Abstract
Microbial arsenate respiration can enhance arsenic release from arsenic-bearing minerals—a process that can cause arsenic contamination of water. In Shewanella sp. strain ANA-3, the arsenate respiration genes (arrAB) are induced under anaerobic conditions with arsenate and arsenite. Here we report how genes that encode anaerobic regulator (arcA and etrA [fnr homolog]) and carbon catabolite repression (crp and cya) proteins affect arsenate respiration in ANA-3. Transcription of arcA, etrA, and crp in ANA-3 was similar in cells grown on arsenate and cells grown under aerobic conditions. ANA-3 strains lacking arcA and etrA showed minor to moderate growth defects, respectively, with arsenate. However, crp was essential for growth on arsenate. In contrast to the wild-type strain, arrA was not induced in the crp mutant in cultures shifted from aerobic to anaerobic conditions containing arsenate. This indicated that cyclic AMP (cAMP)-cyclic AMP receptor (CRP) activates arr operon transcription. Computation analysis for genome-wide CRP binding motifs identified a putative binding motif within the arr promoter region. This was verified by electrophoretic mobility shift assays with cAMP-CRP and several DNA probes. Lastly, four putative adenylate cyclase (cya) genes were identified in the genome. One particular cya-like gene was differentially expressed under aerobic versus arsenate respiration conditions. Moreover, a double mutant lacking two of the cya-like genes could not grow with arsenate as a terminal electron acceptor; exogenous cAMP could complement growth of the double cya mutant. It is concluded that the components of the carbon catabolite repression system are essential to regulating arsenate respiratory reduction in Shewanella sp. strain ANA-3.
Chronic consumption of well water contaminated with arsenic has become a global public health crisis of epidemic proportions (3). In most cases, arsenic contamination originates from natural geological sources (36). Elevated levels of dissolved arsenic in contaminated sediments and aquifers are generally associated with low oxygen tensions (2, 13, 26, 28). Under these conditions, bacteria are able to utilize arsenate as a terminal electron acceptor, reducing it to arsenite, which has greater toxicity and hydrological mobility than arsenate. Microbial respiratory reduction of arsenate is likely a major pathway that leads to the accumulation of toxic arsenite in groundwater (6).
The biochemical mechanism of reduction of arsenate as a terminal electron acceptor involves a molybdenum-containing terminal reductase, ArrA, and a Fe-S subunit, ArrB (1, 18, 21). In Shewanella sp. strain ANA-3, ArrA and ArrB are encoded by a two-gene operon, arrAB (32). Moreover, a membrane-associated tetraheme c-type cytochrome is also required for growth on arsenate as a terminal electron acceptor (25). Little is know about how arr operons are regulated in arsenate-respiring bacteria. In Shewanella sp. strain ANA-3, the expression of the arr operon is greatest in arsenate-grown cells (33). Arsenite is also a strong inducer of arrA expression, but only in anaerobically grown cells. Oxygen and nitrate repress arrA expression. A small arsenite-binding repressor, ArsR, mediates the arsenite-dependent regulation of the Shewanella sp. strain ANA-3 arr operon (J. N. Murphy and C. W. Saltikov, unpublished results).
The oxygen-dependent regulation of arr follows expression patterns similar to those of pathways regulated by redox-sensing regulators like FNR and the two-component sensor/response regulator proteins ArcB and ArcA, respectively (22, 37). Previous work with non-arsenate-respiring Shewanella oneidensis MR-1 has shown that regulation of anaerobic respiration relies mostly on the global regulator cyclic AMP (cAMP) receptor protein (CRP) (30). FNR (referred to as EtrA in Shewanella) and ArcA play less critical roles in regulating pathways for anaerobic electron acceptor utilization in Shewanella (4, 9, 12, 20). The molecular details of EtrA-, ArcA-, and CRP-dependent regulation of anaerobic respiratory pathways in Shewanella are not well understood. In this study, we investigated the functional roles of etrA, arcA, crp, adenylate cyclase-encoding genes (cya), and cAMP-CRP in the regulation and physiology of anaerobic respiration by using the arsenate respiratory reduction pathway of Shewanella sp. strain ANA-3 as a model system.
MATERIALS AND METHODS
Strains and plasmids.
All of the Escherichia coli and Shewanella strains and plasmids used in this study are described in Table 1. E. coli strain BL21 and pGEX-6P-2 were kindly provided by Karen Ottemann, University of California, Santa Cruz.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or markers; characteristics and uses | Source or reference |
|---|---|---|
| E. coli strains | ||
| UQ950 | DH5α λ(pir) host for cloning; F− Δ(argF-lac)169 φ80dlacZ58(ΔM15) glnV44(AS) rfbD1 gyrA96(Nalr) recA1 endA1 spoT1 thi-1 hsdR17 deoR λpir+ | 32 |
| WM3064 | Donor strain for conjugation; thrB1004 pro thi rpsL hsdS lacZΔM15 RP4_1360 Δ(araBAD)567 ΔdapA1341::[erm pir(wt)] | 32 |
| BL21 | F−ompT gal dcm lon hsdSB(rB− mB−) λ(DE3) | GE Healthcare |
| Shewanella strains | ||
| ANA-3 | Isolated from an As-treated wooden pier piling in a brackish estuary (Eel Pond, Woods Hole, MA) | 31 |
| AN-CRP1 | Shewanella sp. strain ANA-3; Δcrp (Shewana3_0617) | This study |
| AN-ETRA1 | Shewanella sp. strain ANA-3; ΔetrA (Shewana3_2115) | This study |
| AN-ARCA1 | Shewanella sp. strain ANA-3; ΔarcA (Shewana3_0650) | This study |
| AN-CYA0387 | Shewanella sp. strain ANA-3; ΔShewana3_0387, predicted adenylate cyclase | This study |
| AN-CYA0814/0815 | Shewanella sp. strain ANA-3; ΔShewana3_0814/0815, predicted adenylate cyclase | This study |
| AN-CYA0823 | Shewanella sp. strain ANA-3; ΔShewana3_0823, predicted adenylate cyclase | This study |
| AN-CYA3045 | Shewanella sp. strain ANA-3; ΔShewana3_3045, predicted adenylate cyclase | This study |
| AN-CYA0387/3045 | Shewanella sp. strain ANA-3; ΔShewana3_0387 ΔShewana3_3045 | This study |
| AN-CYA-ALL | Shewanella sp. strain ANA-3; ΔShewana3_0387, ΔShewana3_0814-0815, ΔShewana3_0823, ΔShewana3_3045 | This study |
| Plasmids/vectors | ||
| pSMV10 | 9.1-kb mobilizable suicide vector; oriR6K mobRP4 sacB Kmr Gmr | 32 |
| pBBR1-MCS2 | 5.1-kb broad-host-range plasmid; KmrlacZ | 17 |
| pGEX-6P-2 | Overexpression vector with the tac promoter; generates N-terminal GST fusions, has PreScission protease site to cleave GST fusion from protein product; Ampr | GE Healthcare |
| pGEX-crp | Shewanella sp. strain ANA-3 crp gene cloned into BamHI site of pGEX-6P-2 | This study |
| pCRP | ANA-3 crp PCR fragment cloned into SpeI site of pBBR1MCS-2 | This study |
| pEtrA | ANA-3 etrA PCR fragment cloned into SpeI site of pBBR1MCS-2 | This study |
| pArcA | ANA-3 arcA PCR fragment cloned into SpeI site of pBBR1MCS-2 | This study |
Growth conditions.
E. coli strains were grown in Luria-Bertani (LB) medium or 2X YT medium (described in reference 34). Shewanella sp. strain ANA-3 was grown at 30°C in LB or minimal salts medium (referred to as TME medium) consisting of 1.5 g liter−1 NH4Cl, 0.6 g liter−1 NaHPO4, 0.1 g liter−1 KCl, 0.5 g liter−1 yeast extract, 10 mM HEPES, 20 mM lactate, and 10 ml liter−1 each trace mineral and vitamin solutions, pH 7 (described in reference 25). ANA-3 aerobic liquid cultures were shaken at 250 rpm. Preparation of anaerobic medium, electron acceptors, medium additions, and anaerobic culturing were done as previously described (25).
Growth experiments.
Aerobic cultures were grown overnight in TME medium. The optical densities at 600 nm (OD600) of the cultures were adjusted to below 0.6 and standardized to each other by the addition of medium to ensure that the inoculation levels of all of the strains were equal. Cells were then inoculated at a 1/100 dilution into anaerobic Balch tubes containing 10 ml medium or, for aerobic growth curves, into 250-ml flasks with 20 ml medium (shaken at 250 rpm). Growth was monitored with a Spectronic 20D+. Control cultures were also grown and monitored in anaerobic medium without electron acceptors.
Mutagenesis.
In-frame, nonpolar deletions of arcA (Shewana3_0650), crp (Shewana3_0617), etrA (Shewana3_2115), cya-0387 (Shewana3_0387), cya-0814 (Shewana3_0814), cya-0823 (Shewana3_0823), and cya-3045 (Shewana3_3045) were generated by previously developed methods (25, 31, 32); for the primers used, see Table S1 in the supplemental material. Because an insertion element (Shewana3_0815) overlapped the 3′ end of cya-0814, the deletion also included the insertion element. To generate stains with multiple deletions, the plasmid carrying the new deletion was conjugated into the appropriate Shewanella sp. strain ANA-3 null mutants.
Complementation plasmids pArcA, pCRP, and pEtrA were generated by cloning PCR products of the genes into the SpeI site of pBBR1-MCS2; for the primers used, see Table S1 in the supplemental material. Conjugation of the complementation vectors into Shewanella sp. strain ANA-3 was performed as previously described (31, 32). All plasmid constructs were verified by PCR, restriction mapping, and sequencing.
Quantification of gene transcription.
The methods for culturing, preparing cells, RNA extraction, and quantitative reverse transcriptase PCR (RT-PCR) have been described previously (25, 33); for the primers used, see Table S1 in the supplemental material. Real-time PCR mixtures (30 μl) consisted of 15 μl 2X HotStart SybrGreen PCR Master Mix (New England BioLabs), 300 nM primers (see Table S1 in the supplemental material), and 4 μl of diluted cDNA (1/4 dilution in nuclease-free water). Reaction products were analyzed with an MJ Research Opticon 2 real-time PCR machine. The thermocycler profile consisted of 95°C for 10 min; 30 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s; and a final extension of 72°C for 5 min.
For aerobic-anaerobic shifting studies, overnight cultures of wild-type and AN-CRP null mutant ANA-3 strains were grown aerobically in TME medium (pH 7), inoculated in duplicate into 20 ml similar medium, and grown aerobically to an OD600 of 0.1. Cultures were then sparged with nitrogen for 10 min, transferred to an anaerobic chamber, and amended with 10 mM arsenate. Samples (1 ml) were removed for RNA extraction (described in reference 25). Arsenate and arsenite analyses of culture filtrates were done by inductively coupled plasma-optical emission spectroscopy and NaBH4-based arsenic speciation methodology (19). RT-PCR and agarose gel electrophoresis were used to monitor arrA and 16S rRNA gene expression at several time points during the aerobic-anaerobic shift experiment. The efficiency of DNase treatment of RNA extracts was assessed from control cDNA reaction mixtures without RT. The RT-PCR products were analyzed on a 2% agarose gel. The intensities of the RT-PCR DNA bands were quantified with the ImageJ software (http://rsb.info.nih.gov/ij/). For semiquantitative analysis, the arrA DNA intensities were normalized to that of the corresponding 16S rRNA gene DNA band.
Protein purification.
A bacterial expression vector for crp was generated by fusing the N-terminal domain of CRP to glutathione S-transferase (GST). The crp gene was amplified with primers crp-BamHI-F1 (5′-GGG GAT CCA TGG CTC TGA TTG GTA AGC-3′) and crp-BamHI-R1 (5′-GGG GAT CCT TAA CGG GTA CCA TAT AC-3′) (BamHI sites are underlined). Products were digested with BamHI and cloned into the BamHI site of overexpression plasmid pGEX-6P-2 to create pGEX-crp. This plasmid was transformed into E. coli BL21 and plated onto LB agar containing ampicillin (100 μg/ml).
Cells carrying the plasmid were grown in 4 liters of 2X YT medium at 37°C until they reached an OD600 of 0.8 to 1.0. The temperature of the culture was brought down to 28°C, and the cells were induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Cells were harvested by centrifugation after 3 h and immediately scraped out and ground to a fine powder with a mortar and pestle under liquid nitrogen. The powdered cells were stored at −80°C until lysis could be performed.
The pulverized cells were placed on ice and resuspended in 5 volumes (wt/vol) of phosphate-buffered saline (PBS) containing 0.5% Tween 20, 1 M NaCl, 1 mM (2-aminoethyl)benzenesulfonyl fluoride (ABESF), and 10 mM dithiothreitol (DTT). The suspension was mixed for 3 to 5 min at 4°C, lysed by sonication for 1 min, and centrifuged at 12,000 × g for 15 min at 4°C. The supernatant was recovered and centrifuged for 1.5 h at 150,000 × g at 4°C. The membrane-free supernatant was loaded onto a 5- to 10-ml glutathione-agarose column (Sigma G-4510) with a fast protein liquid chromatograph (Bio-Rad). The column was washed with 1× PBS buffer containing 0.05% Tween 20, 0.5 mM DTT, and 0.25 M KCl until there was no protein washing off the column. The column was washed with 2 volumes of 1× PBS buffer containing 0.5 mM DTT and 0.25 mM KCl. The protein was eluted with a 50 mM Tris-HCl buffer, pH 8.1, containing 0.25 M KCl and 5 mM reduced glutathione into 1-ml fractions. Fractions were assayed, and peak fractions were pooled and dialyzed into 50 mM Tris-HCl buffer containing 150 mM NaCl and 1 mM DTT, pH 7.5. The amount of dialyzed GST-protein fusion was calculated, and 2 U of PreScission protease (GE Healthcare) was added for every 100 μg of GST-protein fusion; the mixture was allowed to incubate for 4 h at 5°C. Lysate was loaded onto the 5- to 10-ml glutathione column and then washed with dialysis buffer into 1-ml fractions and then with 50 mM Tris-HCl buffer, pH 8.1, containing 0.25 M KCl and 5 mM reduced glutathione to clean the column of GST. Sample fractions were analyzed for purity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and peak fractions were pooled, the protein concentration was calculated, and the fractions were stored in 20% glycerol at −80°C. CRP was concentrated with a Microcon centrifugal filter device (Amicon).
Electrophoretic mobility shift assay (EMSA).
Cy3-labeled (*) DNA probes were generated (for the primers used, see Table S1 in the supplemental material). The F1R1 probe was PCR synthesized with arsRbind-F1* and arsRbind-R1; the probe was purified and concentrated with the QIAquick gel extraction kit according to the manufacturer's PCR purification instructions. The F1R4 probe was generated by PCR with primers arsRbind-F1 and arsRbind-R3 to first generate F1R3. This product was purified and used as the DNA template at a 1:25 dilution in a seminested PCR with arsRbind-F1* and arsRbind-R4 to generate the F1R4 fluorescent probe, which was purified and concentrated as described above. An EMSA was performed with a final volume of 20 μl containing 10 mM Tris-Cl (pH 7.6), 0.5 mM Na2EDTA (pH 8.0), 10% glycerol, 100 mM KCl, 50 μg/ml bovine serum albumin, 50 μg/ml poly(dI-dC), 0.1 pmol DNA probe, 50 μM cAMP (unless otherwise noted), and different amounts of purified protein. The mixtures were incubated on ice for 30 min and loaded onto a nondenaturing 5% polyacrylamide gel. Electrophoresis was carried out at 4°C and 95 V in 0.5× Tris-borate-EDTA buffer. Gels were photographed on a Typhoon variable-mode imager (Amersham Biosciences).
CRP binding site motif analysis.
A 20-nucleotide position-specific frequency matrix for the CRP binding site was obtained from the Prodoric database of prokaryotic gene regulatory regions (23, 24) (Prodoric matrix accession no. MX000093). This CRP binding site motif was derived from 210 known E. coli CRP binding sites (39). A background null motif of the Shewanella sp. strain ANA-3 genome was computed by sliding a 20-nucleotide window across the entire genome and, for each position in the genome, computing the base frequencies for each position in the window starting at that position. A scan for putative binding sites was made by computing, for each position in the Shewanella sp. strain ANA-3 genome, the log likelihood ratio of the CRP binding site motif to the background null motif. According to the following equation, for each xi nucleotide in 20-nucleotide word x in the genome, the score is computed as the log2 of the ratio of the probability (P) of seeing word x given the CRP binding motif (θCRP) to the probability of seeing word x given the null background motif (θnull):
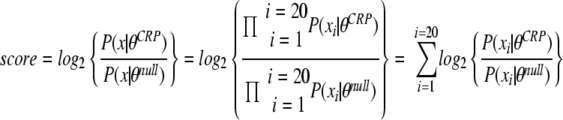 |
Positions with a score above 10 bits were saved as potential candidate binding sites.
RESULTS
Transcription patterns of global regulators.
We focused on three potential genes as possible regulators for the arr operon, arcA (Shewana3_0650), etrA (Shewana3_2115), and crp (Shewana3_0617). These were chosen because previous genetic, microarray, and bioinformatic studies demonstrated the importance of arcA, etrA, and crp as likely global regulators of metabolism in non-arsenate-respiring S. oneidensis MR-1 (4, 12, 20, 30); strain MR-1 lacks the arsenate respiratory reductase gene cluster arrAB and cannot grow with arsenate as a terminal electron acceptor. An initial characterization of the transcription patterns of arcA, etrA, and crp was done for Shewanella sp. strain ANA-3 grown aerobically and with different terminal electron acceptors (Table 2). In general, crp expression was 2 to 4 and 12 to 26 times greater than arcA and etrA gene expression, respectively. Transcription of arcA showed the narrowest range of expression patterns, and expression of etrA was the lowest under all of the conditions we tested. Different terminal electron acceptors also affected the expression patterns of the three regulators. In general, arcA, etrA, and crp expression was highest in cells grown with nitrate. Differential expression of arcA, etrA, and crp was not observed in cells grown aerobically or anaerobically with either arsenate or fumarate. We concluded that expression of the global regulators is not specifically affected by arsenate. However, nitrate appears to have a greater influence on increasing the expression of the global regulators than do other electron acceptors.
TABLE 2.
Expression of crp, etrA, and arcA relative to that of gyrB in wild-type Shewanella sp. strain ANA-3 as determined by quantitative RT-PCR
| TEAb | Relative expressiona
|
||
|---|---|---|---|
| arcA | etrA | crp | |
| Oxygen | 0.69 ± 0.02 | 0.08 ± 0.02 | 1.02 ± 0.05 |
| Nitrate | 0.88 ± 0.11 | 0.16 ± 0.04 | 3.78 ± 0.14 |
| Arsenate | 0.48 ± 0.03 | 0.05 ± 0.004 | 1.30 ± 0.1 |
| Fumarate | 0.43 ± 0.12 | 0.05 ± 0.01 | 1.01 ± 0.13 |
Relative expression refers to the nanogram genomic equivalent of mRNA for each gene normalized to the nanogram genomic equivalent of mRNA for the DNA gyrase gene gyrB. Data points represent the averages and standard deviations of mRNA extracts from triplicate cultures.
TEA, terminal electron acceptor.
The role of the global regulators in growth on arsenate.
To further characterize the functional roles of the global regulators in arsenate respiration, we generated crp, arcA, and etrA null mutants of Shewanella sp. strain ANA-3; these strains were referred to as AN-CRP1, AN-ARCA1, and AN-ETRA1, respectively (Table 1). Figure 1A shows the arsenate growth curve analysis of wild-type ANA-3 versus the three null mutants. The phenotypes ranged from subtle (ΔarcA) to moderate (ΔetrA) to severe (Δcrp) growth defects. Cultures of the ΔarcA mutant strain had a 20% depression in the maximum OD600 in stationary phase compared to that of the wild-type strain. However, the generation times were similar (g = ∼0.9 h). In the ΔetrA mutant strain, the doubling time (g = 2.5 h) was dramatically greater than that of wild-type Shewanella sp. strain ANA-3 (g = 0.86 h); however, growth was not eliminated. The Δcrp mutant Shewanella strain was completely deficient in growth on arsenate (and fumarate [data not shown]). Growth with nitrate, trimethylamine N-oxide (TMAO), and oxygen was also significantly altered but not eliminated (see Fig. S1 in the supplemental material). Complementation of the respective gene deletion strains with plasmids containing arcA, etrA, or crp restored growth on arsenate to wild-type levels (Fig. 1B to D). We concluded that arcA was not required for growth on arsenate, the loss of etrA significantly impaired growth on arsenate but the gene was nevertheless dispensable, and crp was essential to anaerobic respiration of arsenate. These results indicate that cAMP-CRP positively regulates the arr operon.
FIG. 1.
Arsenate respiration in crp, etrA, and arcA null mutants of Shewanella sp. strain ANA-3. (A) Symbols correspond to wild-type (wt) Shewanella sp. strain ANA-3 (•) and arcA (⧫), etrA (▴), and crp (▪) null mutants. (B, C, and D) Growth complementation on arsenate (10 mM) for ΔarcA (B), ΔetrA (C), and Δcrp (D) null mutants (▪, complementing plasmid pArcA, pEtrA, or pCRP; □, vector plasmid pBBR-1-MCS2) and wild-type Shewanella sp. strain ANA-3 with pBBR1-MCS2 (○). The data points and error bars represent means and standard deviations of triplicate cultures, respectively.
DNA-binding activities of cAMP-CRP.
To further investigate the functional role of cAMP-CRP in regulating arsenate respiration, we used computational biology to predict CRP binding sites within the ANA-3 genome. The analysis relied on a positional weight matrix for individual nucleotides among 210 E. coli CRP binding motifs (23). The analysis revealed 1,526 (760 plus strand, 766 negative strand) predicted CRP binding motifs with log odds scores of >10. Many of these motifs were present as the reverse complement on the opposite DNA strand. This would limit the number of predicted CRP motifs to ∼700, assuming that CRP could bind on the forward and reverse DNA strands. Analysis of the frequency distribution of the log odds score of the predicted CRP motifs revealed that ∼75% have low scores ranging from 10 to 12 (Fig. 2A).
FIG. 2.
Computational analysis of CRP binding motifs (A, B, and C) and cAMP-CRP interactions within the ars-arr intergenic region of Shewanella sp. strain ANA-3 (D and E). (A) Histogram of the number of predicted CRP binding motifs with log odds scores of >10 identified in the Shewanella sp. strain ANA-3 genome. (B) Map of the ars-arr intergenic region with a predicted CRP binding motif (gray box) and promoters for the ars (Pars) and arr (Parr) operons. Vertical tick marks indicate 50-bp intervals. F1R1 and F1R4 represent fluorescently labeled (*) DNA probes used in the EMSAs in panels D and E. Nucleotide positions −178 and −92 represent relative distances from the arrA start codon. (C) Comparison of the predicted Shewanella sp. strain ANA-3 CRP binding motif to the consensus for the E. coli CRP binding motif (24). (D) EMSA results for CRP (3 μg, 160 fmol, or 8 nM) with increasing cAMP levels and the F1R1 DNA probe (1 μM). Lanes 1 to 7 correspond to cAMP concentrations of 1 mM, 100 μM, 10 μM, 1 μM, 100 nM, 10 nM, and no cAMP, respectively. Lane 8 contains the DNA probe without cAMP and CRP. (E) EMSA with CRP (with and without cAMP, lanes 1 and 4, respectively) and probes F1R1 (lanes 1 and 2) and F1R4 (lanes 3 and 4). For panels D and E, the bottom and top arrows indicate the free DNA probe and protein-DNA interactions, respectively.
Among the 1,526 predicted CRP motifs, one of these sites (log odds score, 13.6) was located within the ars-arr intergenic region, 155 nucleotides upstream of the arrA start codon (Fig. 2B). The putative CRP binding motif differed from the E. coli consensus by one nucleotide (Fig. 2C). To test if this predicted CRP binding motif was functional, the Shewanella sp. strain ANA-3 CRP was overexpressed and purified as a recombinant protein from E. coli to be used in an EMSA with the F1R1 fluorescently labeled DNA probe. Figure 2D shows that the F1R1 DNA probe shifted upward in the presence of CRP and cAMP (>100 nM cAMP). Increasing the cAMP concentration (1 mM) caused the probe to further shift upward, possibly because of binding of multimers or conformational changes to cAMP-CRP. To further define where CRP binding occurred in the ars-arr intergenic region, an EMSA was performed with the F1R4 DNA probe (Fig. 2E). This probe lacks the predicted CRP binding motif. In contrast to the F1R1 probe, we did not observe a shift with the F1R4 DNA probe and cAMP-CRP. These results indicate that the cAMP-CRP binding site is located within 92 to 178 nucleotides upstream of the arrA start codon.
CRP and arrA expression.
The question of whether cAMP-CRP was functioning to activate arrA transcription under anaerobic conditions remained. Because the strain lacking crp is unable to grow anaerobically on arsenate, we needed to perform an aerobic-anaerobic shifting experiment in order to examine the genetic interactions of crp on arrA expression in Δcrp mutant ANA-3. Both the wild-type and Δcrp mutant Shewanella sp. strain ANA-3 were grown aerobically to mid-log growth phase, shifted to anaerobic conditions, and induced with arsenate. The presence of arrA-specific mRNA was analyzed by RT-PCR at several time points (0 and 8 h) postshift (Fig. 3). At the onset of anaerobic conditions with arsenate, arrA expression was low but similar in both the wild-type and Δcrp mutant strains. After 8 h, arrA expression increased in the wild-type strain (Fig. 3A, top gel, lanes 3 and 4) but not in the Δcrp mutant strain (Fig. 3A, top gel, lanes 7 and 8). The 16S rRNA gene was detectable by RT-PCR with similar intensities for each strain and throughout the time course (Fig. 3A, bottom gel, lanes 1 to 8). No RT-PCR products were detected when RT was excluded from cDNA synthesis reaction mixtures (data not shown). Arsenate reduction was also monitored in wild-type and Δcrp mutant Shewanella sp. strain ANA-3; both were able to reduce arsenate. However, the Δcrp mutant reduced half the amount of arsenate reduced by the wild type, 1 versus 2 mM, respectively, at 8 h post anaerobic shift (data not shown). Our observations thus far support the model in which cAMP-CRP is an activator of arrA gene expression. This activation appears to occur only under anaerobic conditions and is mediated by cAMP-CRP. It is not clear, however, if cAMP-CRP-mediated activation occurs by direct sensing of anaerobic conditions by CRP.
FIG. 3.
RT-PCR analysis (A) of the arrA (top) and 16S rRNA (bottom) genes in Δcrp mutant and wild-type (wt) Shewanella sp. strain ANA-3 during an aerobic-to-anaerobic shift. Duplicate cultures of wild-type Shewanella sp. strain ANA-3 and the Δcrp mutant were grown aerobically with vigorous shaking until they reached an OD600 of 0.1 and then sparged with nitrogen for 10 min. The anaerobic cultures were placed in an anaerobic chamber and supplemented with arsenate (10 mM). RT-PCRs for the arrA and 16S rRNA genes were done with RNA extracts from duplicate cultures sampled at the initial anaerobic shift (lanes: 1 and 2, wild type; 5 and 6, Δcrp mutant) and 8 h postshift (lanes: 3 and 4, wild type; 7 and 8, Δcrp mutant). Control RT-PCRs performed on cDNA reaction mixtures without RT showed no PCR products (data not shown). ANA-3 genomic DNA and no-DNA controls for the arrA and 16S rRNA gene PCRs are shown in lanes 9 and 10, respectively. L indicates the 100-bp region of the DNA ladder. (B) Semiquantitative analysis of arrA gene expression was done by normalizing the band intensities of the arrA RT-PCR products (A, top) to the corresponding 16S rRNA gene RT-PCR products (A, bottom).
Transcription analysis of putative adenylate cyclases.
It is well established that CRP is a cellular cAMP sensor linking cAMP production to transcriptional induction or repression of target genes (22). The adenylate cyclases (e.g., CyaA and its homologs) function to generate cAMP from ATP (5). It is likely that the Shewanella CRP regulon is directly linked to the activities of adenylate cyclase. In the Shewanella sp. strain ANA-3 genome, four genes that encode putative adenylate cyclases were identified: Shewana3_0387, Shewana3_0814, Shewana3_0823, and Shewana3_3045. The predicted protein for Shewana3_0387 is similar to class I adenylate cyclases, which are typified within the family Enterobacteriaceae (35). Shewana3_0814 and Shewana3_0823 have N-terminal CYTH domains, which are commonly associated with some types of adenylate cyclases (15). No predicted CRP binding site was found in the upstream region of Shewana3_0823. Shewana3_0814 is the last gene within a cluster of three that includes cydDC. The latter two genes encode an ABC-type transport system that is essential for cytochrome maturation in E. coli (27). The 3′ region of Shewana3_0814 also contained repetitive DNA that was associated with a putative transposase, Shewana3_0815 (IS4 family), duplicated eight times throughout the genome. A predicted CRP binding motif was identified near the beginning of the 0812-0814 gene cluster. The Shewana3_3045 gene is annotated to encode a putative class III adenylate cyclase, which is common to a variety of diverse microorganisms (35). The predicted protein has three transmembrane helices near the N terminus and a nucleotydyl (adenylyl and guanylyl) binding domain near the C terminus.
The putative cya genes were analyzed for their transcription patterns in ANA-3 wild-type cells grown with vigorous aeration or anaerobically with arsenate as an electron acceptor (Fig. 4). The expression levels of the Shewana3_0387, Shewana3_0814, and Shewana3_0823 genes were generally low relative to that of the housekeeping gene gyrB. The aerobic and anaerobic expression levels of Shewana3_3045 were nearly two times greater than those of the other three cya-like genes. With the exception of Shewana3_3045, aerobic versus arsenate growth conditions did not appear to affect (greater than twofold) the expression of either of the putative cya genes. Additional expression analyses with different terminal electron acceptors are needed to establish a more complete pattern of cya gene expression.
FIG. 4.
Quantitative expression of putative adenylate cyclase-encoding genes Shewana3_0387, Shewana3_0814, Shewana3_0823, and Shewana3_3045. Gene expression was quantified by real-time PCR of cDNA reaction mixtures containing total RNA prepared from aerobic (open bars) versus arsenate-grown (dark bars) Shewanella sp. strain ANA-3 cells, respectively. The expression of each gene was normalized to housekeeping gene gyrB. The data points and error bars represent means and standard deviations of triplicate cultures, respectively.
Growth curve analysis of putative cya gene null mutants.
The functional roles of the putative cya genes were examined by generating various single-gene and multigene null mutants of Shewanella sp. strain ANA-3. Deletion of Shewana3_0814 required deletion of Shewana3_0815 (which encodes an insertion element duplicated eight times in the genome) because of repetitive DNA spanning the 3′ end of Shewana3_0814 to the 5′ end of Shewana3_0815. Growth curve analyses were done to characterize the phenotypes of the various strains (Fig. 5). The growth phenotypes of the Δ0814/0815 and Δ0823 null mutants were indistinguishable from that of the wild-type ANA-3 strain (data not shown). The Shewana3_3045 null mutant (Δ3045) was the only strain to exhibited a subtle increase in generation time (g = 1.24 h) and a depression in the stationary phase of growth compared to the wild-type strain (g = 0.98 h). A strain lacking all four cya genes (AN-CYA-ALL) exhibited very little growth on arsenate and fumarate, in contrast to the single-deletion and wild-type strains. These results indicated that two or more putative cya genes might be involved in cAMP production during growth on arsenate and fumarate.
FIG. 5.
Growth curve analysis of wild-type Shewanella sp. strain ANA-3 (•) and putative adenylate cyclase (cya) null mutant strains Δ3045 (○), Δ0387 (▴), Δ0387/3045 (×), and AN-CYA-ALL (□). Strains were grown anaerobically with (A) arsenate (10 mM) or (B) fumarate (10 mM) as terminal electron acceptors. The data points and error bars represent means and standard deviations of triplicate cultures, respectively.
Because the upstream regions of the coding sequences for the Shewana3_0387 and Shewana3_3045 genes have putative CRP binding sites, we hypothesized that these two genes would be required for anaerobic growth on arsenate. The double cya mutant (Δ0387/3045) was tested for growth on arsenate and fumarate (Fig. 5A and B). The growth curve of the Δ0387/3045 strain was nearly identical to that of the full cya null mutant strain. It is known that cya mutants can be complemented by exogenous additions of cAMP to the growth medium (16). When 10 mM cAMP was added to the full cya and Δ0387/3045 null mutant strains, growth on arsenate was restored to nearly wild-type levels (Fig. 6). These results suggest that the Shewana3_0387 and Shewana3_3045 genes encode adenylate cyclases. Moreover, one of these genes (Shewana3_3045) appears to be upregulated under anaerobic conditions.
FIG. 6.
Effects of extracellular cAMP on growth complementation of cya mutants. Wild-type Shewanella sp. strain ANA-3 (A) and the full cya null mutant AN-CYA-ALL (Δ0387/Δ0814-0815/Δ0823/Δ3045) (B) and double cya null mutant Δ0387/Δ3045 (C) strains were grown anaerobically with arsenate (10 mM) as the terminal electron acceptor. Cultures received no exogenous cAMP (•), or the growth medium was supplemented with 10 mM cAMP (□). The OD600 scale is the same for panels A, B, and C. The data points and error bars represent means and standard deviations of triplicate cultures, respectively.
DISCUSSION
The objective of this work was to identify regulators of the arsenate respiration pathway in the metal-reducing bacterium Shewanella sp. strain ANA-3. In our previous study (33), we determined that the arr operon was highly expressed under anaerobic conditions and in the presence of arsenate and arsenite. Efforts are under way to identify the arsenic-specific regulators of arr. However, in this study we focused on the aerobic-anaerobic dependency of arr expression. The latter form of regulation was thought to involve anaerobic sensor/response regulator FNR and the two-component redox sensor/response regulators ArcA/ArcB (reviewed in reference 37). We identified the fnr (referred to as etrA in Shewanella) and arcA genes in the genome of Shewanella sp. strain ANA-3. Through mutational analysis, we showed that the etrA mutant strain exhibited a moderate growth defect when grown anaerobically on arsenate. Growth on other electron acceptors was also impaired. These physiological outcomes are similar to those observed with the S. oneidensis MR-1 etrA mutant (20); growth defects were subtle, such as increased lag times for growth on nitrate and fumarate, longer generation times, and decreased fumarate and nitrate reductase activities. Mutation of etrA in MR-1 also affected the transcriptome of numerous genes for electron transport, iron uptake, and intermediary carbon metabolism (4). Although etrA does influence respiration in Shewanella, the gene is not essential for arsenate respiration in ANA-3, which suggests that there are other important regulators of this respiratory pathway. One such regulator, arcA, was considered in our study. However, the arcA mutation in ANA-3 had very little effect on anaerobic growth on arsenate. This is in contrast to work done by Gralnick et al. (12) that showed that arcA was necessary for the growth of S. oneidensis strain MR-1 on dimethyl sulfoxide. In general, the Arc system in Shewanella appears to be atypical because the histidine kinase sensor protein ArcB is absent.
Although it appears that EtrA plays a minor role in regulating arr expression, it is clear that cAMP-CRP is an essential regulator of arsenate respiration in Shewanella sp. strain ANA-3 (30). We suspected that crp would have this effect because mutating crp in S. oneidensis MR-1 was previously shown to result in severe defects in the reduction of Fe(III) and Mn(IV) and growth with nitrate, fumarate, and dimethyl sulfoxide as terminal electron acceptors (30). Our results with Shewanella sp. strain ANA-3 showed that the loss of crp also affected growth on oxygen, nitrate, and TMAO (see Fig. S1 in the supplemental material). In further support of the functional role of cAMP-CRP in regulating arr, we showed that cAMP-CRP could bind within the putative arr promoter region.
The physiological role of CRP in Shewanella appears to be quite different from that in E. coli, where cAMP-CRP alters the expression of genes involved in carbon source utilization (7, 10). In E. coli, cAMP-CRP was shown to interact with ∼200 regulatory regions (11). Robison et al. (29) predicted ∼10,000 low-affinity CRP binding sites. Our computational analysis for CRP motifs in the ANA-3 genome showed similar trends in the number of motifs (∼700). Because Shewanella species are generally more limited in their repertoire of carbon sources used for metabolism (14) than E. coli is, it is likely that the CRP regulon has diverged to include respiratory genes. For example, putative CRP motifs were identified in close proximity (100 to 500 nucleotides) to a variety of operons that contain genes involved in respiratory metabolism. Some of these are the genes for nitrate reductase (5′-TTATTGATCATTTTCACCTA [Shewana3_1898-1902] and 5′-AGTGTGAACAACAACACTAT [Shewana3_0658]), TMAO reductase (5′-TTAGCGAAAAAATTCAAAAT [Shewana3_1057-1054]), fumarate reductase (5′-TTTGTGATCCTCTTCTAAGA [Shewana3_0401-0404]), nitrite reductase (5′-AATGTGATCTATGTCAAAAA [Shewana3_3963-3960]), and iron reduction (5′-TATGTGACAAAAATCTCAAT-3′ and 5′-AGTCAGATCTCAATCACATT-3′ [Shewana3_2672-2678]), the latter was confirmed through an EMSA with cAMP-CRP and DNA probes targeting the mtr-omc gene cluster (unpublished data). We also identified predicted CRP binding motifs in upstream regions of genes that encode lactate dehydrogenase (Shewana3_3319); the putative nitrate/nitrite sensor-regulator, narQP (Shewana3_0657-0656); c-type cytochrome maturation (ccm cluster); and heme biosynthesis (hem cluster). Early studies by Dills and Dobrogosz (8) showed that E. coli cya and crp mutants had dramatic reductions in components of oxidative phosphorylation, mainly total flavin content. It was also noted that cytochrome content might also be reduced because the cya and crp mutant colonies were “chalk white” compared to the normal “yellowish color” of wild-type E. coli. Cytochrome contents in S. oneidensis MR-1 crp mutants were also dramatically less than in wild-type strains (30). It is expected that the cytochrome content of the ANA-3 Δcrp strain would also be less than that of the wild-type strain. Decreases in total cytochrome would impact aerobic growth (see Fig. S1 in the supplemental material), but their effect on anaerobic respiration would be more severe; both modes of respiration depend on cytochromes. There is a paucity of information about the role of cAMP-CRP as a regulator of anaerobic respiration pathways. Unden and Duchene (38) reported that in E. coli fumarate reductase expression was not regulated in response to cAMP. In contrast, Saffarini et al. (30) reported that crp was essential to a variety of anaerobic respiration pathways in S. oneidensis strain MR-1. Moreover, it was postulated that cAMP should be equally important in the expression of various respiratory reductases in Shewanella.
To further define the role of cAMP as a coactivator of the arr operon, we identified two putative adenylate cyclase genes (Shewana3_0347 and Shewana3_3045) that in combination were necessary for anaerobic growth with arsenate in ANA-3. Complementation of the double Shewana3_0347/3045 null mutant strain with exogenous cAMP provided evidence that Shewana3_0347 and Shewana3_3045 encode adenylate cyclases. Further work is needed to determine how these adenylate cyclases are activated. One possibility is through posttranslational regulation by the phosphotransferase system of the carbon catabolite repression pathway (10); Shewanella sp. strain ANA-3 has a nearly complete set of phosphotransferase system genes. Lastly, the presence of multiple adenylate cyclase genes is not unique among bacteria. Shenroy and Visweswariah (35) reported that the genomes of a variety of bacteria have multiple adenylate cyclase-like genes. For example, plant and soil bacteria like Sinorhizobium meliloti and Bradyrhizobium japonicum have 26 and 23 predicted adenylate cyclase-encoding genes, respectively. Pathogens like Mycobacterium tuberculosis and related species have three to eight adenylate cyclase-like genes within their genomes. Why there is an apparent redundancy of adenylate cyclases within bacteria remains an open question.
This report represents the first identification of regulators of the arr operon in an arsenate-respiring bacterium. We have characterized the physiological roles of global anaerobic-aerobic regulators (etrA and arcA) and cAMP-CRP in the arsenate respiration pathway in Shewanella. Although the anaerobic regulators are nonessential for growth with arsenate in Shewanella sp. strain ANA-3, redox sensing probably functions to activate and repress pathways necessary for adapting to changes in oxidizing and reducing conditions. The latter environmental condition is known to correlate with high dissolved arsenic concentrations in anaerobic water and sediments (reviewed in reference 26). For stricter anaerobes that respire arsenate, such as Sulfurospirillum and Desulfitobacterium species, it is not known if ArcA and/or FNR would be used to regulate respiratory pathways. Similarly, cAMP-CRP-dependent regulation of respiration and metabolism in other metal reducers has not been reported, even though crp-like genes are present in the genome sequences of several anaerobic bacteria (e.g., Geobacter, Rhodoferax, Desulfitobacterium, and Desulfotomaculum). Additional work needs to be done to determine the interactions of aerobic-anaerobic sensing, carbon catabolite repression, and arsenic-dependent regulation in controlling arr gene expression.
Supplementary Material
Acknowledgments
We thank Jiunn (Nick) Fong, University of California at Santa Cruz, for insightful comments. Carolina Reyes performed the arsenic analysis. Roseanna Dueñas constructed the ΔetrA strain.
This work was supported by National Science Foundation grant EAR-0535392 and the Cancer Research Coordinating Committee of the University of California.
Footnotes
Published ahead of print on 5 December 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Afkar, E., J. Lisak, C. Saltikov, P. Basu, R. S. Oremland, and J. F. Stolz. 2003. The respiratory arsenate reductase from Bacillus selenitireducens strain MLS10. FEMS Microbiol. Lett. 226107-112. [DOI] [PubMed] [Google Scholar]
- 2.Ahmann, D., L. R. Krumholz, H. F. Hemond, D. R. Lovley, and F. M. Morel. 1997. Microbial mobilization of arsenic from sediments of the Aberjona watershed. Environ. Sci. Technol. 312923-2930. [Google Scholar]
- 3.Ahmed, M. F., S. Ahuja, M. Alauddin, S. J. Hug, J. R. Lloyd, A. Pfaff, T. Pichler, C. Saltikov, M. Stute, and A. van Geen. 2006. Epidemiology: ensuring safe drinking water in Bangladesh. Science 3141687-1688. [DOI] [PubMed] [Google Scholar]
- 4.Beliaev, A. S., D. K. Thompson, M. W. Fields, L. Wu, D. P. Lies, K. H. Nealson, and J. Zhou. 2002. Microarray transcription profiling of a Shewanella oneidensis etrA mutant. J. Bacteriol. 1844612-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botsford, J. L., and J. G. Harman. 1992. Cyclic AMP in prokaryotes. Microbiol. Rev. 56100-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell, K. M., D. Malasarn, C. W. Saltikov, D. K. Newman, and J. G. Hering. 2006. Simultaneous microbial reduction of iron(III) and arsenic(V) in suspensions of hydrous ferric oxide. Environ. Sci. Technol. 405950-5955. [DOI] [PubMed] [Google Scholar]
- 7.Deutscher, J. 2008. The mechanisms of carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 1187-93. [DOI] [PubMed] [Google Scholar]
- 8.Dills, S. E., and W. J. Dobrogosz. 1977. Cyclic adenosine 3′,5′-monophosphate regulation of membrane energetics in Escherichia coli. J. Bacteriol. 131854-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao, H., X. Wang, Z. K. Yang, T. Palzkill, and J. Zhou. 2008. Probing regulon of ArcA in Shewanella oneidensis MR-1 by integrated genomic analyses. BMC Genomics 942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Görke, B., and J. Stulke. 2008. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat. Rev. Microbiol. 6613-624. [DOI] [PubMed] [Google Scholar]
- 11.Grainger, D. C., D. Hurd, M. Harrison, J. Holdstock, and S. J. Busby. 2005. Studies of the distribution of Escherichia coli cAMP-receptor protein and RNA polymerase along the E. coli chromosome. Proc. Natl. Acad. Sci. USA 10217693-17698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gralnick, J. A., C. T. Brown, and D. K. Newman. 2005. Anaerobic regulation by an atypical Arc system in Shewanella oneidensis. Mol. Microbiol. 561347-1357. [DOI] [PubMed] [Google Scholar]
- 13.Harvey, C. F., C. H. Swartz, A. B. Badruzzaman, N. Keon-Blute, W. Yu, M. A. Ali, J. Jay, R. Beckie, V. Niedan, D. Brabander, P. M. Oates, K. N. Ashfaque, S. Islam, H. F. Hemond, and M. F. Ahmed. 2002. Arsenic mobility and groundwater extraction in Bangladesh. Science 2981602-1606. [DOI] [PubMed] [Google Scholar]
- 14.Hau, H. H., and J. A. Gralnick. 2007. Ecology and biotechnology of the genus Shewanella. Annu. Rev. Microbiol. 61237-258. [DOI] [PubMed] [Google Scholar]
- 15.Iyer, L. M., and L. Aravind. 2002. The catalytic domains of thiamine triphosphatase and CyaB-like adenylyl cyclase define a novel superfamily of domains that bind organic phosphates. BMC Genomics 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolb, A., S. Busby, H. Buc, S. Garges, and S. Adhya. 1993. Transcriptional regulation by cAMP and its receptor protein. Annu. Rev. Biochem. 62749-795. [DOI] [PubMed] [Google Scholar]
- 17.Kovach, M. E., R. W. Phillips, P. H. Elzer, R. M. Roop, and K. M. Peterson. 1994. pBBR1MCS-a broad-host-range cloning vector. BioTechniques 16800. [PubMed] [Google Scholar]
- 18.Krafft, T., and J. M. Macy. 1998. Purification and characterization of the respiratory arsenate reductase of Chrysiogenes arsenatis. Eur. J. Biochem. 255647-653. [DOI] [PubMed] [Google Scholar]
- 19.Langner, H. W., and W. P. Inskeep. 2000. Microbial reduction of arsenate in the presence of ferrihydrite. Environ. Sci. Technol. 343131-3136. [Google Scholar]
- 20.Maier, T. M., and C. R. Myers. 2001. Isolation and characterization of a Shewanella putrefaciens MR-1 electron transport regulator etrA mutant: reassessment of the role of EtrA. J. Bacteriol. 1834918-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malasarn, D., J. R. Keeffe, and D. K. Newman. 2008. Characterization of the arsenate respiratory reductase from Shewanella sp. strain ANA-3. J. Bacteriol. 190135-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martínez-Antonio, A., and J. Collado-Vides. 2003. Identifying global regulators in transcriptional regulatory networks in bacteria. Curr. Opin. Microbiol. 6482-489. [DOI] [PubMed] [Google Scholar]
- 23.Münch, R., K. Hiller, H. Barg, D. Heldt, S. Linz, E. Wingender, and D. Jahn. 2003. PRODORIC: prokaryotic database of gene regulation. Nucleic Acids Res. 31266-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Münch, R., K. Hiller, A. Grote, M. Scheer, J. Klein, M. Schobert, and D. Jahn. 2005. Virtual footprint and PRODORIC: an integrative framework for regulon prediction in prokaryotes. Bioinformatics 214187-4189. [DOI] [PubMed] [Google Scholar]
- 25.Murphy, J. N., and C. W. Saltikov. 2007. The cymA gene encoding a tetraheme c-type cytochrome is required for arsenate respiration in Shewanella species. J. Bacteriol. 1892283-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oremland, R. S., and J. F. Stolz. 2005. Arsenic, microbes and contaminated aquifers. Trends Microbiol. 1345-49. [DOI] [PubMed] [Google Scholar]
- 27.Pittman, M. S., H. Corker, G. Wu, M. B. Binet, A. J. Moir, and R. K. Poole. 2002. Cysteine is exported from the Escherichia coli cytoplasm by CydDC, an ATP-binding cassette-type transporter required for cytochrome assembly. J. Biol. Chem. 27749841-49849. [DOI] [PubMed] [Google Scholar]
- 28.Polizzotto, M. L., C. F. Harvey, S. R. Sutton, and S. Fendorf. 2005. Processes conducive to the release and transport of arsenic into aquifers of Bangladesh. Proc. Natl. Acad. Sci. USA 10218819-18823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robison, K., A. M. McGuire, and G. M. Church. 1998. A comprehensive library of DNA-binding site matrices for 55 proteins applied to the complete Escherichia coli K-12 genome. J. Mol. Biol. 284241-254. [DOI] [PubMed] [Google Scholar]
- 30.Saffarini, D. A., R. Schultz, and A. Beliaev. 2003. Involvement of cyclic AMP (cAMP) and cAMP receptor protein in anaerobic respiration of Shewanella oneidensis. J. Bacteriol. 1853668-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saltikov, C. W., A. Cifuentes, K. Venkateswaran, and D. K. Newman. 2003. The ars detoxification system is advantageous but not required for As(V) respiration by the genetically tractable Shewanella species strain ANA-3. Appl. Environ. Microbiol. 692800-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saltikov, C. W., and D. K. Newman. 2003. Genetic identification of a respiratory arsenate reductase. Proc. Natl. Acad. Sci. USA 10010983-10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saltikov, C. W., R. A. Wildman, Jr., and D. K. Newman. 2005. Expression dynamics of arsenic respiration and detoxification in Shewanella sp. strain ANA-3. J. Bacteriol. 1877390-7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 35.Shenroy, A. R., and S. S. Visweswariah. 2004. Class III nucleotide cyclases in bacteria and archaebacteria: lineage-specific expansion of adenylyl cyclases and a dearth of guanylyl cyclases. FEBS Lett. 56111-21. [DOI] [PubMed] [Google Scholar]
- 36.Smedley, P. L., and D. G. Kinniburgh. 2002. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 17517-568. [Google Scholar]
- 37.Unden, G., and J. Bongaerts. 1997. Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim. Biophys. Acta 1320217-234. [DOI] [PubMed] [Google Scholar]
- 38.Unden, G., and A. Duchene. 1987. On the role of cyclic AMP and the FNR protein in Escherichia coli growing anaerobically. Arch. Microbiol. 147195-200. [DOI] [PubMed] [Google Scholar]
- 39.Zheng, D., C. Constantinidou, J. L. Hobman, and S. D. Minchin. 2004. Identification of the CRP regulon using in vitro and in vivo transcriptional profiling. Nucleic Acids Res. 325874-5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








