Abstract
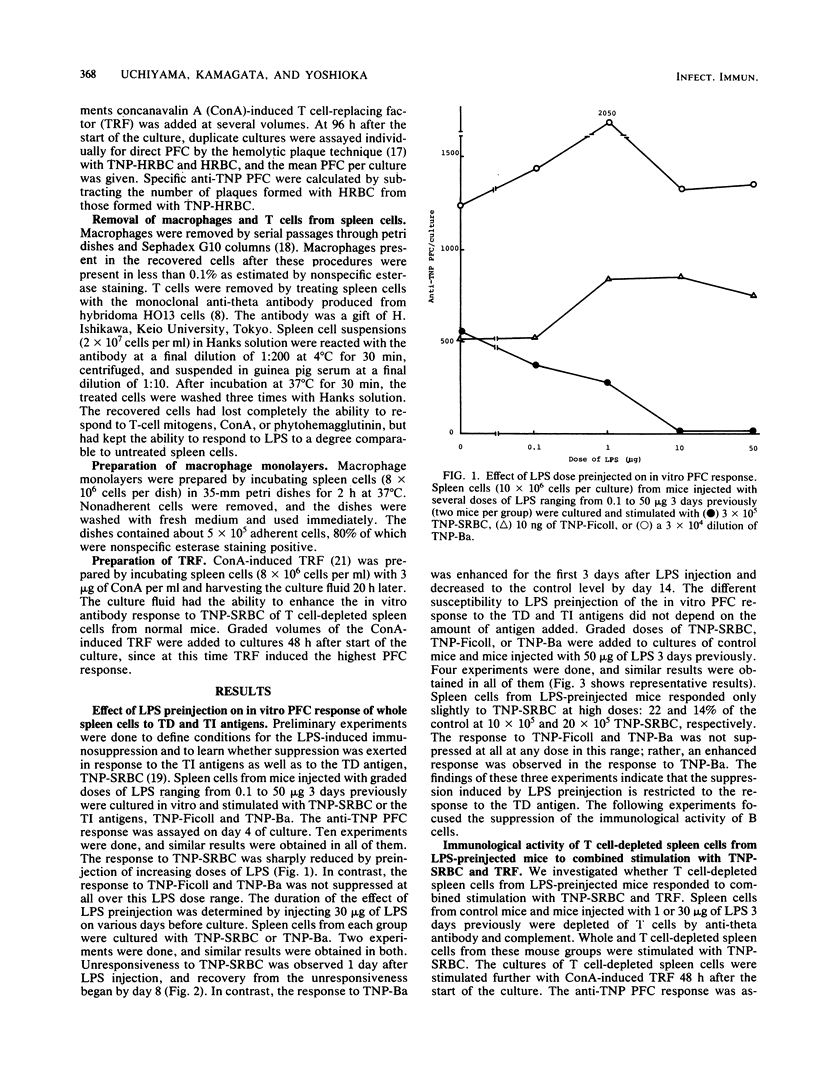
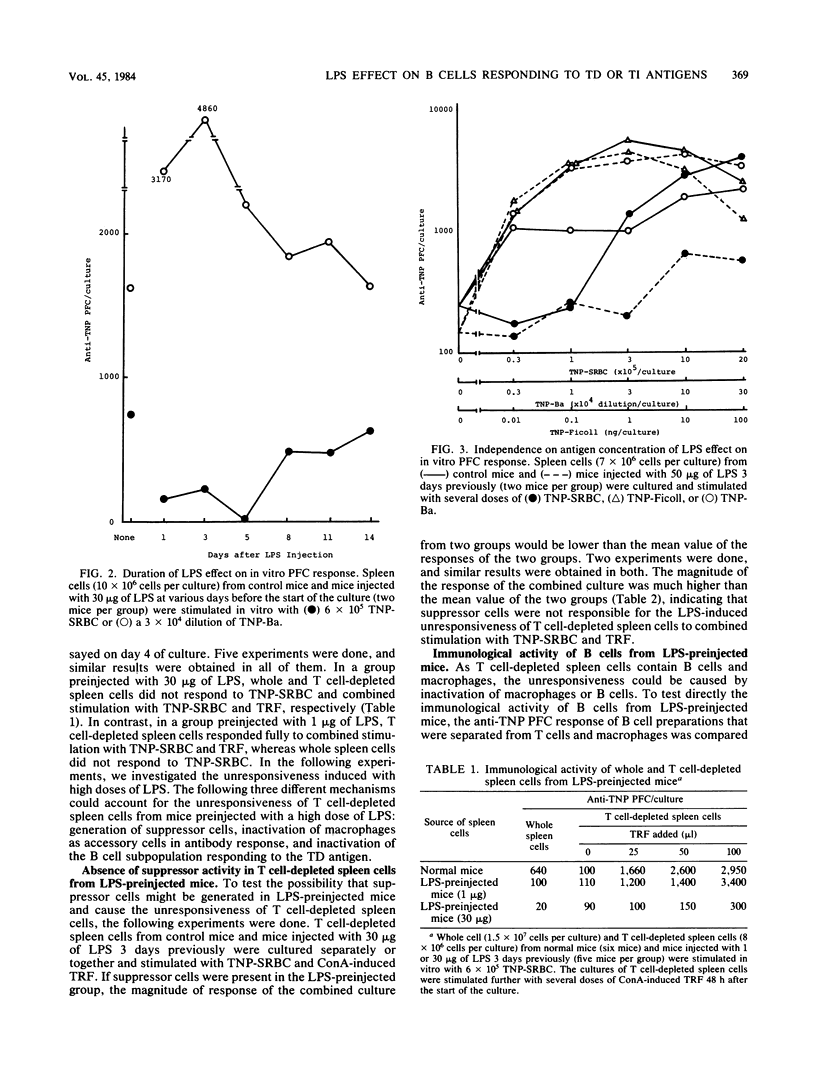
Spleen cells from mice preinjected with high doses of bacterial lipopolysaccharide did not generate anti-trinitrophenyl (TNP) plaque-forming cells in vitro to the T-dependent antigen, TNP-sheep erythrocytes, but did generate fully plaque-forming cells to the T-independent antigens, TNP-Ficoll and TNP-Brucella abortus. The immunological activity of B cells from such lipopolysaccharide-preinjected mice was analyzed in the present study. T cell-depleted spleen cells from mice injected with 30 micrograms of lipopolysaccharide 3 days previously did not respond to combined stimulation with TNP-sheep erythrocytes and concanavalin A-induced T cell-replacing factor and had no suppressive activity on normal T cell-depleted spleen cells. Splenic B cells, which were separated from T cells and macrophages from mice injected with 30 micrograms of lipopolysaccharide 3 days previously, responded only partially (about 25% of the control response) to combined stimulation with TNP-sheep erythrocytes and concanavalin A-induced cell-replacing factor in the presence of normal macrophages, but responded fully to TNP-B. abortus, regardless of the presence of normal macrophages. These results indicate that B cells responding to the T-dependent antigens are rendered unresponsive to antigenic stimulation in mice preinjected with lipopolysaccharide, whereas B cells responding to the T-independent antigens are kept intact.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Blomgren H. T-cell response to polyvinyl pyrrolidone is linked to maturity of B cells. Nature. 1975 Feb 6;253(5491):476–477. doi: 10.1038/253476a0. [DOI] [PubMed] [Google Scholar]
- Galanaud P., Crevon M. C., Dormont J. Effect of azathioprine on in vitro antibody response. Differential effect on B cells involved in thymus-dependent and independent responses. Clin Exp Immunol. 1975 Oct;22(1):139–152. [PMC free article] [PubMed] [Google Scholar]
- Gorczynski R. M., Feldmann M. B Cell heterogeneity - difference in the size of B lymphocytes responding to T dependent and T independent antigens. Cell Immunol. 1975 Jul;18(1):88–97. doi: 10.1016/0008-8749(75)90039-8. [DOI] [PubMed] [Google Scholar]
- Hamaoka T., Katz D. H. Cellular site of action of various adjuvants in antibody responses to hapten-carrier conjugates. J Immunol. 1973 Nov;111(5):1554–1563. [PubMed] [Google Scholar]
- Hoffmann M. K., Koenig S., Mittler R. S., Oettgen H. F., Ralph P., Galanos C., Hammerling U. Macrophage factor controlling differentiation of B cells. J Immunol. 1979 Feb;122(2):497–502. [PubMed] [Google Scholar]
- Huber B. T. B cell differentiation antigens as probes for functional B cell subsets. Immunol Rev. 1982;64:57–79. doi: 10.1111/j.1600-065x.1982.tb00418.x. [DOI] [PubMed] [Google Scholar]
- JOHNSON A. G., GAINES S., LANDY M. Studies on the O antigen of Salmonella typhosa. V. Enhancement of antibody response to protein antigens by the purified lipopolysaccharide. J Exp Med. 1956 Feb 1;103(2):225–246. doi: 10.1084/jem.103.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota E., Ishikawa H., Saito K. Evidence for a cloned hybridoma (HO-13-4-9) producing 2 kinds of antibody molecules different in specificity, one directed to Thy 1.2 gene products and the other to antigen on a subpopulation of Thy 1.1 thymocytes. J Immunol. 1981 Oct;127(4):1498–1503. [PubMed] [Google Scholar]
- McGhee J. R., Kiyono H., Michalek S. M., Babb J. L., Rosenstreich D. L., Mergenhagen S. E. Lipopolysaccharide (LPS) regulation of the immune response: T lymphocytes from normal mice suppress mitogenic and immunogenic responses to LPS. J Immunol. 1980 Apr;124(4):1603–1611. [PubMed] [Google Scholar]
- Michalek S. M., Kiyono H., Wannemuehler M. J., Mosteller L. M., McGhee J. R. Lipopolysaccharide (LPS) regulation of the immune response: LPS influence on oral tolerance induction. J Immunol. 1982 May;128(5):1992–1998. [PubMed] [Google Scholar]
- Mond J. J., Scher I., Mosier D. E., Baese M., Paul W. E. T-independent responses in B cell-defective CBA/N mice to Brucella abortus and to trinitrophenyl (TNP) conjugates of Brucella abortus. Eur J Immunol. 1978 Jul;8(7):459–463. doi: 10.1002/eji.1830080703. [DOI] [PubMed] [Google Scholar]
- Nakano M., Tanabe M. J., Saito T., Shimizu T. Immunosuppressive effect of bacterial lipopolysaccharide on antibody response. Jpn J Microbiol. 1976 Feb;20(1):53–58. doi: 10.1111/j.1348-0421.1976.tb00907.x. [DOI] [PubMed] [Google Scholar]
- Persson U. Lipopolysaccharide-induced suppression of the primary immune response to a thymus-dependent antigen. J Immunol. 1977 Mar;118(3):789–796. [PubMed] [Google Scholar]
- Portnoï D., Motta I., Truffa-Bachi P. Immune unresponsiveness of spleen cells from lipopolysaccharide-treated mice to particulate thymus-dependent antigen. I. Evidence for differentiation signal defect. Eur J Immunol. 1981 Feb;11(2):156–158. doi: 10.1002/eji.1830110218. [DOI] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Uchiyama T. B cell tolerance: B cells rendered tolerant are present in the immune system in a potentially responsive form. Microbiol Immunol. 1980;24(12):1211–1222. doi: 10.1111/j.1348-0421.1980.tb02925.x. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Jacobs D. M. Modulation of immune response by bacterial lipopolysaccharide (LPS): cellular basis of stimulatory and inhibitory effects of LPS on the in vitro IgM antibody response to a T-dependent antigen. J Immunol. 1978 Dec;121(6):2347–2351. [PubMed] [Google Scholar]
- Uchiyama T., Jacobs D. M. Modulation of immune response by bacterial lipopolysaccharide (LPS): multifocal effects of LPS-induced suppression of the primary antibody response to a T-dependent antigen. J Immunol. 1978 Dec;121(6):2340–2346. [PubMed] [Google Scholar]
- Uchiyama T. Modulation of immune response by bacterial lipopolysaccharide (LPS): roles of macrophages and T cells in vitro adjuvant effect of LPS on antibody response to T cell-dependent and T cell-independent antigens. Microbiol Immunol. 1982;26(3):213–225. doi: 10.1111/j.1348-0421.1982.tb00173.x. [DOI] [PubMed] [Google Scholar]
- Watson J., Aarden L. A., Lefkovits I. The purification and quantitation of helper T cell-replacing factors secreted by murine spleen cells activated by concanavalin A. J Immunol. 1979 Jan;122(1):209–215. [PubMed] [Google Scholar]


