Abstract
Hyperthermophilic crenarchaea in the genus Pyrobaculum are notable for respiratory versatility, but relatively little is known about the genetics or regulation of crenarchaeal respiratory pathways. We measured global gene expression in Pyrobaculum aerophilum cultured with oxygen, nitrate, arsenate and ferric iron as terminal electron acceptors to identify transcriptional patterns that differentiate these pathways. We also compared genome sequences for four closely related species with diverse respiratory characteristics (Pyrobaculum arsenaticum, Pyrobaculum calidifontis, Pyrobaculum islandicum, and Thermoproteus neutrophilus) to identify genes associated with different respiratory capabilities. Specific patterns of gene expression in P. aerophilum were associated with aerobic respiration, nitrate respiration, arsenate respiration, and anoxia. Functional predictions based on these patterns include separate cytochrome oxidases for aerobic growth and oxygen scavenging, a nitric oxide-responsive transcriptional regulator, a multicopper oxidase involved in denitrification, and an archaeal arsenate respiratory reductase. We were unable to identify specific genes for iron respiration, but P. aerophilum exhibited repressive transcriptional responses to iron remarkably similar to those controlled by the ferric uptake regulator in bacteria. Together, these analyses present a genome-scale view of crenarchaeal respiratory flexibility and support a large number of functional and regulatory predictions for further investigation. The complete gene expression data set can be viewed in genomic context with the Archaeal Genome Browser at archaea.ucsc.edu.
With the exception of the euryarchaeal halophiles, many archaeal model organisms are either obligate aerobes (e.g., Sulfolobus spp.) or obligate anaerobes (e.g., the Thermococcales), with limited respiratory flexibility. As a consequence, relatively little is known about the genetics, regulation, and enzymology of archaeal respiratory pathways compared to those of bacteria. Species in the genus Pyrobaculum are notable both for respiratory versatility, exemplified by Pyrobaculum aerophilum, and for diversity in respiratory capabilities between closely related species (6, 28, 29, 31, 65), presenting a potential model system for investigating respiratory pathways in the crenarchaea.
Pyrobaculum species grow optimally at 90 to 100°C and are common constituents of microbial communities in neutral pH geothermal springs and shallow marine hydrothermal vents (39, 42, 61). Pyrobaculum aerophilum is a facultative chemoautotroph that can use oxygen, nitrate, nitrite, arsenate, selenate, selenite, soluble ferric iron citrate, and insoluble ferric iron oxide as respiratory electron acceptors. The complete genome sequence of P. aerophilum was published in 2002 (23). The genome sequences of four additional Pyrobaculum species (P. arsenaticum, P. calidifontis, P. islandicum, and Thermoproteus neutrophilus [to be reclassified as a true Pyrobaculum]) have recently been completed (T. M. Lowe et al., unpublished data), providing a valuable resource for comparing gene content to respiratory characteristics within a closely related group of crenarchaea (Table 1).
TABLE 1.
Genomic and respiratory characteristics of selected Pyrobaculum species
| Species | Genome size (Mbp), gene count, GC % | Oxygen tolerance | Terminal electron acceptors supporting growth | Terminal electron acceptors not supporting growth | Reference(s) |
|---|---|---|---|---|---|
| P. aerophilum | 2.2, 2,706, 51 | Facultative microaerobe | O2, NO3−, NO2−, As(V), Se(VI), Se(IV), S2O32−,a Fe(III)-citrate, FeOOH | S0, N2Ob | 2, 20, 21, 29, 31, 65 |
| P. arsenaticum | 2.1, 2,408, 55 | Strict anaerobe | S0, S2O32−, As(V), Se(VI), Fe(III)-citrate, FeOOH | O2, NO3−, Se(IV) | 21, 29 |
| P. calidifontis | 2.0, 2,200, 57 | Facultative aerobe | O2, NO3−, As(V),d Fe(III)-citrate, FeOOH | NO2−,c S0, S2O32−, SO32−, SO42− | 6, 21 |
| P. islandicum | 1.8, 2,062, 50 | Strict anaerobe | S0, S2O32−, SO32−, l-cystine, oxidized glutathione, As(V),a Fe(III)-citrate, FeOOH | dl-Lanthionine, fumarate, (CH3)2SO2, S4O62−, SO42− | 21, 28, 31 |
| T. neutrophilus | 1.8, 2,053, 60 | Strict anaerobe | S0 | 22 |
Weak growth, final densities of ≤107 cells/ml.
P. aerophilum reduced NO3− and NO2− to N2 during growth (2, 65) and N2O reductase activity has been demonstrated in P. aerophilum cells (16), but N2O provided as the sole electron acceptor did not support growth (65).
P. calidifontis reduced NO3− to N2 during growth, but NO2− as the sole electron acceptor did not support growth (6).
See Results and Discussion.
A number of Pyrobaculum gene products have been characterized by heterologous expression or purification of native proteins, helping to identify genes for aerobic growth, oxidative stress, and denitrification (1, 4, 5, 18, 27, 44, 66). Although Pyrobaculum species are among the best-studied archaeal iron respirers, and are the only known archaeal arsenate respirers, the genes required for archaeal arsenate and iron respiration have not been identified (20, 21, 29, 31).
We used DNA microarrays to compare genome-wide expression patterns in P. aerophilum cultures with oxygen, nitrate, arsenate, or ferric iron-citrate as terminal electron acceptors. The results support previous observations related to aerobic respiration and suggest new candidate gene functions and regulatory relationships for denitrification and arsenate respiration. The genes for iron respiration remain enigmatic; however, genes repressed by iron suggest a transcriptionally regulated homeostatic response not previously described in the archaea.
MATERIALS AND METHODS
Culture conditions for P. aerophilum.
P. aerophilum IM2 cultures derived from DSMZ strain 7523 were generously provided by Christopher House, Penn State University. Cultures of P. aerophilum were grown in media containing (per liter) 10 g NaCl, 1.3 g (NH4)2SO4, 0.28 g KH2PO4, 0.25 g MgSO4·7H2O, 1 mg NaSeO4, 0.1 mg Na2WO4·2H2O, 70 mg CaCl2·2H2O, 1 mg (NH4)2Fe(SO4)2·6H2O, 0.5 mg resazurin, 0.5 g yeast extract, and 10 ml of a trace element solution. The trace element solution (DSM141) was prepared by combining the following components (per liter) in a 78.5 μM solution of nitriloacetic acid adjusted to pH 6.5: 3 g MgSO4·7H2O, 0.5 g MnSO4·H2O, 1 g NaCl, 0.1 g FeSO4·7H2O, 0.18 g CoSO4·7H2O, 0.1 g CaCl2·2H2O, 0.18 g ZnSO4·7H2O, 0.01 g CuSO4·5H2O, 0.02 g KAl(SO4)2·12H2O, 0.01 g H3BO3, 0.01 g Na2MoO4·2H2O, 0.025 g NiCl2·6H2O, and 0.0003 g NaSeO3·5H2O. The complete medium preparation was adjusted to pH 6.8 and sterilized by autoclaving.
All P. aerophilum cultures were incubated at 96°C, typically with 200 ml of medium in 0.5-liter wide-mouth bottles under a gas headspace of N2 and shaking at 200 rpm. For the aerobic cultures, 5% (vol/vol) of atmospheric air was added to the headspace for a final concentration of approximately 1% O2. Three replicate respiratory induction experiments were performed in which cultures were grown with a 1% O2 gas headspace, followed by amendment with different respiratory substrates when the cultures consumed the initially supplied O2. No reducing agents were used. The depletion of O2 in cultures was monitored by recording changes in the redox indicator dye resazurin from pink to clear. After the O2 was depleted, the cultures were cooled to the ambient temperature, diluted with a 25% volume of fresh growth medium, and dispensed in 200-ml volumes to 0.5-liter bottles in an anaerobic chamber. Separate bottles were prepared for collection at each of three time points. The bottles received 1% (vol/vol) additions from anoxic 1 M stocks of NaNO3, Na2HAsO4, or Fe(III)-citrate (pH 6); 5% (vol/vol) of atmospheric air in the gas headspace; or no added electron acceptor in each induction experiment. These were reequilibrated to the growth temperature (96°C), and after 2.5 h (T1), 4.5 h (T2), or 7.5 h (T3), one bottle from each respiratory condition was collected for RNA preparation. The cells were collected for RNA analysis by centrifugation at 10,000 × g for 10 min. The cell pellets were frozen in liquid N2 and stored at −80°C.
Metabolite analyses.
Subsamples for Fe(III) and Fe(II) analysis were collected directly into an equal volume of 1 M HCl and stored at −20°C. Subsamples for the analysis of other metabolites were filtered using 0.2-μm Costar Spin-X filters (Corning) and stored at −20°C. NO2− was measured spectrophotometrically using a Lachat Instruments Quickchem FIA+ autoanalyzer. The total concentrations of NO3− plus NO2− were measured by the prereduction of NO3− on a cadmium column, and NO3− concentrations were calculated as the difference between the reduced and untreated samples (67). As(III) and total inorganic As were measured by hydride generation followed by inductively coupled plasma optical emission spectrometry (ICP-OES) using a Perkin Elmer Optima 4300. The selective hydride generation from As(III) was achieved by diluting samples 1:100 in a 1 M citrate-citric acid buffer (pH 5.3) before mixing in equal volumes with a 1% NaBH4-0.5% NaOH solution to generate hydrides (7). Total inorganic As was measured by diluting the samples 1:100 in a 5% solution of KI in 3.8 M HCl to reduce As(V) to As(III) prior to hydride generation and ICP-OES. Fe(II) was measured using the ferrozine assay, and Fe(III) was measured indirectly as the difference between the levels of Fe(II) in the reduced and untreated samples (46).
Microarray production and hybridization.
Microarray probes for the P. aerophilum genome were prepared by the PCR amplification of P. aerophilum genomic DNA using primers picked by a custom PERL program (T. M. Lowe, unpublished data) utilizing the Primer 3 oligonucleotide selection software (48). Probe features included 2,726 annotated open reading frames (ORFs), 243 potential ORFs (dubbed “alternative ORFs”) (S. Fitz-Gibbon, personal communication), and 894 intergenic regions more than 60 nucleotides (nt) long. Probes for the ORFs and alternative ORFs were designed as 250- to 400-nt tags covering the most unique portions of genes and were biased toward the 3′ end of transcripts. The probes for intergenic regions were tiled as overlapping segments of no more than 700 nt each. PCR-amplified probes were evaluated by gel electrophoresis. Ninety-three loci (∼2% of the total) with PCR probe products of unexpected sizes or multiple bands were flagged and removed from analysis (see Table S1 in the supplemental material). The majority of these flagged features are intergenic regions, repetitive gene families, and alternative ORFs. The probes were suspended in 3× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) buffer and printed in quadruplicate on GAPS II slides (Corning), which were stored in a desiccating chamber until use.
Total RNA was extracted from the frozen cell pellets using a Polytron tissue homogenizer and Tri reagent (Sigma-Aldrich). RNA samples were treated with DNase I (Ambion) to remove any residual DNA, reextracted with Tri reagent, and normalized to 1 μg/μl before proceeding with the preparation of the cDNA for microarray analysis. Microarray analyses were performed for all 45 RNA samples generated in the three replicate induction experiments, using a pool of all samples as a universal reference for all hybridizations. Aminoallyl-labeled cDNAs were prepared from 5 μg of sample RNA using 5 μg of random hexamers (3 μg/μl) and the Superscript III labeling kit (Invitrogen). These were subsequently labeled with Cy3-N-hydroxysuccinimide ester or Cy5-N-hydroxysuccinimide ester (GE Healthcare). Spotted microarray slides were hydrated for 3 min in a humidified chamber, snap-dried at 100°C, covalently linked with 250 mJ in a UV cross-linker (Stratagene), and incubated at 42°C in a solution of 5× SSC, 1% bovine serum albumin, and 0.1% sodium dodecyl sulfate (SDS) for 45 min prior to hybridization. For each hybridization, Cy3-labeled cDNA derived from 5 μg of experimental RNA and Cy5-labeled cDNA derived from 5 μg reference pool RNA were combined with 1 mg/ml herring sperm DNA, 0.38 mg/ml yeast tRNA, 4× SSC, and 0.5% SDS in a total volume of 55 μl and hybridized for 14 to 18 h at 60°C in sealed hybridization chambers. After hybridization, the slides were washed for 2 min each in 0.05% SDS plus 0.1× SSC and in 0.05× SSC and dried by centrifugation. The slides were scanned at a 5-μm resolution immediately after washing using a Genepix 4000B microarray scanner (Molecular Devices).
Microarray data analysis.
Microarray features were analyzed using Genepix 6 software (Molecular Devices). The median pixel intensities for each spotted feature were normalized using the LOESS function of the open-source Bioconductor package “marray,” so that the normalized global and print-tip median log2(Ch1/Ch2) ratio of each array was zero (25). For each individual array feature, the median intensity values of four replicate spots were used for subsequent analyses. An intra-experimental normalization was performed before statistical and clustering analyses by subtracting the average log2 ratio at each locus within the experiment from the log2 ratio representing each condition and time point. A statistical analysis of differential expression was performed using Edge software and the SAM package for R (57, 58, 63). A false discovery rate (FDR) of 3% from the Edge analysis was used as an upper limit for calling differentially expressed genes statistically significant. The clustering of gene expression profiles and the production of heat maps were performed using Genesis software (59). Normalized log2 ratios for all loci represented in the microarrays and the results of the statistical analyses are compiled in Table S2A, S2B, and S2C in the supplemental material.
Northern analysis.
RNA samples from each of the three replicate respiratory induction experiments were combined in equal amounts to generate 15 samples representing each condition and time point. Ten micrograms of total RNA from each of these samples was denatured for 45 min at 50°C with glyoxal loading buffer (Ambion) and resolved by electrophoresis in 1% agarose gels buffered with 30 mM bis-Tris, 10 mM PIPES, 1 mM EDTA (pH 6.5) (51). Size-separated RNAs were blotted onto Hybond N+ nylon membranes (GE healthcare) using capillary transfer. The PCR products and primers identical to those used to generate microarray probes were used for single-stranded DNA probe preparation. Single-stranded DNA probes were prepared by linear PCR with Strip-EZ PCR reagents (Ambion) and body labeled using [α-32P]ATP. Hybridizations were carried out at 42°C using Ultrahyb buffer (Ambion). The membranes were stripped of probes and prepared for rehybridization using Strip EZ PCR reagents and protocols.
Computational genomics.
Gene features and organization were evaluated using the Archaeal Genome Browser (53). Orthology was inferred by identifying reciprocal best BlastP hits (3) in which protein pairs were required to align at least 85% of full length and have at least 45% amino acid identity. Predicted orthologs for P. aerophilum genes in the other sequenced Pyrobaculum genomes are listed in Table S3 in the supplemental material. Putative twin-arginine translocation signals were identified using TatP (9). The promoter regions of coregulated genes were screened for possible regulatory motifs using BioProspector (32). The P. aerophilum genome was screened for additional occurrences of identified motifs using position-specific scoring matrices. Basal regulatory elements and TATA boxes were identified using position-specific scoring matrices (P. P. Chan and T. M. Lowe, unpublished data). Motif logo figures were generated using Weblogo (13).
Culture conditions for P. arsenaticum and P. calidifontis.
P. arsenaticum strain DSM 13514 was grown at 95°C in DSM390 medium reduced with 0.01% Na2S and maintained with 20 mM Na2S2O3 as the terminal electron acceptor. Ten millimeter NaNO2 and 10 mM NaNO3 were each tested separately as alternative terminal electron acceptors.
P. calidifontis strain JCM 11548 was grown at 95°C in TY medium (10 g tryptone, 2 g yeast extract per liter) without Na2S2O3 and maintained with 10 mM NaNO3 as a terminal electron acceptor. 10 mM Na2HAsO4 was tested as an alternative terminal electron acceptor. Solid medium was prepared using 1.2% Gelrite and 10 mM MgSO4 as solidifying agents.
Sequence analysis of the P. calidifontis Pcal_1601 locus.
P. calidifontis cells were lysed using SNET lysis buffer, and genomic DNA was extracted using the phenol:chloroform:isoamyl alcohol method (51). Genomic DNA was treated with RNase A (Sigma) and reextracted before analysis. A 1-kb segment of the Pcal_1601 locus (chromosomal position 1489399 to 1490424) was PCR amplified using the forward primer 5′-GAGAGCTACACAGAGATCTTCG, the reverse primer 5′-GATAAGCACGAAGTTCTCAGG, and Phusion high fidelity polymerase (Finnzymes).
Microarray data accession numbers.
A description of the microarray platform has been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (NCBI GEO) database under the accession number GPL6755. The microarray data produced from this study are deposited in the NCBI GEO database under the accession number GSE11366.
RESULTS AND DISCUSSION
Initial microarray experiments comparing the gene expression patterns of P. aerophilum cultured to similar cell densities with nitrate, arsenate, or ferric citrate as the terminal electron acceptor showed differences in gene expression of twofold or greater at approximately one-third of all loci (data not shown). We observed that the growth rates and maximum densities varied with different terminal electron acceptors, which is consistent with other reports (20, 21), and attributed these global changes in gene expression to differences in growth phase as well as responses related to respiratory metabolism.
To focus on genes that are specifically affected by changes in terminal electron acceptors, we analyzed gene expression in five parallel time courses with cultures shifted from aerobic growth to nitrate, arsenate, ferric iron-citrate, or additional O2. A group that received no additional terminal electron acceptor was also included. Gene expression and substrate usage were measured at three time points (2.5, 4.5, and 7.5 h). This interval was designed to allow sufficient time for changes in respiratory substrate usage while minimizing differences in growth phase between the treatment groups.
Substrate usage and growth.
Changes in medium oxygenation, monitored using the redox indicator dye resazurin, showed that cultures that received no additional electron acceptor and those amended with 1% O2 depleted the available oxygen at different stages during the time course. Cultures that received no terminal electron acceptor became anoxic between T1 and T2. Cultures amended with 1% O2 became anoxic between T2 and T3.
Analysis of the culture medium showed that cultures amended with nitrate, arsenate, or ferric citrate reduced each of these substrates during the time course. Cultures shifted to NO3− produced approximately 1 mM NO2− by T3, measured spectrophotometrically (see Fig. S1A and S1B in the supplemental material). Decreases in the total concentration of NO3− plus NO2− varied from 0 mM to 0.5 mM between replicate induction experiments, suggesting a low and variable reduction of NO2− to gaseous NO, N2O, or N2 (see Fig. S1A in the supplemental material). Concentrations of NO3− and NO2− in the cultures that were induced with O2, As(V), Fe(III)-citrate, or no electron acceptor were below 0.04 mM, and no significant changes were detected over the time course (data not shown). Cultures shifted to As(V) reduced approximately 1 mM As(V) to As(III) by T3, as measured by ICP-OES (see Fig. S1C and S1D in the supplemental material). Cultures shifted to Fe(III)-citrate produced approximately 1 mM Fe(II) by T2 and 3.5 mM Fe(II) by T3, as measured by the ferrozine assay (see Fig. S1E in the supplemental material).
With the exception of that of the Fe(III)-treated group, the optical densities of all the treatment groups varied by less than 20% by the end of the time course (see Fig. S1F in the supplemental material). The optical densities of the cultures amended with O2 or NO3− increased more than those amended with As(V) or those that received no additional electron acceptor. The growth medium of the Fe(III)-treated cultures darkened substantially over the time course compared to the uninoculated Fe(III)-amended medium, confounding the use of optical absorbance as a proxy for cell density in this group. However, the total RNA yields and relative intensities for 16S and 23S rRNA bands were similar for cultures shifted to Fe(III) and those shifted to As(V), suggesting similar growth for these two groups (data not shown).
Identification of differentially expressed genes.
Statistical analysis using Edge identified 543 features (14% of the total) with significant differences in expression (FDR of <3%) between respiratory treatments. The hierarchical clustering of expression profiles from this group revealed eight major coexpressed clusters that are the focus of subsequent sections. Edge also identified 1,013 loci (∼27% of the total) with significant (FDR of <1%) changes in gene expression over the time course, irrespective of respiratory treatment, suggesting that changes in growth phase between sampling time points affected gene expression at a large proportion of loci. The majority of the most dynamic loci were highly ranked in the respiratory response group (median 9.3-fold range for the top 50 respiratory response loci compared to 3.4-fold for the top 50 temporal response genes). The gene expression profiles for the complete set of loci in each group and the genome-wide data set (see File S1 in the supplemental material) can be viewed interactively using the free Genesis software (http://genome.tugraz.at/genesisclient/genesisclient_description.shtml) (59).
The ranked lists of differentially expressed loci reported by SAM were highly correlated with the lists reported by Edge (R2 = 0.92 and R2 = 0.98 for respiratory treatment and time course analyses, respectively). The results from statistical analyses and normalized log2 expression ratios from microarray analyses are compiled in Table S2A, S2B, and S2C in the supplemental material. All expression data and views of key respiration loci are also readily accessible in the Archaeal Genome Browser at archaea.ucsc.edu (53).
Opposing regulation of aerobic cytochrome oxidases.
P. aerophilum grows microaerobically under gas headspace O2 concentrations up to 5% (vol/vol) (65). The P. aerophilum genome contains two closely spaced clusters of genes for aerobic cytochrome oxidase complexes (Fig. 1A), described hereafter using Sox nomenclature originating from studies of another crenarchaeal aerobe, Sulfolobus acidocaldarius (33, 34). One group of cytochrome oxidase genes encodes components of a SoxB-type enzyme (PAE1355 and PAE1357). Conserved domains in a flanking set of genes (PAE1358-PAE1362) suggest that these may be involved in the biosynthesis of the SoxB complex. A second group of cytochrome oxidase genes (PAE1337-PAE1340) encodes a SoxM-type complex. Pyrobaculum oguniense and Pyrobaculum calidifontis are both capable of growth under atmospheric O2 concentrations, and each have syntenic clusters of genes orthologous to the cytochrome oxidase complexes found in P. aerophilum (Table 2) (6, 49). The SoxB complex, SoxM complex, superoxide dismutase, and catalase proteins of P. aerophilum show strong similarity (≥85%, ≥67%, 88%, and 88% identity, respectively) to those of P. oguniense and P. calidifontis.
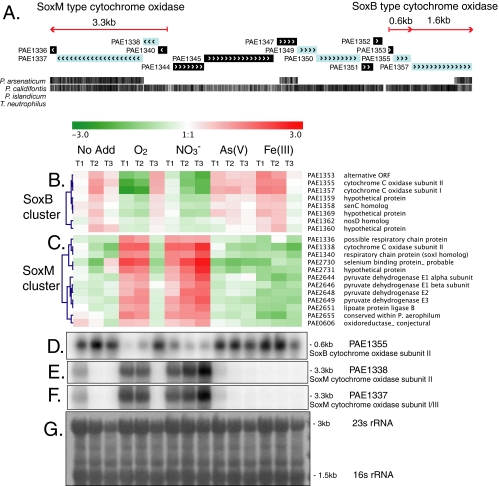
FIG. 1.
Genomic organization (A) and transcriptional expression (B to F) of aerobic SoxM-type and SoxB-type cytochrome oxidase genes in P. aerophilum. The red arrows in panel A indicate transcripts detected by Northern analysis (D to F). Individual genes are shown in shaded boxes, with arrow marks indicating the predicted direction of transcription. The black hatch marks at the bottom of panel A indicate regions of nucleotide sequence similarity in multiple genome alignments with other Pyrobaculum species and show an extended region of similarity in the aerobe P. calidifontis. Hierarchically clustered microarray gene expression profiles for SoxB cytochrome oxidase genes (B) and for SoxM cytochrome oxidase genes (C) during time courses of growth on different terminal electron acceptors are shown (No Add, no terminal electron acceptor added). Positive (red) and negative (green) changes in gene expression are shown on a log2 scale, with the scale bar shown at the top. Expression values for each time course represent the average log2 ratio of three replicate respiratory induction experiments. The blue dendrograms show relationships based on complete linkage clustering using the Pearson correlation. Northern analyses of the SoxB-type cytochrome oxidase (D) and the SoxM-type cytochrome oxidase genes (E and F), with lanes corresponding to sample growth conditions indicated above the heat maps, are shown. (G) Methylene blue stain of total RNA showing 23S and 16S rRNA bands on a representative blot used for Northern analysis.
TABLE 2.
Proposed functions and predicted orthologs for differentially expressed P. aerophilum genes
| Locus (annotation) | Proposed function | Conserved domaina | Predicted ortholog in Pyrobaculum species
|
|||
|---|---|---|---|---|---|---|
| P. arsenaticum | P. calidifontis | P. islandicum | T. neutrophilus | |||
| PAE1353 (alternative ORF) | O2-scavenging oxidoreductase | Region 5′ of Pcal_1950 | ||||
| PAE1355 (cytochrome c oxidase subunit II) | O2-scavenging oxidoreductase | cl08244: cytochrome c oxidase subunit II | Pcal_1950 | |||
| PAE1357 (cytochrome c oxidase subunit I) | O2-scavenging oxidoreductase | cd01660: ba3-like heme-copper oxidase subunit I | Pcal_1949 | |||
| PAE1336 (possible respiratory chain protein) | Aerobic O2 reductase | Pars_0585 | ||||
| PAE1337 (cytochrome c oxidase subunit I/III) | Aerobic O2 reductase | cl00275: heme-copper oxidase subunit I; cd00386: heme-copper oxidase subunit III | Pcal_1943 | |||
| PAE1338 (cytochrome c oxidase subunit II) | Aerobic O2 reductase | cl08244: cytochrome c oxidase subunit II | Pcal_1944 | |||
| PAE1340 (respiratory chain protein [SoxI homolog]) | Aerobic O2 reductase | Pcal_1945 | ||||
| PAE2679 (transcriptional regulator) | NO-responsive transcriptional regulator | cl00088: HTH ARSR superfamily | Pars_0504 | |||
| PAE2680 (hypothetical protein) | NO-responsive transcriptional regulator | COG3945: uncharacterized conserved protein | Pars_0505 | Pcal_1917 | ||
| PAE1888 (multicopper oxidase) | NO2− or N2O reductase | cl06664: multicopper oxidase | ||||
| PAE1263 (molybdopterin oxidoreductase, iron-sulfur-binding subunit) | Arsenate respiratory reductase | COG0437: HybA, Fe-S-cluster-containing hydrogenase | Pars_0387 | Pcal_1599 | Pisl_1986 | Tne_0510 |
| PAE1264 (hypothetical membrane protein) | Arsenate respiratory reductase | cl01295: polysulfide reductase integral transmembrane protein NrfD | Pars_0388 | Pcal_1600 | ||
| PAE1265 (molybdopterin oxidoreductase, molybdopterin-binding subunit) | Arsenate respiratory reductase | cd02758: MopB tetrathionate reductase; cd02780: molybdopterin-binding, C-terminal (MopB_CT) region of tetrathionate reductase (TtrA); respiratory arsenate As(V) reductase (ArrA) | Pars_0389 | Pcal_1601b | ||
| PAE2859 (molybdopterin oxidoreductase, molybdopterin-binding subunit) | Thiosulfate or polysulfide reductase | cd02755: MopB_thiosulfate-R-like; cd02775: molybdopterin-binding, C-terminal (MopB_CT) | Pars_0938 | Pcal_1500 | Pisl_0162 | Tne_1951 |
| PAE2860 (molybdopterin oxidoreductase, iron-sulfur subunit) | Thiosulfate or polysulfide reductase | COG0437: HybA, Fe-S-cluster-containing hydrogenase | Pars_0937 | Pcal_1499 | Pisl_0161 | Tne_1952 |
| PAE2861 (molybdopterin oxidoreductase, membrane subunit) | Thiosulfate or polysulfide reductase | cl01295: polysulfide reductase integral transmembrane protein NrfD | Pars_0936 | Pcal_1498 | ||
| PAE2696 (Mn catalase) | Iron-responsive regulon | cd01051: Mn catalase | Pars_1142 | Pcal_0729 | ||
| PAE2697 (putative ABC transport protein) | Iron-responsive regulon | cl00427: binding protein-dependent transport system inner membrane component | ||||
| PAE2699 [putative Fe(III) ABC transporter ATP-binding protein] | Iron-responsive regulon | cd03255: ABC_MJ0796_Lo1CDE_FtsE | ||||
| PAE2700 (putative ABC transport protein) | Iron-responsive regulon | cl02460: transferrin; cl00115: periplasmic binding protein (PBPb) | ||||
| PAE0600 (hypothetical protein) | Iron-responsive regulon | cl00437: ZIP zinc transporter | Pars_1201 | Pcal_1350 | ||
From the Conserved Domain Database (CDD). The CDD identifier and product are given. HTH, helix-turn-helix; ARSR, arsenical resistance operon repressor.
See Results and Discussion.
Subunits I (PAE1357) and II (PAE1355) of the P. aerophilum SoxB-type cytochrome oxidase complex were upregulated under all conditions in the respiratory time course experiment except the first two time points after amendment with O2 and all three time points after amendment with nitrate. These oxygen- and nitrate-amended samples showed expression levels up to 15-fold lower (Fig. 1B and D). A small alternative ORF upstream of PAE1355 (PAE1353) and directly downstream of a strong promoter signal showed an identical expression pattern, suggesting that this may represent a small, cotranscribed gene. Northern analysis of PAE1355 showed a band at approximately 600 nt, consistent with the cotranscription of PAE1353 (144 nt) and PAE1355 (477 nt) (Fig. 1D), and a minor band at 2.3 kb (not shown), consistent with the cotranscription of these genes with PAE1357 (1,674 nt). Downstream genes, including a senC homolog (PAE1358) and several hypothetical proteins (PAE1359, PAE1360, and PAE1369), were expressed in a similar pattern (Fig. 1B).
The second group of cytochrome oxidase genes was expressed in a pattern that was essentially the opposite of the PAE1353 to -1357 SoxB-type cytochrome oxidase (Fig. 1C). These genes, annotated as a possible respiratory chain protein (PAE1336), a SoxM-type fusion of cytochrome oxidase subunits I and III (PAE1337), a cytochrome oxidase subunit II (PAE1338), and a SoxI homolog (PAE1340) were upregulated up to 11-fold in oxic (the first two time points following induction with oxygen) versus anoxic samples and upregulated up to 16-fold after induction with nitrate (Fig. 1C, E, and F). Genes encoding subunits of a pyruvate dehydrogenase complex (PAE2644, PAE2646, PAE2648, and PAE2649), as well as an adjacent hypothetical protein with a putative DNA binding domain (PAE2655), were upregulated in a very similar pattern, suggesting enhanced pyruvate consumption associated with the expression of the SoxM cluster of genes (Fig. 1C).
The differential expression of the SoxM-type I/III fusion protein (PAE1337) was not detected in the microarrays, but the coexpression of adjacent genes PAE1336, PAE1338, and PAE1340 prompted us to verify the expression pattern for PAE1337. Northern analyses of PAE1338 and PAE1337 (Fig. 1E and F) showed identical banding patterns that agree with the microarray results for PAE1338, and a major band at approximately 3.3 kb, consistent with the cotranscription of a polycistronic mRNA for PAE1336, PAE1337, PAE1338, and PAE1340 (annotated sizes of 195, 2,403, 450, and 240 nt, respectively). Northern analyses of 20 other key loci showed very good agreement with the expression patterns indicated by the microarray results (Fig. 1 and 2; see also Fig. S2 to S5 in the supplemental material).
FIG. 2.
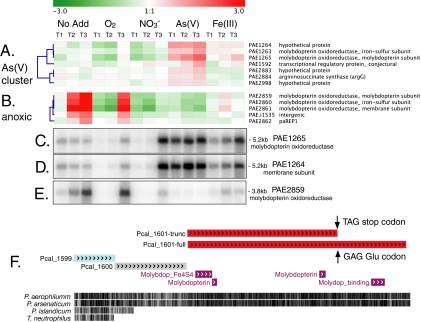
(A to E) Contrasting transcriptional regulation of two putative molybdopterin oxidoreductases. Microarray gene expression profiles for a molybdopterin oxidoreductase complex (PAE1263, PAE1264, and PAE1265) and other genes upregulated during growth on arsenate (A) and a molybdopterin oxidoreductase complex (PAE2859, PAE2860, and PAE2861) upregulated during anoxia (B). Northern analysis of PAE1265 (C), PAE1264 (D), and PAE2859 (E). (F) P. calidifontis genome alignment showing truncated (Pcal_1601-trunc) and full-length (Pcal_1601-full) orthologs for PAE1265 identified before and after selection for growth of P. calidifontis on arsenate, respectively. The conserved protein domains predicted by Pfam are shown in magenta. The black hatch marks show primary sequence similarity to P. aerophilum and P. arsenaticum in multiple genome alignments. No Add, no terminal electron acceptor added.
Many facultatively aerobic bacteria possess more than one respiratory oxygen reductase for growth under a range of oxygen tensions (47). The regulation of two cytochrome oxidases in the facultative aerobe Pyrobaculum oguniense (45) suggests an analogous specialization in the Pyrobaculum genus: the P. oguniense SoxM-type oxidase was induced under aerobic conditions, whereas the SoxB-type oxidase was induced under anaerobic, thiosulfate-respiring conditions (45). This suggested that the terminal reductase for aerobic growth is the SoxM complex and that the SoxB complex functions in oxygen scavenging with a possible regulatory role.
The opposing expression pattern of the two homologous cytochrome oxidase complexes in P. aerophilum corroborates the expression patterns observed in P. oguniense and provides support for the distinct functions proposed by Nunoura and colleagues (45). In the current study, P. aerophilum SoxB complex genes were upregulated under suboxic conditions, consistent with encoding an oxygen scavenger or sensor (Fig. 1B and D). The P. aerophilum SoxM complex genes were expressed in a strikingly opposite pattern, consistent with their primary role in aerobic respiration (Fig. 1C, E, and F). Because P. oguniense can grow under atmospheric oxygen concentrations, whereas P. aerophilum is a microaerobe, Nunoura et al. speculated that the SoxB complex might be the primary terminal reductase for the microaerobic growth of P. aerophilum. Instead, our results indicate that the SoxM-type complex is the terminal reductase for P. aerophilum under microaerobic conditions.
Given this conclusion, the upregulation of the SoxM complex after induction with nitrate was unexpected. Aerobic cytochrome oxidases are structurally and evolutionarily related to nitric oxide reductases, and cytochrome oxidases from several bacteria have been shown to reduce nitric oxide (24, 26, 52, 64). However, a direct role for this SoxM-type oxidase in nitrate respiration or denitrification would be novel. Whether the expression of SoxM complex genes is characteristic of steady-state growth on nitrate or is a transitional effect induced by the shift from oxygen to nitrate remains to be determined.
Genes for nitrate respiration and denitrification in Pyrobaculum.
P. aerophilum can reduce nitrate to dinitrogen during growth, and reductase activities for each individual substrate (nitrate [NO3−], nitrite [NO2−], nitric oxide [NO], and nitrous oxide [N2O]) in this stepwise reaction have been demonstrated in association with P. aerophilum cell membranes (2, 17, 65). The P. aerophilum genome contains genes for a narG-type nitrate reductase (PAE3611-PAE3613), a cd1-type nitrite reductase (PAE3598 and PAE3600), and a heme O-containing nitric oxide reductase (PAE3603) (1, 18, 23) (see Fig. S2A in the supplemental material). However, the P. aerophilum genome lacks a recognizable homolog of nosZ, which encodes nitrous oxide reductase in bacteria, and a functional alternative has not been identified (70).
Orthologs for the nitrate reductase, nitrite reductase and nitric oxide reductase were found in the genomes of both P. calidifontis and P. arsenaticum (see Table S3 in the supplemental material). P. calidifontis also has a nosZ-type nitrous oxide reductase (Pcal_1928) (55). P. calidifontis produced dinitrogen when grown anaerobically on nitrate, indicating a capacity for denitrification consistent with the complement of denitrification genes in the P. calidifontis genome (6). In contrast, despite a large complement of genes for nitrate respiration and denitrification, P. arsenaticum was reported to be incapable of growth using nitrate (29). Our attempts to cultivate P. arsenaticum with nitrate or nitrite as electron acceptors were also unsuccessful (not shown). Further investigation will be required to determine whether P. arsenaticum is missing genes essential for nitrate respiration and denitrification or whether it requires specific culture conditions for growth with N-oxyanions.
Limited modulation of the nitrate reductase operon.
Despite a >250-fold difference in nitrate concentration between cultures shifted to growth with nitrate versus those shifted to other electron acceptors (∼10 mM compared to <0.04 mM), microarray analysis showed relatively little variation in the expression of the nitrate reductase alpha subunit across all conditions, with only moderate upregulation (up to 2.7-fold) after induction with nitrate (see Fig. S2B in the supplemental material). Northern analysis showed that the nitrate reductase alpha subunit (PAE3611) was expressed under all conditions in the respiratory induction experiment (see Fig. S2D in the supplemental material). Northern analysis also showed that PAE3611 is part of an approximately 7-kb transcript, consistent with a polycistronic operon that includes an iron-sulfur subunit (PAE3612), a cytochrome b-containing membrane subunit (PAE3613) (1), and a downstream ORF of unknown function (PAE3614) (see Fig. S2A in the supplemental material).
The expression of the nitrate reductase operon under all respiratory conditions contrasts with that of other respiratory complexes identified in this study and may reflect an adaptive preference for nitrate as a respiratory substrate. Previous studies have shown large (10-fold) differences in nitrate reductase activity in P. aerophilum cultures grown to late log phase with nitrate compared to cultures grown with ferric iron and a distinct redox optimum for growth on nitrate (20, 21). These results suggest that steady-state growth under controlled redox conditions may produce larger differences in nitrate reductase expression.
Coexpression of denitrification genes.
Denitrification genes (PAE3600, the cytochrome d1 subunit of the cd1-type nitrite reductase; PAE3602, a hypothetical protein; PAE3603, the nitric oxide reductase) were upregulated up to 15-fold by the end of the time course after induction with nitrate, compared to aerobic cultures (see Fig. S2C in the supplemental material). A putative transcriptional regulator with homology to MarR-family transcriptional regulators (PAE2679) and an adjacent hypothetical protein with two hemerythrin binding domains (PAE2680) were also strongly upregulated (up to 17-fold) late in the time course after induction with nitrate (see Fig. S2C in the supplemental material). Two other flanking genes (PAE3598, the cytochrome c subunit of the cd1-type nitrite reductase; PAE3601, uroporphyrin-III C-methyltransferase, possibly involved in cytochrome heme synthesis) showed similar expression patterns but were less strongly upregulated.
Northern analysis of PAE3600 showed a major band at 1.6 to 1.7 kb and a minor band at 2.2 kb, consistent with a monocistronic transcript of PAE3600 and cotranscription with PAE3601, respectively (see Fig. S2E in the supplemental material). PAE3598 and PAE3603 showed similar expression patterns (see Fig. S2F and S2G in the supplemental material). Northern analysis of the putative MarR-like transcriptional regulator PAE2679 showed a primary band at approximately 1.1 kb, consistent with the cotranscription of PAE2679-PAE2680 (predicted sizes of 526 nt and 543 nt, respectively), and a pattern of expression that resembles those of the nitrite and nitric oxide reductases (see Fig. S2H in the supplemental material). The results of the microarrays and Northern analyses are consistent in showing strong upregulation of these genes at the third time point during growth on nitrate and more moderate upregulation during growth on arsenate (see Fig. S2B to S2H in the supplemental material).
Conserved promoter motif for denitrification genes.
An inspection of the PAE3598-PAE3603 locus revealed a conserved promoter motif that may be involved in regulating the transcription of these genes. The motif is palindromic (see Fig. S2I in the supplemental material), which is typical for targets of helix-turn-helix transcription factors, and occurs between the cytochrome c and d1 components of the nitrite reductase (PAE3598 and PAE3600), in the promoter region of the nitric oxide reductase (PAE3603), and in the promoter region of a hypothetical protein (PAE3602) (see Fig. S2A in the supplemental material). The palindromic halves of the motif show particularly strong conservation between the two nitrite reductase genes, which are divergently transcribed in each of the three Pyrobaculum genomes where they occur (see Fig. S2J in the supplemental material).
A candidate nitric oxide-responsive transcriptional regulator.
The coexpression of genes for putative MarR-like and hemerythrin-binding proteins (PAE2679 and PAE2680) and genes for denitrification (see Fig. S2C and S2E to S2H in the supplemental material) suggests that these may be regulated by common factors. The upregulation of these genes when nitrite accumulated to ∼1 mM in the culture medium (see Fig. S1B in the supplemental material) suggests a possible role for nitrite in their regulation, with relatively low sensitivity to concentrations less than 1 mM. An alternative possible effector is nitric oxide. Bacterial transcriptional regulators of denitrification generally fall in the Crp-Fnr family, are responsive to both oxygen and nitric oxide, and can be sensitive to nanomolar concentrations of nitric oxide (69). In contrast, bacterial MarR family transcriptional regulators typically regulate antibiotic resistance, virulence, and oxidative stress (60). Hemerythrins are di-iron-containing proteins that can reversibly bind oxygen or nitric oxide (43). These annotations suggest that the PAE2679-PAE2680 operon encodes a transcriptional regulator that controls either stress responses or denitrification genes in response to nitric oxide. P. calidifontis has an ortholog for only the hemerythrin-like PAE2860, located within a region of the genome that contains the complete suite of genes for denitrification, while P. arsenaticum has orthologs for both PAE2679 and PAE2680 (Table 2), flanked on either side by genes for nitrate reductase and nitric oxide reductase.
Upregulation of a multicopper oxidase during nitrate respiration.
A putative multicopper oxidase (PAE1888) was among the genes most specifically upregulated (up to fivefold) by growth on nitrate and was highly ranked by statistical analyses (see Fig. S3A and Table S2C in the supplemental material). Northern analysis with a probe for PAE1888 showed a band consistent with the annotated size of 1,434 nt, as well as increasing expression during the time course on nitrate (see Fig. S3B in the supplemental material). The putative 53-kDa enzyme encoded by PAE1888 contains a predicted N-terminal twin-arginine translocation signal, a predicted binuclear Cu-3-type center similar to those in Cu-containing nitrite reductases, and a predicted mononuclear C-terminal Cu-2-type center (36). The twin-arginine signal suggests that this enzyme is exported after assembly to the outer membrane. No orthologs of this gene were found in the other Pyrobaculum or Thermoproteales genomes (Table 2). The closest BlastP hits (∼40% identity) were annotated as bilirubin oxidases or multicopper oxidases and were from bacteria that are notable for thermophily (Thermus, Aquifex), catabolism of complex organic substrates (Polaromonas naphthalenivorans, “Candidatus Desulfococcus oleovorans”), or for ammonia oxidation and denitrification (Nitrosomonas, Nitrosospira).
Computational scans identified an overrepresented sequence motif (see Fig. S3C in the supplemental material) that may coregulate PAE1888, a hypothetical protein with a thioredoxin-like domain (PAE2750), and two downstream genes (encoding a putative cytochrome c-type biogenesis protein [PAE2751] and a potential noncoding RNA within intergenic region PAE.i1473) that showed similar expression patterns (see Fig. S3A in the supplemental material). The motif is located immediately upstream from the predicted TATA box for both PAE1888 and PAE2750 and is conserved in the promoter regions of genes orthologous to PAE2750 in the genomes P. calidifontis and P. arsenaticum (see Fig. S3D to S3E in the supplemental material). A putative cytoplasmic siroheme-type ferredoxin-nitrite reductase (PAE2577), typically involved in the assimilatory or detoxifying reduction of nitrite to ammonium (41, 54), also showed an expression pattern similar to PAE1888 (see Fig. S3A in the supplemental material) but lacked this promoter motif.
Enzymes in the multicopper oxidase superfamily are diverse and typically use oxygen as an electron acceptor but also include bacterial copper-containing nitrite reductases encoded by nirK genes and nitrous oxide reductases encoded by nosZ genes (70). Based on the specific upregulation of PAE1888 during nitrate respiration and the putative domain structure of the encoded protein, we propose that PAE1888 encodes a novel nitrite or nitrous oxide reductase. The similarity between PAE1888 and genes in Nitrosomonas species, which also reduce nitrous oxide and lack identified nitrous oxide reductase genes, provides potential support for a role in nitrous oxide reduction (56, 70). The coregulated, putative thioredoxin (PAE2750) and cytochrome c biosynthesis (PAE2751) genes may function in the biosynthesis of cofactors or electron donors for this enzyme.
A predicted ARR.
Bacterial arsenate respiratory reductases (ARRs) are members of the molybdopterin oxidoreductase superfamily (50). Degenerate PCR primers that reliably amplify bacterial ARR genes do not detect them in P. aerophilum or P. arsenaticum, the only archaeal arsenate respirers identified to date (29, 35). We identified nine putative molybdopterin oxidoreductase genes in the P. aerophilum genome, four of which (PAE1265, PAE2002, PAE2839/PAE2840, and PAE2859) show limited similarity to functionally characterized genes. We predicted that one of these encodes an ARR similar to those found in bacteria.
BlastP searches of the P. aerophilum genome using the Mo-containing subunit of ARR from diverse bacteria (Shewanella sp. strain ANA-3, Bacillus arseniciselenatis, Chrysiogenes arsenatis, Sulfurospirillum barnesii) as query sequences all returned PAE2859 as the strongest match. However, the PAE2859 operon was upregulated under anoxic conditions only in the absence of nitrate, arsenate, or ferric iron (Fig. 2B and E). In contrast, a group of genes encoding a putative 132-kDa molybdopterin oxidoreductase (PAE1265), a putative 23-kDa iron-sulfur-protein (PAE1263), and a possible 40.7-kDa membrane-anchoring subunit (PAE1264) were strongly upregulated (up to eightfold) in cultures induced with arsenate (Fig. 2A). These cultures reduced approximately 1 mM arsenate to arsenite (see Fig. S1C and S1D in the supplemental material). Northern analysis with probes for the Mo-containing subunit PAE1265 and the membrane subunit PAE1264 showed transcripts of approximately 5.2 kb and identical patterns of upregulation, consistent with the transcription of an operon containing all three of these coregulated genes (Fig. 2C and D). Northern analysis also showed increasing expression in cultures induced with ferric iron and upregulation at the last time point after induction with nitrate.
The predicted active site domain of PAE1265 is conserved in bacterial tetrathionate reductases and ARRs (36). Both the iron-sulfur subunit and the molybdopterin-binding subunit contain predicted N-terminal twin-arginine translocation signals, suggesting that the gene products are translocated to the outer membrane after translation. Although there are orthologs of PAE1263 in several Pyrobaculum species, orthologs for all three components of the PAE1263-PAE1265 operon were found only in P. arsenaticum, the only other archaeon known to grow robustly on arsenate (Table 2) (29). Based on these data, we propose that the PAE1263-PAE1264-PAE1265 operon encodes a membrane-bound ARR.
Few other genes were specifically upregulated by arsenate (Fig. 2A). These included a putative arsR homolog (PAE1592), which may be involved in the regulation of arsenite-responsive genes, and a putative argininosuccinate synthase (PAE2884). Genes annotated as an arsenical pump-driving ATPase (PAE1427) and an arsenite permease (PAE2457) were not upregulated in arsenate-respiring P. aerophilum cultures, even when arsenite accumulated to 1 mM (see Table S2B in the supplemental material).
Recovery of a variant P. calidifontis strain after growth on arsenate.
The P. calidifontis genome (Refseq NC_009073) contains orthologs for both PAE1263 and PAE1264 (Pcal_1599 and Pcal_1600) (Table 2). An ORF adjacent to these orthologs (Pcal_1601-trunc; currently annotated as a pseudogene) with strong primary sequence similarity to PAE1265, is truncated after 810 amino acids by a stop codon (TAG) at P. calidifontis chromosomal position 1489855 (Fig. 2F). Fourteen of 15 sequencing reads used to assemble the P. calidifontis genome in this region unambiguously show a TAG stop at this position, indicating that this was not a sequencing error. However, one read (NCBI trace archive ID number 1617717348) shows a GAG at this position (Glu; the consensus amino acid at the corresponding site in P. aerophilum and P. arsenaticum), which would produce a 1,170-amino-acid protein with 83% identity to the 1,173-amino-acid PAE1265 protein (Pcal_1601-full) (Fig. 2F). The stop codon at position 1489855 occurs approximately 500 nt upstream of what would be a molybdopterin-binding domain in the full-length Pcal_1601, if translated. Although the P. calidifontis culture used for whole-genome sequencing was grown from a single colony, the anomalous sequencing record showing the key GAG Glu codon suggests that this culture may have contained a subpopulation of cells with a variant Pcal_1601 locus encoding a full-length, functional ortholog of PAE1265.
We resequenced the Pcal_1601 locus in our laboratory stocks of P. calidifontis, which were directly derived from cultures used for whole-genome sequencing. The PCR amplification of the genomic DNA confirmed the presence of the TAG stop codon, with no evidence for alternative bases at position 1489855. We tested the growth of these P. calidifontis cultures with arsenate to determine whether a full-length ortholog of the PAE1265 protein is required for arsenate respiration. P. calidifontis grew very slowly and to low densities during the first two passages with arsenate as the only electron acceptor. No growth was observed when arsenate was omitted. Growth during subsequent passages with arsenate was more rapid and produced higher cell densities (≥108 cells/ml). After the sixth passage of P. calidifontis on arsenate, we extracted genomic DNA and sequenced the PCR-amplified Pcal_1601 locus again. This revealed a GAG Glu codon at position 1489855. No other differences relative to the reference sequence were observed in the 1-kb PCR amplicon, suggesting that the cultivation of P. calidifontis with arsenate as the only electron acceptor selected for a strain with a full-length ortholog of the proposed ARR encoded by PAE1265. We have isolated clonal cultures of each strain from single colonies and confirmed that only the strain with the full-length Pcal_1601 grows on arsenate. We are currently investigating the variant strains to describe these in more detail.
Upregulation of a putative thiosulfate or polysulfide reductase during anoxia.
Components of the putative membrane molybdopterin oxidoreductase encoded by PAE2859, PAE2860, and PAE2861, were specifically upregulated in P. aerophilum following the onset of anoxia in cultures lacking an alternative respiratory electron acceptor. These included the second and third time points in cultures that received no added terminal electron acceptor, and the last time point in the cultures that received additions of oxygen (Fig. 2B and E). The putative 91-kDa Mo-containing subunit encoded by PAE2859 contains a twin-arginine translocation signal and a MopB-thiosulfate-R-like domain that is conserved in thiosulfate, polysulfide, and sulfur reductases. The putative 31-kDa membrane subunit encoded by PAE2861 contains a domain that is conserved in membrane subunits of polysulfide reductases. Orthologs of the PAE2859-PAE2860-PAE2861 complex were found in the P. arsenaticum and P. calidifontis genomes, whereas P. islandicum and T. neutrophilus were missing the membrane subunit (Table 2). Northern analysis of the molybdopterin-containing subunit PAE2859 showed a single band of approximately 3.8 kb, a size consistent with the cotranscription of these three genes, and possibly a small 3′ untranslated region (PAE.i1535) (Fig. 2E).
The domain structure and expression patterns of this operon suggest that it is a respiratory reductase of an electron acceptor such as thiosulfate or polysulfide that is not used by P. aerophilum in the presence of oxygen, nitrate, arsenate or ferric iron. P. aerophilum is poisoned by the presence of elemental sulfur and grows weakly using thiosulfate as an electron acceptor (29, 65). Growth on polysulfide has not been tested. The basal growth medium contained 11 mM sulfate and no other sulfur compounds aside from those present as trace constituents of the yeast extract used as the carbon and energy source for growth. Physiological studies of Pyrobaculum species have shown that their growth with different terminal electron acceptors is optimized at different reducing potentials (21). This observation suggests that reducing potential may directly modulate the regulation of respiratory reductases in Pyrobaculum and might explain the upregulation of the PAE2859-PAE2861 molybdopterin oxidoreductase in the absence of its cognate substrate.
Limited upregulation during iron respiration.
Cultures shifted to ferric-citrate reduced approximately 3.5 mM Fe(III) to Fe(II) during the time course (see Fig. S1E in the supplemental material). A large number of genes were moderately upregulated in these cultures (see Fig. S4A in the supplemental material). However, we were unable to identify genes that exhibited strong and specific upregulation in response to iron, and positive transcriptional responses to iron (see Fig. S4A in the supplemental material) were generally weaker than the negative responses (see Fig. S5A in the supplemental material). A region containing three potential genes (PAE.i1354, PAE2526, and PAE2527) showed a strong initial response to iron that declined over the time course and opposite trends in cultures amended with oxygen or no electron acceptor (see Fig. S4A in the supplemental material). Other genes upregulated by iron exhibited increasing trends over the time course that were also apparent under other respiratory conditions. These included a putative 40.9-kDa monoheme c-type cytochrome (PAE0090), a putative thiosulfate sulfurtransferase (PAE2582), and a number of loci adjacent to each of these (see Fig. S4A in the supplemental material). Genes including a succinate dehydrogenase complex (PAE0716-PAE0725) (see Fig. S4B in the supplemental material) exhibited positive responses that appeared to be more specific but were also relatively modest.
Previous studies have shown that respiratory ferric reductase activity is regulated and primarily associated with the cytoplasmic cell fraction in P. aerophilum and P. islandicum (20, 21). Specialized membrane proteins and c-type cytochromes are important in bacterial iron respiration but were found to be lacking in iron-respiring Pyrobaculum cultures. We were similarly unable to identify a specific oxidoreductase for ferric iron respiration. It is possible that genes for respiratory iron reduction, like the genes in the nitrate reductase operon, were expressed under all conditions in our study with only modest differences between respiratory treatments. Alternatively, the lack of apparent specialization for ferric iron respiration may support the theory that respiratory iron reduction evolved early as a primitive process mediated by a variety of oxidoreductases and electron carriers rather than a single specific complex (47).
Transcriptional repression by iron.
A large proportion of genes that were differentially expressed between respiratory conditions at a statistically significant level were downregulated in cultures amended with ferric iron. A number of these have annotations suggesting roles in oxidative stress response or iron uptake and homeostasis. These included genes for superoxide dismutase (PAE0274), Mn-catalase (PAE2696), a putative antioxidant protein (PAE0877), a putative ABC transporter for Fe(III) (PAE2699), and a gene annotated as a bacterioferritin comigratory protein (PAE2732) (see Fig. S5A to S5D in the supplemental material). Genes for putative GTP cyclohydrolases (PAE1588-PAE1589, PAE2984-PAE2986), which are involved in folate and flavin biosynthesis, were also repressed in cultures amended with iron (see Fig. S5A, S5F, and S5G in the supplemental material).
The Mn-catalase (PAE2696) was the top-ranked gene in statistical analyses for differential expression between respiratory conditions (see Table S2C in the supplemental material) and was very strongly downregulated by iron (up to 40-fold compared to cultures induced with oxygen) (see Fig. S5A and S5C in the supplemental material). An examination of the catalase gene and an adjacent cluster of iron-repressed genes (PAE2697-PAE2702) revealed a strikingly conserved palindromic promoter motif (see Fig. S5J in the supplemental material). Three nearly identical versions of this motif are located directly over predicted basal regulatory elements for PAE2696, PAE2699, and PAE2700 (see Fig. S5K and S5L in the supplemental material). A genome-wide search for similar motifs revealed a perfect match in the promoter region for a predicted metal transporter that was upregulated at the end of the time course with nitrate and downregulated by iron (PAE0600) (see Fig. S2C and S5E in the supplemental material). No orthologs for PAE2697, PAE2699, or PAE2700 were found in the other Pyrobaculum genomes (Table 2). However, the promoter motif is largely conserved in regions upstream of the P. arsenaticum and P. calidifontis orthologs for PAE2696 (see Fig. S5L in the supplemental material) and PAE0600 (not shown).
Northern analysis of the putative Fe(III) transporter gene PAE2699 showed a band at approximately 0.8 kb, somewhat larger than the annotated size of 624 nt, and a band at 1.3 kb, consistent with the cotranscription of the downstream putative ABC transporter gene (PAE2697) (see Fig. S5D in the supplemental material). Northern analysis of the predicted metal transporter PAE0600 (see Fig. S5E in the supplemental material) showed a very similar expression pattern, with strong upregulation at the end of the time course with nitrate, when denitrification genes were induced, and downregulation after amendment with iron. Northern analysis also confirmed microarray data indicating that a ferritin-like gene (PAE2701) located between the PAE2696-PAE2700 cluster and another iron-repressed gene, PAE2702, was not downregulated by iron (see Fig. S5H in the supplemental material).
Evidence for an archaeal ferric uptake regulator system.
Iron concentrations in the P. aerophilum cultures amended with ferric citrate exceeded the concentration in the basal growth medium by more than 1,000-fold (∼9 mM compared to ∼7 μM). Iron is a cofactor for numerous enzymes and an essential nutrient for nearly all microorganisms. However, the co-occurrence of high intracellular Fe(II) and O2 can generate reactive oxygen species, requiring organisms to tightly regulate the uptake and storage of iron in the cell (8). In bacteria, many genes involved in iron homeostasis and oxidative stress are controlled by the ferric uptake regulator (Fur) protein, which binds to DNA and acts as a transcriptional repressor when complexed to Fe(II) (8, 30, 62).
The primary targets of transcriptional repression by bacterial Fur are remarkably similar to those found in this study to be repressed by iron in P. aerophilum: genes for the oxidative stress response and for the acquisition, transport, and storage of iron. GTP cyclohydrolases, which were repressed by iron in P. aerophilum, are involved in reductive iron assimilation in bacteria and may also be controlled by Fur (14, 68). Bacterial Fur systems are mechanistically complex, with posttranscriptional regulatory roles for small regulatory RNAs and for RNA chaperones that are similar to eukaryotic and archaeal small nuclear ribonucleoproteins (37, 38, 40). Whether similar Fur-regulated systems operate in archaea has not been directly investigated, but proteins with Fur-like domains can be found in more than a dozen archaeal genomes, including all those in the Pyrobaculum clade.
The E. coli Fur binding site consensus sequence has been alternatively modeled as an FFnR repeat of GATAAT (where F stands for the forward strand, R for the reverse, and n for any nucleotide) (19) and an FnR repeat of AATGATAAT, which shows good agreement with the consensus sites of Bacillus subtilis and Pseudomonas aeruginosa (12). The motif identified in the PAE2696-PAE2700 locus shows some consistency with both models. The putative P. aerophilum motif consists of the sequence ATTAAC repeating in the pattern FnnnR, which can be viewed in reverse complement as GTTAAT repeated RnnnF (see Fig. S5J in the supplemental material), remarkably similar to the bacterial GATAAT motif. Whether this motif is the target of the P. aerophilum Fur homolog remains to be determined. Fur is autoregulatory in many, but not all, bacteria, repressing its own promoter in the presence of iron (16, 15). However, no match for the PAE2696-PAE2700 promoter motif was found in the promoter region of the P. aerophilum Fur homolog (PAE2309), and the transcript was not strongly downregulated after induction with iron (see Table S2B and Fig. S5I in the supplemental material). Nevertheless, similarities with the bacterial Fur system suggest a potentially homologous regulation system in archaea.
A transcriptional map of crenarchaeal respiratory versatility.
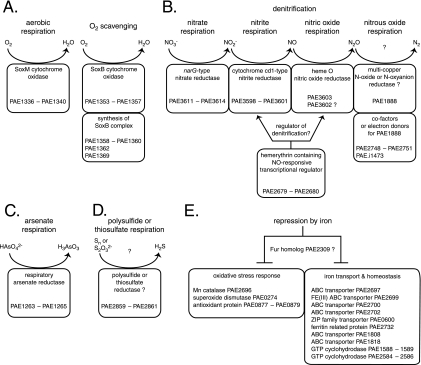
The results of this study provide a map of gene expression for multiple respiratory pathways in P. aerophilum and suggest a number of functional and regulatory predictions for further study. Two cytochrome oxidases were expressed in opposing patterns, suggesting that a SoxB-type complex is for oxygen scavenging and a SoxM-type complex is for aerobic growth (Fig. 3A). The nitrate reductase operon was expressed under all conditions examined. In contrast, genes for nitrite reductase and nitric oxide reductase were coordinately upregulated when nitrite accumulated to ∼1 mM and were coexpressed with a putative hemerythrin-containing transcriptional regulator that may control nitric oxide-responsive genes (Fig. 3B). A multicopper oxidase upregulated during nitrate respiration may play a specific role in denitrification, possibly as a nitrous oxide reductase (Fig. 3B). During arsenate respiration, P. aerophilum upregulated a molybdopterin oxidoreductase complex (Fig. 3C). A full-length ortholog of this complex appears to be required for the growth of P. calidifontis on arsenate, providing support for the proposed role as a crenarchaeal ARR. P. aerophilum expressed an alternative molybdopterin oxidoreductase operon, which may encode a thiosulfate or polysulfide reductase, in anoxic cultures lacking an alternative electron acceptor (Fig. 3D). Although a number of loci were moderately upregulated during ferric iron respiration, we were unable to identify genes specific for this process.
FIG. 3.
Summary schematic showing proposed roles for P. aerophilum genes in aerobic respiration and O2 scavenging (A), denitrification (B), arsenate respiration (C), polysulfide or thiosulfate respiration (D), and iron-mediated transcriptional repression (E).
P. aerophilum cultures shifted to growth with ∼9 mM iron exhibited transcriptional repression at many loci with annotations suggesting roles in iron homeostasis and oxidative stress (Fig. 3E). These responses are similar to those controlled by the ferric uptake regulator in bacteria and suggest either convergent evolution, an ancient global regulatory system, or some combination of the two. Evidence for an ancient Fur system would have interesting evolutionary and paleogeochemical implications. The bacterial Fur regulatory system apparently evolved to cope with iron scarcity caused by the low solubility of Fe(III) oxides in an oxidizing environment and the toxicity posed by intracellular Fe(II) and free O2. The presumed divergence of the Bacteria and Archaea occurred well before the global oxidation of the atmosphere and oceans 2.3 to 2.7 billion years ago, when soluble ferrous iron was plentiful and free O2 was scarce. However, based on the phylogeny of cytochrome oxidases, it has been proposed that the capacity for aerobic growth evolved in the last universal ancestor of Bacteria and Archaea prior to the broader oxidation of the biosphere, within localized environments enriched in O2 (10, 11). A conserved, vertically transmitted Fur system in the Archaea might provide additional support for evolutionary adaptation to oxidizing environments in the last universal ancestor and therefore an early emergence of free O2.
Supplementary Material
Acknowledgments
Support for this work was provided in part by an NSF graduate research fellowship to A.E.C. and an Alfred P. Sloan research fellowship to T.M.L. We thank Jeffrey H. Miller for providing funding for the oligonucleotide primers and other materials used in the construction of the DNA microarrays.
We thank the Department of Energy's Community Sequencing Program and the other members of the Pyrobaculum Sequencing Consortium (Chad Saltikov, Christopher House, and Sorel Fitz-Gibbon) for making comparative genomics possible in Pyrobaculum. We thank Jeffrey Savas, Lily Shiu, and Sofie Salama for their help in constructing the microarrays and Rob Franks and Carolina Reyes for assistance with analytical chemistry. Finally, we thank Chad Saltikov and Jonathan Trent for helpful discussions about the manuscript.
Footnotes
Published ahead of print on 1 December 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Afshar, S., E. Johnson, S. de Vries, and I. Schroder. 2001. Properties of a thermostable nitrate reductase from the hyperthermophilic archaeon Pyrobaculum aerophilum. J. Bacteriol. 1835491-5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afshar, S., C. Kim, H. G. Monbouquette, and I. I. Schroder. 1998. Effect of tungstate on nitrate reduction by the hyperthermophilic archaeon Pyrobaculum aerophilum. Appl. Environ. Microbiol. 643004-3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amo, T., H. Atomi, and T. Imanaka. 2003. Biochemical properties and regulated gene expression of the superoxide dismutase from the facultatively aerobic hyperthermophile Pyrobaculum calidifontis. J. Bacteriol. 1856340-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amo, T., H. Atomi, and T. Imanaka. 2002. Unique presence of a manganese catalase in a hyperthermophilic archaeon, Pyrobaculum calidifontis VA1. J. Bacteriol. 1843305-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amo, T., M. Paje, A. Inagaki, S. Ezaki, H. Atomi, and T. Imanaka. 2002. Pyrobaculum calidifontis sp. nov., a novel hyperthermophilic archaeon that grows in atmospheric air. Archaea 1113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson, R. K., M. Thompson, and E. Culbard. 1986. Selective reduction of arsenic species by continuous hydride generation. 1. Reaction media. Analyst 1111143-1152. [Google Scholar]
- 8.Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27215-237. [DOI] [PubMed] [Google Scholar]
- 9.Bendtsen, J. D., H. Nielsen, D. Widdick, T. Palmer, and S. Brunak. 2005. Prediction of twin-arginine signal peptides. BMC Bioinformatics 6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castresana, J. 2001. Comparative genomics and bioenergetics. Biochim. Biophys. Acta 1506147-162. [DOI] [PubMed] [Google Scholar]
- 11.Castresana, J., and D. Moreira. 1999. Respiratory chains in the last common ancestor of living organisms. J. Mol. Evol. 49453-460. [DOI] [PubMed] [Google Scholar]
- 12.Chen, Z., K. A. Lewis, R. K. Shultzaberger, I. G. Lyakhov, M. Zheng, B. Doan, G. Storz, and T. D. Schneider. 2007. Discovery of Fur binding site clusters in Escherichia coli by information theory models. Nucleic Acids Res. 356762-6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crooks, G. E., G. Hon, J. M. Chandonia, and S. E. Brenner. 2004. WebLogo: a sequence logo generator. Genome Res. 141188-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crossley, R. A., D. J. Gaskin, K. Holmes, F. Mulholland, J. M. Wells, D. J. Kelly, A. H. van Vliet, and N. J. Walton. 2007. Riboflavin biosynthesis is associated with assimilatory ferric reduction and iron acquisition by Campylobacter jejuni. Appl. Environ. Microbiol. 737819-7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delany, I., G. Spohn, A. B. Pacheco, R. Ieva, C. Alaimo, R. Rappuoli, and V. Scarlato. 2002. Autoregulation of Helicobacter pylori Fur revealed by functional analysis of the iron-binding site. Mol. Microbiol. 461107-1122. [DOI] [PubMed] [Google Scholar]
- 16.De Lorenzo, V., M. Herrero, F. Giovannini, and J. B. Neilands. 1988. Fur (ferric uptake regulation) protein and CAP (catabolite-activator protein) modulate transcription of fur gene in Escherichia coli. Eur. J. Biochem. 173537-546. [DOI] [PubMed] [Google Scholar]
- 17.de Vries, S., and I. Schroder. 2002. Comparison between the nitric oxide reductase family and its aerobic relatives, the cytochrome oxidases. Biochem. Soc. Trans. 30662-667. [DOI] [PubMed] [Google Scholar]
- 18.de Vries, S., M. J. Strampraad, S. Lu, P. Moenne-Loccoz, and I. Schroder. 2003. Purification and characterization of the MQH2:NO oxidoreductase from the hyperthermophilic archaeon Pyrobaculum aerophilum. J. Biol. Chem. 27835861-35868. [DOI] [PubMed] [Google Scholar]
- 19.Escolar, L., J. Perez-Martin, and V. de Lorenzo. 1998. Binding of the Fur (ferric uptake regulator) repressor of Escherichia coli to arrays of the GATAAT sequence. J. Mol. Biol. 283537-547. [DOI] [PubMed] [Google Scholar]
- 20.Feinberg, L. F., and J. F. Holden. 2006. Characterization of dissimilatory Fe(III) versus NO3− reduction in the hyperthermophilic archaeon Pyrobaculum aerophilum. J. Bacteriol. 188525-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feinberg, L. F., R. Srikanth, R. W. Vachet, and J. F. Holden. 2008. Constraints on anaerobic respiration in the hyperthermophilic archaea Pyrobaculum islandicum and Pyrobaculum aerophilum. Appl. Environ. Microbiol. 74396-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer, F., W. Zillig, K. O. Stetter, and G. Schreiber. 1983. Chemolithoautotrophic metabolism of anaerobic extremely thermophilic archaebacteria. Nature 301511-513. [DOI] [PubMed] [Google Scholar]
- 23.Fitz-Gibbon, S. T., H. Ladner, U. J. Kim, K. O. Stetter, M. I. Simon, and J. H. Miller. 2002. Genome sequence of the hyperthermophilic crenarchaeon Pyrobaculum aerophilum. Proc. Natl. Acad. Sci. USA 99984-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forte, E., A. Urbani, M. Saraste, P. Sarti, M. Brunori, and A. Giuffre. 2001. The cytochrome cbb3 from Pseudomonas stutzeri displays nitric oxide reductase activity. Eur. J. Biochem. 2686486-6491. [DOI] [PubMed] [Google Scholar]
- 25.Gentleman, R. C., V. J. Carey, D. M. Bates, B. Bolstad, M. Dettling, S. Dudoit, B. Ellis, L. Gautier, Y. Ge, J. Gentry, K. Hornik, T. Hothorn, W. Huber, S. Iacus, R. Irizarry, F. Leisch, C. Li, M. Maechler, A. J. Rossini, G. Sawitzki, C. Smith, G. Smyth, L. Tierney, J. Y. Yang, and J. Zhang. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giuffre, A., G. Stubauer, P. Sarti, M. Brunori, W. G. Zumft, G. Buse, and T. Soulimane. 1999. The heme-copper oxidases of Thermus thermophilus catalyze the reduction of nitric oxide: evolutionary implications. Proc. Natl. Acad. Sci. USA 9614718-14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henninger, T., S. Anemuller, S. Fitz-Gibbon, J. H. Miller, G. Schafer, and C. L. Schmidt. 1999. A novel Rieske iron-sulfur protein from the hyperthermophilic crenarchaeon Pyrobaculum aerophilum: sequencing of the gene, expression in E. coli and characterization of the protein. J. Bioenerg. Biomembr. 31119-128. [DOI] [PubMed] [Google Scholar]
- 28.Huber, R., J. K. Kristjansson, and K. O. Stetter. 1987. Pyrobaculum gen. nov., a new genus of neutrophilic, rod-shaped archaebacteria from continental solfataras growing optimally at 100°C. Arch. Microbiol. 14995-101. [Google Scholar]
- 29.Huber, R., M. Sacher, A. Vollmann, H. Huber, and D. Rose. 2000. Respiration of arsenate and selenate by hyperthermophilic archaea. Syst. Appl. Microbiol. 23305-314. [DOI] [PubMed] [Google Scholar]
- 30.Kadner, R. J. 2005. Regulation by iron: RNA rules the rust. J. Bacteriol. 1876870-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kashefi, K., and D. R. Lovley. 2000. Reduction of Fe(III), Mn(IV), and toxic metals at 100°C by Pyrobaculum islandicum. Appl. Environ. Microbiol. 661050-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, X., D. L. Brutlag, and J. S. Liu. 2001. BioProspector: discovering conserved DNA motifs in upstream regulatory regions of co-expressed genes. Pac. Symp. Biocomput. 6127-138. [PubMed] [Google Scholar]
- 33.Lubben, M., S. Arnaud, J. Castresana, A. Warne, S. P. Albracht, and M. Saraste. 1994. A second terminal oxidase in Sulfolobus acidocaldarius. Eur. J. Biochem. 224151-159. [DOI] [PubMed] [Google Scholar]
- 34.Lubben, M., B. Kolmerer, and M. Saraste. 1992. An archaebacterial terminal oxidase combines core structures of two mitochondrial respiratory complexes. EMBO J. 11805-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malasarn, D., C. W. Saltikov, K. M. Campbell, J. M. Santini, J. G. Hering, and D. K. Newman. 2004. arrA is a reliable marker for As(V) respiration. Science 306455. [DOI] [PubMed] [Google Scholar]
- 36.Marchler-Bauer, A., J. B. Anderson, P. F. Cherukuri, C. DeWeese-Scott, L. Y. Geer, M. Gwadz, S. He, D. I. Hurwitz, J. D. Jackson, Z. Ke, C. J. Lanczycki, C. A. Liebert, C. Liu, F. Lu, G. H. Marchler, M. Mullokandov, B. A. Shoemaker, V. Simonyan, J. S. Song, P. A. Thiessen, R. A. Yamashita, J. J. Yin, D. Zhang, and S. H. Bryant. 2005. CDD: a conserved domain database for protein classification. Nucleic Acids Res. 33D192-D196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masse, E., and S. Gottesman. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. USA 994620-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masse, E., C. K. Vanderpool, and S. Gottesman. 2005. Effect of RyhB small RNA on global iron use in Escherichia coli. J. Bacteriol. 1876962-6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer-Dombard, D. R., E. L. Shock, and J. P. Amend. 2005. Archaeal and bacterial communities in geochemically diverse hot springs of Yellowstone National Park, USA. Geobiology 3211-227. [Google Scholar]
- 40.Moller, T., T. Franch, P. Hojrup, D. R. Keene, H. P. Bachinger, R. G. Brennan, and P. Valentin-Hansen. 2002. Hfq: a bacterial Sm-like protein that mediates RNA-RNA interaction. Mol. Cell 923-30. [DOI] [PubMed] [Google Scholar]
- 41.Moreno-Vivian, C., P. Cabello, M. Martinez-Luque, R. Blasco, and F. Castillo. 1999. Prokaryotic nitrate reduction: molecular properties and functional distinction among bacterial nitrate reductases. J. Bacteriol. 1816573-6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niederberger, T. D., R. S. Ronimus, and H. W. Morgan. 2008. The microbial ecology of a high-temperature near-neutral spring situated in Rotorua, New Zealand. Microbiol. Res. 163594-603. [DOI] [PubMed] [Google Scholar]
- 43.Nocek, J. M., D. M. Kurtz, Jr., J. T. Sage, Y. M. Xia, P. Debrunner, A. K. Shiemke, J. Sanders-Loehr, and T. M. Loehr. 1988. Nitric oxide adducts of the binuclear iron site of hemerythrin: spectroscopy and reactivity. Biochemistry 271014-1024. [DOI] [PubMed] [Google Scholar]
- 44.Nunoura, T., Y. Sako, T. Wakagi, and A. Uchida. 2005. Cytochrome aa3 in facultatively aerobic and hyperthermophilic archaeon Pyrobaculum oguniense. Can. J. Microbiol. 51621-627. [DOI] [PubMed] [Google Scholar]
- 45.Nunoura, T., Y. Sako, T. Wakagi, and A. Uchida. 2003. Regulation of the aerobic respiratory chain in the facultatively aerobic and hyperthermophilic archaeon Pyrobaculum oguniense. Microbiology 149673-688. [DOI] [PubMed] [Google Scholar]
- 46.Phillips, E. J. P., and D. R. Lovley. 1987. Determination of Fe(III) and Fe(II) in oxalate extracts of sediment. Soil Sci. Soc. Am. J. 51938-941. [Google Scholar]
- 47.Richardson, D. J. 2000. Bacterial respiration: a flexible process for a changing environment. Microbiology 146551-571. [DOI] [PubMed] [Google Scholar]
- 48.Rozen, S., and H. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132365-386. [DOI] [PubMed] [Google Scholar]
- 49.Sako, Y., T. Nunoura, and A. Uchida. 2001. Pyrobaculum oguniense sp. nov., a novel facultatively aerobic and hyperthermophilic archaeon growing at up to 97 degrees C. Int. J. Syst. Evol. Microbiol. 51303-309. [DOI] [PubMed] [Google Scholar]
- 50.Saltikov, C. W., and D. K. Newman. 2003. Genetic identification of a respiratory arsenate reductase. Proc. Natl. Acad. Sci. USA 10010983-10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 52.Saraste, M., and J. Castresana. 1994. Cytochrome oxidase evolved by tinkering with denitrification enzymes. FEBS Lett. 3411-4. [DOI] [PubMed] [Google Scholar]
- 53.Schneider, K. L., K. S. Pollard, R. Baertsch, A. Pohl, and T. M. Lowe. 2006. The UCSC Archaeal Genome Browser. Nucleic Acids Res. 34D407-D410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simon, J. 2002. Enzymology and bioenergetics of respiratory nitrite ammonification. FEMS Microbiol. Rev. 26285-309. [DOI] [PubMed] [Google Scholar]
- 55.Simon, J., O. Einsle, P. M. Kroneck, and W. G. Zumft. 2004. The unprecedented nos gene cluster of Wolinella succinogenes encodes a novel respiratory electron transfer pathway to cytochrome c nitrous oxide reductase. FEBS Lett. 5697-12. [DOI] [PubMed] [Google Scholar]
- 56.Stein, L. Y., D. J. Arp, P. M. Berube, P. S. Chain, L. Hauser, M. S. Jetten, M. G. Klotz, F. W. Larimer, J. M. Norton, H. J. Op den Camp, M. Shin, and X. Wei. 2007. Whole-genome analysis of the ammonia-oxidizing bacterium, Nitrosomonas eutropha C91: implications for niche adaptation. Environ. Microbiol. 92993-3007. [DOI] [PubMed] [Google Scholar]
- 57.Storey, J. D., J. Y. Dai, and J. T. Leek. 2007. The optimal discovery procedure for large-scale significance testing, with applications to comparative microarray experiments. Biostatistics 8414-432. [DOI] [PubMed] [Google Scholar]
- 58.Storey, J. D., W. Xiao, J. T. Leek, R. G. Tompkins, and R. W. Davis. 2005. Significance analysis of time course microarray experiments. Proc. Natl. Acad. Sci. USA 10212837-12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sturn, A., J. Quackenbush, and Z. Trajanoski. 2002. Genesis: cluster analysis of microarray data. Bioinformatics 18207-208. [DOI] [PubMed] [Google Scholar]
- 60.Sulavik, M. C., L. F. Gambino, and P. F. Miller. 1995. The MarR repressor of the multiple antibiotic resistance (mar) operon in Escherichia coli: prototypic member of a family of bacterial regulatory proteins involved in sensing phenolic compounds. Mol. Med. 1436-446. [PMC free article] [PubMed] [Google Scholar]
- 61.Takai, K., and K. Horikoshi. 1999. Molecular phylogenetic analysis of archaeal intron-containing genes coding for rRNA obtained from a deep-subsurface geothermal water pool. Appl. Environ. Microbiol. 655586-5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Touati, D., M. Jacques, B. Tardat, L. Bouchard, and S. Despied. 1995. Lethal oxidative damage and mutagenesis are generated by iron in Δfur mutants of Escherichia coli: protective role of superoxide dismutase. J. Bacteriol. 1772305-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tusher, V. G., R. Tibshirani, and G. Chu. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 985116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Oost, J., A. P. de Boer, J. W. de Gier, W. G. Zumft, A. H. Stouthamer, and R. J. van Spanning. 1994. The heme-copper oxidase family consists of three distinct types of terminal oxidases and is related to nitric oxide reductase. FEMS Microbiol. Lett. 1211-9. [DOI] [PubMed] [Google Scholar]
- 65.Volkl, P., R. Huber, E. Drobner, R. Rachel, S. Burggraf, A. Trincone, and K. O. Stetter. 1993. Pyrobaculum aerophilum sp. nov., a novel nitrate-reducing hyperthermophilic archaeum. Appl. Environ. Microbiol. 592918-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whittaker, M. M., and J. W. Whittaker. 2000. Recombinant superoxide dismutase from a hyperthermophilic archaeon, Pyrobaculum aerophilum. J. Biol. Inorg. Chem. 5402-408. [PubMed] [Google Scholar]
- 67.Wood, E. D., F. A. J. Armstrong, and F. A. Richards. 1967. Determination of nitrate in sea water by cadmium-copper reduction to nitrite. J. Mar. Biol. Assoc. UK 4723-31. [Google Scholar]
- 68.Worst, D. J., M. M. Gerrits, C. M. Vandenbroucke-Grauls, and J. G. Kusters. 1998. Helicobacter pylori ribBA-mediated riboflavin production is involved in iron acquisition. J. Bacteriol. 1801473-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zumft, W. G. 2002. Nitric oxide signaling and NO dependent transcriptional control in bacterial denitrification by members of the FNR-CRP regulator family. J. Mol. Microbiol. Biotechnol. 4277-286. [PubMed] [Google Scholar]
- 70.Zumft, W. G., and P. M. Kroneck. 2007. Respiratory transformation of nitrous oxide (N2O) to dinitrogen by Bacteria and Archaea. Adv. Microb. Physiol. 52107-227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.