a conclusion that physiologic but not pathologically elevated MMP-8 levels exert protective and anti-inflammatory functions possibly by processing growth factors and protective endogenous proteinase inhibitors (58). Our data further suggest that MMP-8 deficiency may influence leukocyte accumulation in the gingiva by regulating increased cell migration or, alternatively, by reduced resolution of inflammation after bacterial challenge. We can speculate, in respect to treatment of periodontitis, that a complete inhibition of MMP-8 may not be a desirable goal, but instead a reduction from pathologically excessive MMP-8 close to the physiological levels would be optimal (17, 49, 60). To verify this concept and to find out the connection of MMP-8 to lipid metabolism will require more detailed studies.
Acknowledgments
The MMP8−/− mice were kindly provided by Carlos Lopéz-Otín, Universidad de Oviedo, Oviedo, Spain. The antibody for laminin-322 was kindly provided by Sirpa Salo, University of Oulu, Oulu, Finland.
This study was funded by grants from the Academy of Finland (grant 118391 to P.J.P., grant 210722 to T.S., and grant 108717 to E.P.), the Helsinki University Central Hospital EVO (T.S.), the K. Albin Johanssons Foundation, the Finnish Dental Society Apollonia, the Finnish Female Dentists Association, the Biomedicum Helsinki Foundation (H.K.), and the Finnish Foundation for Cardiovascular Research (M.J.).
We have no financial conflicts of interest.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 24 November 2008.
REFERENCES
- 1.Assuma, R., T. Oates, D. Cochran, S. Amar, and D. T. Graves. 1998. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J. Immunol. 160403-409. [PubMed] [Google Scholar]
- 2.Balbín, M., A. Fueyo, A. M. Tester, A. M. Pendás, A. S. Pitiot, A. Astudillo, C. M. Overall, S. D. Shapiro, and C. Lopéz-Otín. 2003. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat. Genet. 35252-257. [DOI] [PubMed] [Google Scholar]
- 3.Barlage, S., D. Fröhlich, A. Böttcher, M. Jauhiainen, H. P. Müller, F. Noetzel, G. Rothe, C. Schütt, R. P. Linke, K. J. Lackner, C. Ehnholm, and G. Scmitz. 2001. ApoE-containing high density lipoproteins and phospholipid transfer protein activity increase in patients with a systemic inflammatory response. J. Lipid Res. 42281-290. [PubMed] [Google Scholar]
- 4.Beck, J., R. Garcia, G. Heiss, P. S. Vokonas, and S. Offenbacher. 1996. Periodontal disease and cardiovascular disease. J. Periodontol. 671123-1137. [DOI] [PubMed] [Google Scholar]
- 5.Bezerra, M. M., V. de Lima, V. B. M. Alencar, I. B. Vieira, G. A. C. Brito, R. A. Ribeiro, and F. A. C. Rocha. 2000. Selective cyclooxygenase-2 inhibition prevents alveolar bone loss in experimental periodontitis in rats. J. Periodontol. 711009-1014. [DOI] [PubMed] [Google Scholar]
- 6.Birkedal-Hansen, H. 1993. Role of cytokines and inflammatory mediators in tissue destruction. J. Periodontal Res. 28500-510. [DOI] [PubMed] [Google Scholar]
- 7.Bostanci, N., R. Allaker, U. Johansson, M. Rangarajan, M. A. Curtis, F. J. Hughes, and I. J. McKay. 2007. Interleukin-1α stimulation in monocytes by periodontal bacteria: antagonistic effects of Porphyromonas gingivalis. Oral Microbiol. Immunol. 2252-60. [DOI] [PubMed] [Google Scholar]
- 8.Brousseau, M. E., G. P. Eberhart, J. Dupuis, B. F. Asztalos, A. L. Goldkamp, E. J. Schaefer, and M. W. Freeman. 2000. Cellular cholesterol efflux in heterozygotes for Tangier disease is markedly reduced and correlates with high density lipoprotein cholesterol concentration and particle size. J. Lipid Res. 411125-1135. [PubMed] [Google Scholar]
- 9.Brunham, L. R., J. K. Kruit, J. Iqbal, C. Fievet, J. M. Timmins, T. D. Pape, B. A. Coburn, N. Bissada, B. Staels, A. K. Groen, M. M. Hussain, J. S. Parks, F. Kuipers, and M. R. Hayden. 2006. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Investig. 1161052-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campus, G., A. Salem, S. Uzzau, E. Baldoni, and G. Tonolo. 2005. Diabetes and periodontal disease: a case control study. J. Periodontol. 76418-425. [DOI] [PubMed] [Google Scholar]
- 11.Chang, K. M., N. S. Ramamurthy, T. F. Mc Namara, R. T. Evans, B. Klausen, P. A. Murray, and L. M. Golub. 1994. Tetracyclines inhibit Porphyromonas gingivalis-induced alveolar bone loss in rats by a non-antimicrobial mechanism. J. Periodontal Res. 29242-249. [DOI] [PubMed] [Google Scholar]
- 12.Dasanayake, A. P., D. Boyd, P. N. Madianos, S. Offenbacher, and E. Hills. 2001. The association between Porphyromonas gingivalis-specific maternal serum IgG and low birth weight. J. Periodontol. 721491-1497. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich, T., M. Jimenez, E. A. Krall Kaye, P. S. Vokonas, and R. I. Garcia. 2008. Age-dependent associations between chronic periodontitis/edentulism and risk of coronary heart disease. Circulation 1171668-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumitrescu, A. L., S. Abd El-Aleem, B. Morales-Aza, and L. F. Donaldson. 2004. A model of periodontitis in the rat: effect of lipopolysaccharide on bone resorption, osteoclast activity, and local peptidergic innervation. J. Clin. Periodontol. 31596-603. [DOI] [PubMed] [Google Scholar]
- 15.Ebersole, J. L. 1990. Systemic humoral immune responses in periodontal disease. Crit. Rev. Oral Biol. 1283-331. [DOI] [PubMed] [Google Scholar]
- 16.Garlet, G. P., M. J. Avila-Campos, C. M. Milanezi, B. R. Ferreira, and J. S. Silva. 2005. Actinobacillus actinomycetemcomitans-induced periodontal disease in mice: patterns of cytokine, chemokine, and chemokine receptor expression and leukocyte migration. Microbes Infect. 7738-747. [DOI] [PubMed] [Google Scholar]
- 17.Golub, L. M., H. M. Lee, J. A. Stoner, T. Sorsa, R. A. Reinhardt, M. S. Wolff, M. E. Ryan, P. V. Nummikoski, and J. B. Payne. 2008. Subantimicrobial-dose doxycycline modulates gingival crevicular fluid biomarkers of periodontitis in postmenopausal osteopenic women. J. Periodontol. 791409-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gueders, M. M., M. Balbin, N. Rocks, J. M. Foidart, P. Gosset, R. Louis, S. Shapiro, C. Lopéz-Otín, A. Noël, and D. D. Cataldo. 2005. Matrix metalloproteinase-8 deficiency promotes granulocytic allergen-induced airway inflammation. J. Immunol. 1752589-2597. [DOI] [PubMed] [Google Scholar]
- 19.Gutiérrez-Fernandéz, A., M. Inada, M. Balbín, A. Fuyeo, A. S. Pitiot, A. Astudillo, K. Hirose, M. Hirata, S. D. Shapiro, A. Noël, Z. Werb, S. M. Krane, C. Lopéz-Otín, and X. S. Puente. 2007. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8). FASEB J. 212580-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutiérrez-Fernandés, A., A. Fuyeo, A. R. Folgueras, C. Garabaya, C. J. Pennington, S. Pilgrim, D. R. Edwards, D. L. Holliday, J. L. Jones, P. N. Span, F. C. Sweep, X. S. Puente, and C. López-Otín. 2008. Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer Res. 682755-2763. [DOI] [PubMed] [Google Scholar]
- 21.Hammad, S. M., S. Stefansson, W. O. Twal, C. J. Drake, P. Fleming, A. Remaley, H. B. Brewer, and W. S. Argraves. 1999. Cubilin, the endocytic receptor for intrinsic factor-vitamin B12 complex, mediates high-density lipoprotein holoparticle endocytosis. Proc. Natl. Acad. Sci. USA 9610158-10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernichel-Gorbach, E., K. S. Kornman, S. C. Holt, F. Nichols, H. Meador, J. T. Kung, and C. A. Thomas. 1994. Host responses in patients with generalized refractory periodontitis. J. Periodontol. 658-16. [DOI] [PubMed] [Google Scholar]
- 23.Holt, S. C, L. Kesavalu, S. Walker, and C. A. Genco. 1999. Virulence factors of Porphyromonas gingivalis. Periodontol. 2000 20168-238. [DOI] [PubMed] [Google Scholar]
- 24.Jessup, W., I. C. Gelissen, K. Gaus, and L. Kritharides. 2006. Roles of ATP binding cassette transporters A1 and G1, scavenger receptor BI and membrane lipid domains in cholesterol export from macrophages. Curr. Opin. Lipidol. 17247-257. [DOI] [PubMed] [Google Scholar]
- 25.Kiili, M., S. W. Cox, H. W. Chen, J. Wahlgren, P. Maisi, B. M. Eley, T. Salo, and T. Sorsa. 2002. Collagenase-2 (MMP-8) and collagenase-3 (MMP-13) in adult periodontitis: molecular forms and levels in gingival crevicular fluid and immunolocalization in gingival tissue. J. Clin. Periodontol. 29224-232. [DOI] [PubMed] [Google Scholar]
- 26.Khovidhunkit, W., M.-S. Kim, R. A. Memon, J. K. Shigenaga, A. H. Moser, K. R. Feingold, and C. Grunfeld. 2004. Effects of infection inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 451169-1196. [DOI] [PubMed] [Google Scholar]
- 27.Korpi, J. T., V. Kervinen, H. Mäklin, A. Väänänen, M. Lahtinen, E. Läärä, A. Ristimäki, G. Thomas, M. Ylipalosaari, P. Åström, C. Lopéz-Otín, T. Sorsa, S. Kantola, E. Pirilä, and T. Salo. 2008. Collagenase-2 (matrix metalloproteinase-8) plays a protective role in tongue cancer. Br. J. Cancer 98766-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krupinski, J., M. M. Turu, M. A. Font, N. Ahmed, M. Sullivan, A. Luque, F. Rubio, L. Badimon, and M. Slevin. 2007. Increased tissue factor, MMP-8, and D-dimer expression in diabetic patients with unstable advanced carotid atherosclerosis. Vasc. Health Risk Manag. 3405-412. [PMC free article] [PubMed] [Google Scholar]
- 29.Lalla, E., I. B. Lamster, M. A. Hofmann, L. Bucciarelli, A. P. Jerud, S. Tucker, Y. Lu, P. N. Papapanou, and A. M. Schmidt. 2003. Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 231405-1411. [DOI] [PubMed] [Google Scholar]
- 30.Lindy, O., Y. T. Konttinen, T. Sorsa, Y. Ding, S. Santavirta, A. Ceponis, and C. Lopéz-Otín. 1997. Matrix metalloproteinase-13 (collagenase-3) in human rheumatoid synovium. Arthritis. Rheum. 401391-1999. [DOI] [PubMed] [Google Scholar]
- 31.Luan, Q., T. Desta, L. Chehab, V. J. Sanders, J. Plattner, and D. T. Graves. 2008. Inhibition of experimental periodontitis by a topical boron-based antimicrobial. J. Dent. Res. 87148-152. [DOI] [PubMed] [Google Scholar]
- 32.Määttä, M., Y. Soini, P. Pääkkö, S. Salo, K. Tryggvason, and H. Autio-Harmainen. 1999. Expression of the laminin γ2 chain in different histological types of lung carcinoma. A study by immunohistochemistry and in situ hybridisation. J. Pathol. 188361-368. [DOI] [PubMed] [Google Scholar]
- 33.Moilanen, M., E. Pirilä, R. Grenman, T. Sorsa, and T. Salo. 2002. Expression and regulation of collagenase-2 (MMP-8) in head and neck squamous cell carcinomas. J. Pathol. 19772-81. [DOI] [PubMed] [Google Scholar]
- 34.Morton, R. S., and A. I. Dongari-Bagtzoglou. 2001. Cyclooxygenase-2 is upregulated in inflamed gingival tissues. J. Periodontol. 72461-469. [DOI] [PubMed] [Google Scholar]
- 35.Mouton, C., M. Desclauriers, H. Allard, and M. Bouchard. 1987. Serum antibodies to Bacteroides gingivalis in periodontitis: a longitudinal study. J. Periodontal Res. 22426-430. [DOI] [PubMed] [Google Scholar]
- 36.O'Brien-Simpson, N. M., R. D. Pathirana, R. A. Paolini, Y. Chen, P. D. Veith, V. Tam, N. Ally, R. N. Pike, and E. C. Reynolds. 2005. An immune response directed to proteinase and adhesion functional epitopes against Porphyromonas gingivalis-induced bone loss. J. Immunol. 1753980-3989. [DOI] [PubMed] [Google Scholar]
- 37.Owen, C. A., Z. Hu, C. Lopéz-Otín, and S. D. Shapiro. 2004. Membrane-bound matrix metalloproteinase-8 on activated polymorphonuclear cells is a potent, tissue inhibitor of metalloproteinase-resistant collagenase and serpinase. J. Immunol. 1727791-7803. [DOI] [PubMed] [Google Scholar]
- 38.Pirilä, E., T. P. Maisi, Salo, E. Koivunen, and T. Sorsa. 2001. In vivo localization of gelatinases (MMP-2 and -9) by in situ zymography with selective gelatinase inhibitor. Biochem. Biophys. Res. Commun. 287766-774. [DOI] [PubMed] [Google Scholar]
- 39.Pirilä, E., N. S. Ramamurthy, T. T. Sorsa, Salo, J. Hietanen, and P. Maisi. 2003. Gelatinase A (MMP-2), collagenase-2 (MMP-8), and laminin-5 gamma2-chain expression in murine inflammatory bowel disease (ulcerative colitis) Dig. Dis. Sci. 4893-98. [DOI] [PubMed] [Google Scholar]
- 40.Prikk, K., P. Maisi, E. Pirilä, R. Sepper, T. Salo, J. Wahlgren, and T. Sorsa. 2001. In vivo collagenase-2 (MMP-8) expression by human bronchial epithelial cells and monocytes/macrophages in bronchiectasis. J. Pathol. 194232-238. [DOI] [PubMed] [Google Scholar]
- 41.Prikk, K., P. Maisi, E. Pirilä, M.-A. Reintam, T. Salo, T. Sorsa, and R. Sepper. 2002. Airway obstruction correlates with collagenase-2 (MMP-8) expression and activation in bronchial asthma. Lab. Investig. 821535-1545. [DOI] [PubMed] [Google Scholar]
- 42.Pussinen, P. J., T. Vilkuna-Rautiainen, G. Alfthan, K. Mattila, and S. Asikainen. 2002. Multiserotype enzyme-linked immunosorbent assay as a diagnostic aid for periodontitis in large-scale studies. J. Clin. Microbiol. 40512-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pussinen, P. J., M. Jauhiainen, T. Vilkuna-Rautiainen, J. Sundvall, M. Vesanen, K. Mattila, T. Palosuo, G. Alfthan, and S. Asikainen. 2004. Periodontitis decreases the antiatherogenic potency of high density lipoprotein. J. Lipid Res. 45139-147. [DOI] [PubMed] [Google Scholar]
- 44.Pussinen, P. J., T. Vilkuna-Rautiainen, G. Alfthan, T. Palosuo, M. Jauhiainen, J. Sundvall, M. Vesanen, K. Mattila, and S. Asikainen. 2004. Severe periodontitis enhances macrophage activation via increased serum lipopolysaccharide. Arterioscler. Thromb. Vasc. Biol. 242174-2180. [DOI] [PubMed] [Google Scholar]
- 45.Pussinen, P. J., S. Paju, P. Mäntylä, and T. Sorsa. 2007. Serum microbial- and host-derived markers of periodontal diseases: a review. Curr. Med. Chem. 142402-2412. [DOI] [PubMed] [Google Scholar]
- 46.Rams, T. E., M. A. Listgarten, and J. Slots. 2006. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis subgingival presence, species-specific serum immunoglobulin G antibody levels, and periodontitis disease recurrence. J. Periodontal Res. 41228-234. [DOI] [PubMed] [Google Scholar]
- 47.Smith, W. L. 1989. The eicosanoids and their biochemical mechanism of action. Biochem. J. 259315-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sorsa, T., L. Tjäderhane, and T. Salo. 2004. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 10311-318. [DOI] [PubMed] [Google Scholar]
- 49.Sorsa, T., and L. M. Golub. 2005. Is the excessive inhibition of matrix metalloproteinases (MMPs) by potent synthetic MMP inhibitors (MMPIs) desirable in periodontitis and other inflammatory diseases? That is, ‘Leaky’ MMPIs versus excessively efficient drugs. Oral Dis. 11408-409. [DOI] [PubMed] [Google Scholar]
- 50.Sorsa, T., L. Tjäderhane, Y. T. Konttinen, A. Lauhio, T. Salo, H.-M. Lee, L. M. Golub, D. L. Brown, and P. Mäntylä. 2006. Matrix metalloproteinases: contribution to pathogenesis, diagnosis, and treatment of periodontal inflammation. Ann. Med. 38306-312. [DOI] [PubMed] [Google Scholar]
- 51.Tall, A. R. 2007. CETP inhibitors to increase HDL cholesterol levels. N. Engl. J. Med. 3561304-1316. [DOI] [PubMed] [Google Scholar]
- 52.Tervahartiala, T., E. Pirilä, A. Ceponis, P. Maisi, T. Salo, G. Tuter, P. Kallio, J. Törnwall, R. Srinivas, Y. T. Konttinen, and T. Sorsa. 2000. The in vivo expression of the collagenolytic matrix metalloproteinases (MMP-2, -8, -13, and -14) and matrilysin (MMP-7) in adult and localized juvenile periodontitis. J. Dent. Res. 791969-1977. [DOI] [PubMed] [Google Scholar]
- 53.Timmins, J. M., J.-Y. Lee, E. Boudyguina, K. D. Klucman, L. R. Brunham, A. Mulya, A. K. Gebre, J. M. Coutinho, P. L. Colvin, T. L. Smith, M. R. Hayden, N. Maeda, and J. Parks. 2005. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Investig. 1151333-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tiwari, R. L., V. Singh, and M. K. Barthwal. 2007. Macrophages: an elusive yet emerging therapeutic target of atherosclerosis. Med. Res. Rev. 28483-544. [DOI] [PubMed] [Google Scholar]
- 55.Tuomainen, A. M., K. Nyyssönen, J. A. Laukkanen, T. Tervahartiala, T. P. Tuomainen, J. T. Salonen, T. Sorsa, and P. J. Pussinen. 2007. Serum matrix metalloproteinase-8 concentrations are associated with cardiovascular outcome in men. Arterioscler. Thromb. Vasc. Biol. 272722-2728. [DOI] [PubMed] [Google Scholar]
- 56.Tuomainen, A. M., M. Jauhiainen, P. T. Kovanen, J. Metso, S. Paju, and P. J. Pussinen. 2008. Aggregatibacter actinomycetemcomitans induces MMP-9 expression and proatherogenic lipoprotein profile in apoE-deficient mice. Microb. Pathog. 44111-117. [DOI] [PubMed] [Google Scholar]
- 57.van Haperen, R., A. van Tol, P. Vermeulen, M. Jauhiainen, T. van Gent, P. van der Berg, S. Ehnholm, F. Grosveld, A. van der Kamp, and R. de Crom. 2000. Human plasma phospholipid transfer protein increases the antiatherogenic potential of high density lipoproteins in transgenic mice. Arterioscler. Thromb. Vasc. Biol. 201082-1088. [DOI] [PubMed] [Google Scholar]
- 58.Van Lint, P., and C. Libert. 2006. Matrix metalloproteinase-8: cleavage can be decisive. Cytokine Growth Factor Rev. 17217-223. [DOI] [PubMed] [Google Scholar]
- 59.van Winkelhoff, A. J., B. G. Loos, W. A. van der Reijden, and U. van der Velden. 2002. Porphyromonas gingivalis, Bacteroides forsythus, and other putative periodontal pathogens in subjects with or without periodontal destruction. J. Clin. Periodontol. 291023-1028. [DOI] [PubMed] [Google Scholar]
- 60.Vardar-Sengul, S., E. Buduneli, O. Turkoglu, N. Buduneli, G. Atilla, J. Wahlgren, T. Sorsa, and H. Baylas. 2008. The effects of selective cyclooxygenase-2 inhibitor/celecoxib and omega-3 fatty acid on matrix metalloproteinases, tissue inhibitor of matrix metalloproteionase-1 and laminin-5μ2-chain immunolocalization in experimental periodontitis. J. Periodontol. 791934-1941. [DOI] [PubMed] [Google Scholar]
- 61.Wahlgren, J., P. Maisi, T. Sorsa, M. Sutinen, T. Tervahartiala, E. Pirilä, O. Teronen, J. Hietanen, L. Tjäderhane, and T. Salo. 2001. Expression and induction of collagenases (MMP-8 and -13) in plasma cells associated with bone-destructive lesions. J. Pathol. 194217-224. [DOI] [PubMed] [Google Scholar]
- 62.Wu, T., M. Trevisan, R. J. Genco, J. P. Dorn, K. L. Falkner, and C. T. Sempos. 2000. Periodontal disease and risk of cerebrovascular disease. Arch. Intern. Med. 1602749-2755. [DOI] [PubMed] [Google Scholar]
- 63.Zhang, F., S. P. Engebretson, R. S. Morton, P. F. Cavanaugh, Jr., K. Subbaramaiah, and A. J. Dannenberg. 2003. The overexpression of cyclo-oxygenase-2 in chronic periodontitis. J. Am. Dent. Assoc. 134861-867. [DOI] [PubMed] [Google Scholar]
- 64.Zubery, Y., C. R. Dunstan, B. M. Story, L. Kesavalu, J. L. Ebersole, S. C. Holt, and B. F. Boyce. 1998. Bone resorption caused by three periodontal pathogens in vivo in mice is mediated in part by prostaglandin. Infect. Immun. 664158-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]