Abstract
The electroencephalogram (EEG) recorded from the human scalp is widely used to study cognitive and brain functions in schizophrenia. Current research efforts are primarily devoted to the assessment of event-related potentials (ERPs) and event-related oscillations (EROs), extracted from the ongoing EEG, in patients with schizophrenia and in clinically unaffected individuals who, due to their family history and current mental status, are at high risk for developing schizophrenia. In this article, we discuss the potential usefulness of ERPs and EROs as genetic vulnerability markers, as pathophysiological markers, and as markers of possible ongoing progressive cognitive and cortical deterioration in schizophrenia. Our main purpose is to illustrate that these neurophysiological measures can offer valuable quantitative biological markers of basic pathophysiological mechanisms and cognitive dysfunctions in schizophrenia, yet they may not be specific to current psychiatry's diagnosis and classification. These biological markers can provide unique information on the nature and extent of cognitive and brain dysfunction in schizophrenia. Moreover, they can be utilized to gain deeper theoretical insights into illness etiology and pathophysiology and may lead to improvements in early detection and more effective and targeted treatment of schizophrenia. We conclude by addressing several key methodological, conceptual, and interpretative issues involved in this research field and by suggesting future research directions.
Keywords: human brain electrophysiology, event-related potentials, event-related oscillations, cognition, neuropsychiatric disorders, biological marker
Introduction
The electroencephalogram (EEG) recorded from the human scalp provides a powerful noninvasive tool for studying the brain mechanisms of attention and information processing in health and disease.1–7 In contrast to blood flow neuroimaging techniques, such as magnetic resonance imaging (MRI), the EEG provides a direct and “real-time” index of neuronal activities at a millisecond scale of resolution that is relatively easy and inexpensive to implement. Due to its high temporal resolution, the EEG is ideally suited to examine the rapidly changing patterns of brain activities that underlie human cognitive function and dysfunction.
The scalp EEG is believed to reflect mainly the summated postsynaptic potentials from large synchronously activated populations of pyramidal cells in the cerebral cortex.1–7 The recorded EEG activities show changes over time, which are often rhythmic or oscillatory in the sense that they alternate regularly. The rhythmic activities in the resting or “spontaneous” EEG are usually divided into several frequency bands (delta: <4 Hz; theta: 4–8 Hz; alpha: 8–12 Hz; beta: 12–30 Hz; and gamma: 30–70 Hz or higher, centered at 40 Hz), which are associated with different behavioral states, ranging from sleep and drowsiness to relaxation and heightened alertness and mental concentration,1,2,7,8 yet there exists little consensus on the precise frequency limits of each band. The EEG has a well-established value and role in the clinical assessment, diagnosis, and management of patients with certain neurological disorders, such as sleep disorders and epilepsy.7
The EEG also shows systematic changes when a person processes a specific external or internal stimulus event, such as a light flash or a sound or an internal thought. In general, 2 types of changes temporally related to a sensory, cognitive, or motor event may occur in the EEG (figure 1).9 One type of change involves the more traditional, well-examined event-related potentials (ERPs). The other type of event-related EEG change is the focus of more recent research efforts and variously referred to as event-related synchronization and event-related desynchronization,9 event-related spectral perturbation,10 or event-related oscillations (EROs).11–13
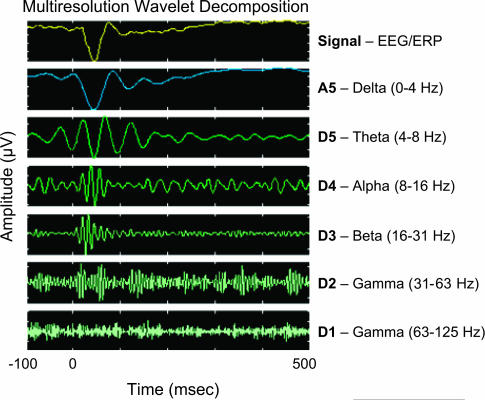
Fig. 1.
Five-level multiresolution decomposition of a human averaged auditory event-related potential (ERP) based on the discrete wavelet transform (DWT), using the Daubechies D5 wavelet as a mother wavelet. The application of a 5-level, dyadic DWT decomposes the scalp-recorded event-related electroencephalogram (EEG) signal (upper panel) in the time-frequency plane at different resolution levels, or scales, into a set of basic orthogonal individual signal components (lower panels) that approximately correspond to the main rhythmic activities traditionally used to classify the ongoing EEG. The result of the DWT yields 5 detail functions and corresponding sets of wavelet detail coefficients (D1–D5), representing the energy of the EEG signal as a function of time in the 63- to 125-Hz (D1, gamma), 31- to 63-Hz (D2, gamma), 16- to 31-Hz (D3, beta), 8- to 16-Hz (D4, alpha), and 4- to 8-Hz (D5, theta) frequency bands, and one final set of wavelet approximation coefficients (A5), representing the activity of the remaining part of the signal in the 0- to 4-Hz (delta) frequency band. Note that the frequency band limits are rounded and that the y-axis is scaled differently for each frequency band. By applying the inverse DWT, the detail (D1–D5) and approximation (A5) signal components, as well as the original signal (ERP = A5 + D5 + D4 + D3 + D2 + D1), can be reconstructed from the wavelet coefficients for each scale.184,189 The ERP was recorded at the midline central scalp electrode from a healthy adult subject in response to task-irrelevant frequent standard 1000-Hz tones (100-ms duration, 10-ms rise/fall, using a variable interstimulus interval of 1300–1700 ms), while the subject performed a visual oddball task. The data indicate that the auditory ERP (upper panel) coincides with auditory transient-evoked (phase locked) oscillations in multiple frequency bands (lower panels), including early evoked gamma (31–63 Hz)-band responses that peak around 20 and 60 ms and an early evoked beta (16–31 Hz)-band response that peaks around 30 ms after tone onset.
ERPs are a series of scalp-positive and -negative voltage deflections, waves, or components that are strictly time and phase locked to the onset of a particular stimulus event. ERPs can be extracted from the EEG by time domain analysis and averaging the EEG activity following multiple stimulus repetitions. The obtained ERPs may be broadly subdivided into early or sensory-evoked components (eg, auditory-evoked brainstem potentials, P50, N100), which emerge within the first 50–100 ms or so after stimulus onset and basically reflect stimulus detection, and later or cognitive-related components (eg, mismatch negativity [MMN], P300, N400), which primarily index stimulus context.3–6 While some of the early sensory-evoked components are well established as a clinical test to assess the integrity of the afferent sensory pathways (eg, hearing, vision) of individual patients,7,14 the cognitive-related components reflect more complex brain functions and have, to date, mainly been utilized as a research tool instead of a clinical tool.
EROs are changes in the frequency power spectrum of the ongoing EEG, which can be either strictly time and phase locked (evoked) or more loosely time-related and non-phase locked (induced) to the eliciting event.15 EROs can be extracted by means of time-frequency domain analysis and averaging the obtained EEG power spectrograms following the same stimuli. An important issue awaiting clarification is whether ERPs reflect a transient neural-evoked response that is added, but unrelated, to the ongoing EEG oscillations, result from a reorganization or phase resetting of ongoing oscillations by a given stimulus event, or are generated by a combination of both or other mechanisms.9–12,16 In general, it seems that ERPs and EROs can give valuable complementary insights into the basic mechanisms of cognitive and higher brain functions, such as perception, attention, memory, motor control, and language.
In this article, we discuss the application of ERPs and EROs to the study of cognitive and brain functions in schizophrenia. We focus on the results of more recent studies of patients with schizophrenia as well as of clinically unaffected persons who, by reason of their family history and current mental status, are at high risk for developing schizophrenia (for excellent reviews of older EEG/ERP work on schizophrenia, see Zubin et al,17 Pritchard,18 and Friedman19). We discuss selected examples of the potential usefulness of ERPs and EROs as genetic vulnerability markers, as pathophysiological markers, and as markers of possible ongoing progressive cognitive and cortical deterioration in schizophrenia. Our main purpose is to illustrate that these neurophysiological measures can offer valuable biological markers of basic pathophysiological mechanisms and cognitive dysfunctions in schizophrenia, yet they may not be specific to current psychiatry's diagnosis and classification. These quantitative biological markers can be utilized to gain deeper theoretical insights into the etiology and pathophysiology of complex and heterogeneous psychiatric disorders, such as schizophrenia, and may lead to more accurate early detection and more effective and targeted treatments.20–27 We conclude by addressing briefly several important methodological, conceptual, and interpretative issues involved in this research field and by suggesting future research directions.
ERPs and EROs in Schizophrenia
A variety of ERPs and EROs have been examined in schizophrenia, including the auditory-evoked P50 “sensory gating” response.6,25,27,28 Here, we select to consider the P300, MMN, and EROs in the gamma-band frequency range, referred to as the gamma-band response (GBR), as biological markers of potentially distinct pathophysiological mechanisms and cognitive dysfunctions in schizophrenia.
P300 as a Vulnerability Marker
A vulnerability marker, or endophenotype,24 may be defined as “a heritable trait, associated with a causative pathophysiological factor in an inherited disease.”20 A vulnerability marker may be distinguished from a risk factor, which refers to any characteristic that has predictive validity, but not etiological significance, for developing a psychiatric disorder.29 Thus, in theory, while both can be used to predict psychiatric disease, only a vulnerability marker can offer basic insights into illness etiology and pathophysiology and may contribute to the rational development of psychiatric diagnostic systems and therapeutic treatments that are based on the underlying illness causes and biology rather than on the final overt complex clinical phenotypes.20–27
Basic Studies
The P300 (P3 or P3b) refers to a late scalp-positive ERP component that is usually recorded in an auditory or visual “oddball” experimental paradigm in which a subject detects an infrequent deviant or task-relevant “target” stimulus (eg, a 1000-Hz tone) randomly presented within a series of frequent nontarget or “standard” stimuli (eg, 1500-Hz tones).30–33 The size or amplitude of the P300 elicited by task-relevant target stimuli is typically largest over the medial central and parietal scalp locations, and its peak latency, depending on stimulus, task, and subject factors, may occur between about 300 and 1000 ms after stimulus onset. The P300 is a relatively slow, low-frequency neuroelectrical event and linked to stimulus-evoked delta and theta oscillations.13,34
The P300 is believed to index stimulus significance and the amount of attention allocated to the eliciting stimulus event, being maximal to task-relevant or attended stimuli and being absent or small to task-irrelevant or unattended stimuli.31–33,35 While the P300 reflects primarily cognitive factors, this component can also be sensitive to constitutional factors (eg, age, sex) and to natural (eg, circadian rhythm, menstrual cycle) and environmentally induced (eg, exercise, caffeine, nicotine, psychotropic medications) changes in the subject's arousal state.33,35,36 Similarly, the P300 can be sensitive to individual differences in major personality trait dimensions, such as sensation seeking or novelty seeking.37,38
The intracerebral origin of the P300 is poorly understood but most likely involves the complex summation of activity from multiple brain regions, particularly the various association areas of the cerebral cortex and the limbic system.32,39–41 Similarly, the neurochemical substrates of the P300 are unclear but presumably involve various neurotransmitter systems in the brain.32,42 The P300 most likely reflects the summation of multiple, simultaneously occurring cognitive and brain processes39 that are engaged during the active processing of behaviorally significant stimulus events and functionally linked to attentional resource allocation and memory updating operations in the brain.31,33,36,40,43
Genetically, the P300 is considered multifactorial and a complex quantitative trait. Family and twin studies have indicated that P300 characteristics are heritable,26,44–50 but reported heritability estimates vary greatly (ranging between about 0.3 and 0.7) as a function of experimental task paradigm, P300 measure (eg, amplitude vs latency), scalp location (eg, frontal vs parietal), stimulus type (eg, target vs nontarget), stimulus modality (eg, visual vs auditory), age, and sex. Information about the molecular genetic basis of P300 is thus far limited and comes mostly from psychiatric genetic research, which will be discussed later.
Clinical Studies
Numerous studies have shown that schizophrenia patients display a smaller than normal auditory P300 over the midline central and parietal scalp electrode locations,17,18,44,47,50–57 as well as a distinct left-smaller-than-right voltage asymmetry at temporal scalp sites.58–65 Auditory P300 amplitude abnormalities over the posterior scalp have been detected in schizophrenia patients at the initial and advanced stages of illness60,64,65 and remain detectable even in patients free of clinical symptoms and in relative remission.55 Additionally, schizophrenia patients often show a prolonged auditory P300 latency relative to healthy control subjects.50,56,57
Schizophrenia patients have also been found to generate a significantly smaller and/or delayed visual P300 over the posterior scalp than healthy control subjects,56 but this finding seems less robust55 and the size of patient-control group differences is usually smaller.56,63 As compared with the auditory P300, the visual P300 in schizophrenia seems more sensitive to clinical state variables, such as current antipsychotic medication status and clinical symptom severity.53,55 Accordingly, it has been suggested that the visual P300 could serve primarily as a clinical state marker, whereas the auditory P300 could offer a trait or vulnerability marker of schizophrenia.18,52,53,55,63
Additional supporting evidence for the idea that the auditory P300 indexes a genetic and biological vulnerability to schizophrenia has come from several, though not all,19,61,66 family-based “high-risk” studies showing that this ERP component is also impaired in clinically unaffected family members who, by reason of their family history, are at high risk for developing schizophrenia.44,47,50,67 Similarly, persons with either psychometrically68 or clinically69,70 defined schizotypal personality traits, who are assumed to carry the same latent vulnerability as schizophrenia patients,71–73 display P300 amplitude abnormalities.
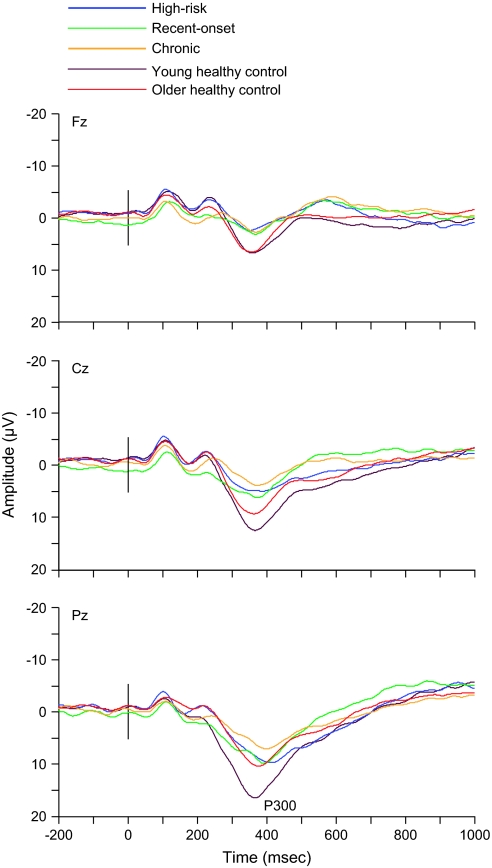
Similarly, we recently observed that auditory P300 amplitude abnormalities over the posterior scalp, similar to those detected under identical experimental conditions in recently ill and chronically ill schizophrenia patients, are present in putatively “prodromal” patients who, on the basis of both their family history and current mental status,74 are at ultrahigh risk for developing a first psychotic episode (figure 2).64 Longitudinal follow-up data and large patient samples, however, are required to determine whether the auditory P300 has indeed predictive validity for later schizophrenia or other psychiatric disorders in these ultrahigh-risk populations. An earlier family-based longitudinal prospective study reported that both the auditory and visual P300 recorded from adolescent subjects, irrespective of their family history risk status, has predictive validity, not for a diagnostically specific psychiatric outcome but for later global behavioral maladjustment in general.75
Fig. 2.
Group mean event-related potentials (ERPs) elicited by auditory target stimuli recorded at midline frontal (Fz), central (Cz), and parietal (Pz) scalp locations, superimposed for subjects at high risk for schizophrenia (n = 10; mean age = 22.1 years, SD = 4.3), subjects with recent-onset schizophrenia (n = 10; mean age = 21.3 years, SD = 3.2; mean illness duration = 0.6 years, SD = 0.3), subjects with chronic schizophrenia (n = 14; mean age = 37.5 years, SD = 7.5; mean illness duration = 12.2 years, SD = 6.8), young healthy control subjects (n = 14; mean age = 22.5 years, SD = 2.0) who are age matched to the high-risk and recent-onset groups, and older healthy control subjects (n = 14; mean age = 34.1 years, SD = 10.9) who are age matched to the chronic patient group. The ERPs were collected while subjects performed an auditory oddball task. The auditory stimuli were pure sinusoidal tones (100-ms duration, 10-ms rise/fall), consisting of frequent standard stimuli (1000-Hz tones, P = 91.5%) and infrequent deviant or target stimuli (1064-Hz tones, P = 8.5%), presented binaurally through inserted earphones in a random order using a constant interstimulus interval of 1500 ms. Study participants were instructed to pay attention to the auditory stimuli and to make a button-press response only to the infrequent target stimuli, emphasizing both speed and accuracy. P300 refers to the late scalp-positive ERP component associated with target detection. High-risk, recent-onset, and chronic schizophrenia patient groups all showed significantly smaller P300 amplitudes at Pz and/or Cz relative to healthy age-matched control subjects. Older healthy control subjects displayed significantly smaller P300 amplitudes at Pz compared with younger healthy control subjects. Reprinted with permission from Schizophr Res.,64 Copyright 2005, Elsevier.
The auditory and visual P300 seem to be impaired not only in schizophrenia but in a variety of psychiatric and neurological disorders, including bipolar affective disorder, attention-deficit hyperactivity disorder, and substance use disorders.19,26,32,33,52,76–86 In particular, impaired generation of the visual P300 has been found to characterize individuals with alcoholism as well as clinically unaffected family members, such as children of alcoholics, who are at high genetic risk for developing alcoholism.26,76,77,80–85 Longitudinal follow-up data from this and related research fields indicate that the visual P300 recorded from children and adolescents, regardless of their family history risk status, has predictive validity, not specifically for alcoholism but more generally for a spectrum of “disinhibitory” behavioral syndromes, including childhood externalizing disorders and adult antisocial personality disorder and substance abuse.81,85,86
Relatively little is yet known about the molecular genetic bases of the P300 and of its disruption in schizophrenia. Blackwood et al67 reported that within a large Scottish family, a translocation breakpoint region on chromosome 1q42 shows evidence for linkage to schizophrenia as well as to unipolar and bipolar affective disorders and that translocation carriers, either clinically affected or not, exhibit auditory P300 abnormalities. This chromosomal site harbors 2 recently described genes labeled “Disrupted In Schizophrenia 1 and 2” (DISC1 and DISC2), which seem to be involved in both early brain development and adult neurogenesis and neural plasticity.87,88 Additionally, a significant association has been observed in schizophrenia patient and healthy subject populations between the auditory P300 and catechol-O-methyltransferase (COMT) genotype,89,90 a gene that also has been implicated in schizophrenia vulnerability,87,88 but another study failed to observe a significant association between the auditory P300 and COMT polymorphism.91
Genetic linkage analyses of data from the Consortium on the Genetics of Alcoholism (COGA), a large, multicenter family-based study, have yielded several chromosomal regions, in particular on chromosomes 2, 4, 5, 6, 13, and 17, that appear to be linked to the visual P300 amplitude.26,48,49 The chromosomal regions linked to the visual P300 each contain many genes, including those encoding glutamate and acetylcholine receptors, and usually show also linkage, though in a wider region of linkage, to clinical diagnoses of alcoholism and related disorders.92 The reported linkage of the visual P300 amplitude to an area on chromosome 5 is noteworthy because this region has also been implicated in schizophrenia93 and contains the Engrailed-1 gene (En1), which codes for a protein that is brain expressed and implicated in neuronal differentiation.94
Furthermore, COGA has reported both linkage and association between visual-evoked theta and delta oscillations accompanying the visual P300 generation and a muscarinic cholinergic receptor M2 gene (CHRM2) on chromosome 7.95 While variants within or close to the CHRM2 locus have also been found to influence risk for alcoholism and major depressive disorder,96 these findings suggest that central muscarinic acetylcholine receptor systems influence the generation of event-related theta and delta neuronal oscillations associated with the P300 and with basic cognitive and memory functions in healthy subjects.13,97 These findings may have relevance to the visual P300 abnormalities observed in schizophrenia because central muscarinic acetylcholine receptor abnormalities have also been implicated in the pathophysiology and cognitive dysfunction of schizophrenia.98,99
Conclusion
The visual and auditory P300 seem to be impaired not only in schizophrenia but in a variety of psychiatric disorders characterized by cognitive and brain dysfunction. Initial data from psychiatric genetic studies similarly suggest that the putative genes (eg, DISC1, COMT, CHRM2) that underlie these neuroelectrical measures are not diagnostically specific and are involved, to a lesser or greater extent, in multiple psychiatric clinical phenotypes. These findings suggest that P300 abnormalities detected over the posterior scalp in schizophrenia reflect a more general biological and cognitive vulnerability or risk factor that cuts across current psychiatric diagnostic categories. Conceivably, the actual behavioral outcome in any particular individual carrying this vulnerability depends on other genetic and environmental factors specific to the individual.37,72,75–77 This interpretation does not preclude the possibility that the P300 also reflects, in its scalp topography or subcomponents, other or more diagnostically specific illness pathology,100 such as the left-smaller-than-right temporal scalp voltage asymmetry often observed in schizophrenia patients.58–65
In addition, evidence is emerging that P300 can be utilized successfully as an intermediate phenotype in identifying clinically unaffected vulnerability-gene carriers26,67 and, hence, increasing the power of genetic linkage and association studies of complex psychiatric disorders.92 From a clinical perspective, the P300 while unlikely providing a diagnostically specific marker of risk could become useful for the early detection and prediction of psychopathology in general. It remains to be determined, however, whether P300 represents a risk factor or truly a vulnerability marker that is causally related to later manifestation of psychopathology. Furthermore, several other key conceptual and methodological issues remain to be addressed, which will be considered later.
MMN as a Marker of Possible Progressive Pathology
Current theories about the origin of schizophrenia postulate that not only early neurodevelopmental processes but also later progressive, perhaps neurodegenerative, processes are involved in the etiology and pathogenesis of the illness.101–106 The possibility has recently been raised that the MMN component of the ERP could offer a biological marker of postonset progressive cognitive and cortical deterioration in schizophrenia.107,108 If empirically validated, the MMN could have profound theoretical and clinical implications for understanding and treating schizophrenia.
Basic Studies
The MMN refers to a scalp-negative ERP component that is usually recorded in a “passive” auditory oddball paradigm.3,109–112 In this paradigm, subjects passively listen to a series of frequent standard and infrequent deviant auditory stimuli while they are resting or are involved in the attentive processing of visual information, such as reading a book or performing a visual discrimination task. The MMN typically occurs between about 100 and 250 ms following the infrequent, physically deviant auditory stimuli and reaches maximal voltages over frontal and central scalp locations. Although this ERP component may be affected by subject's attention in certain situations,113,114 the MMN is essentially an automatic, preattentive brain response because its generation is not dependent on the subject's attention toward the eliciting deviant auditory stimuli.3,109–112 Human and animal studies suggest that the MMN is generated primarily within the supratemporal plane in or near the primary auditory cortex, with possible additional generating sources in the frontal lobe.3,109–117
The MMN is believed to reflect a “mismatch” or comparison process between the current deviant acoustic input and a neuronal sensory memory trace representing the physical features of the preceding standard stimuli.3,109,110,112 The MMN represents the initial processing step in a biologically important series of cognitive and brain events involved in alerting and redirecting the organism's attention toward novel or deviant, potentially significant, auditory stimulus events in the environment. Because the MMN provides a unique objective measure of auditory discrimination and sensory memory, this component offers a powerful research tool for basic cognitive neuroscience as well as for clinical and other applications.3,109–120
Clinical Studies
Shelley et al121 were the first to report that the MMN elicited by a deviation in tone duration is substantially reduced in chronically ill, medicated schizophrenia patients. Since then many studies have confirmed that MMN responses elicited by a change in tone duration as well as in tone frequency are often markedly reduced in chronic schizophrenia patients as compared with healthy control subjects.54,65, 107,108,116,122–128 The MMN amplitude deficits seen in chronic schizophrenia patients seem to parallel deficits in tone-matching performance122 and do not seem to be affected by antipsychotic medications.108 Moreover, MMN deficits appear to be relatively specific to schizophrenia in that they are not prominent features of unipolar and bipolar affective disorders.126 Abnormalities of MMN, however, have also been observed in dyslexia,129 as well as in normal aging and in various neurological disorders,130 although the observed patterns of MMN deficits in these conditions appear to be different than the pattern seen in chronic schizophrenia.
Additionally, impaired MMN generation in chronically ill schizophrenia patients is associated with higher order cognitive deficits54 and global impairments in social and everyday functioning127,128 but generally does not show a consistent relationship to clinical symptoms.108 Moreover, schizophrenia-like deficits in MMN generation can be experimentally induced in nonhuman primates following either systemic or local infusion of N-methyl-D-aspartate (NMDA) antagonists directly in the auditory cortex,116 as well as in healthy humans following administration of the NMDA receptor antagonist ketamine,131 supporting recent glutamate/NMDA pathophysiological theories of schizophrenia.87,88,116,132,133
The genetics of MMN are as yet poorly understood. It has been reported that the tone duration–deviant MMN is smaller than normal, not only in schizophrenia patients but also in clinically unaffected biological relatives,123 but another family study was unable to confirm the latter finding.134 MMN abnormalities similar to those seen in schizophrenia have been found in adolescents and young adults with 22q11 deletion or velocardiofacial syndrome, which is associated with markedly elevated rates of major psychiatric disorders, including schizophrenia, in early adulthood, with COMT polymorphism modifying the severity of MMN abnormalities in this syndrome.135 These findings seem to implicate that genetically mediated alterations of catecholaminergic, especially dopaminergic, neurotransmission contribute to MMN abnormalities and elevated neuropsychiatric risk in this syndrome. However, a study of twin pairs discordant for schizophrenia reports that MMN amplitudes are smaller than normal in schizophrenia patients but are normal in their unaffected co-twins,136 suggesting that MMN abnormalities reflect state rather than trait characteristics of schizophrenia.
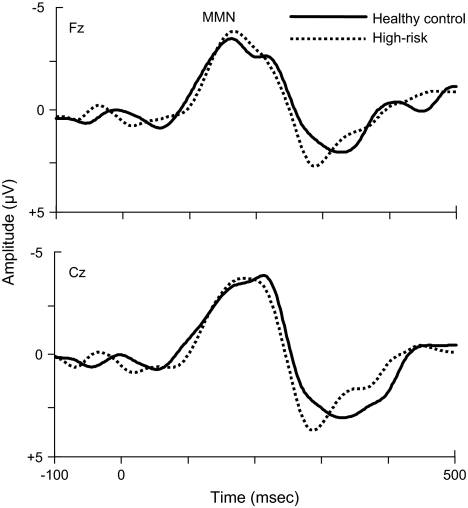
Cross-sectional studies of schizophrenia patients at the initial and advanced stages of illness suggest that MMN abnormalities are present selectively or predominantly in chronically ill patients, whereas recently ill and/or first-episode patients display MMN potentials that do not differ markedly from those recorded in healthy age-matched control subjects.63,65,107 Similarly, in accordance with the findings of a recent study,137 we observed that putatively prodromal patients at high imminent risk for developing a first psychotic episode exhibit, as a group, tone frequency–deviant MMN responses that do not differ significantly from those seen in healthy age-matched control subjects (figure 3), while marked differences between these 2 groups existed in the auditory P300 (figure 2).
Fig. 3.
Group mean event-related potential (ERP) difference waveforms, formed by subtracting the ERPs to auditory standard stimuli from the ERPs to auditory deviant stimuli, recorded at midline frontal (Fz) and central (Cz) scalp locations, superimposed for subjects at high risk for schizophrenia (n = 9; mean age = 21.9 years, SD = 4.5) and healthy age-matched control subjects (n = 10; mean age = 21.9 years, SD = 3.5). The ERPs were collected using a passive auditory oddball paradigm while subjects performed a visual oddball task. The auditory stimuli were pure sinusoidal tones (100-ms duration, 10-ms rise/fall), consisting of frequent standard stimuli (1000-Hz tones, P = 97.1%) and infrequent deviant stimuli (1064-Hz tones, P = 2.9%), presented binaurally through inserted earphones in a random order using a variable interstimulus interval (ISI) of 1300–1700 ms. The visual stimuli were pictures, consisting of frequent standard stimuli (squares), infrequent novel stimuli (unique familiar objects), and infrequent deviant or target stimuli (circles), and were presented on a computer screen in a random order using a constant ISI of 1500 ms. Study participants were instructed to pay attention to the visual stimuli, while ignoring the auditory stimuli, and to make a button-press response only to the infrequent visual target stimuli, emphasizing both speed and accuracy. Mismatch negativity (MMN) refers to the mismatch negativity ERP component associated with auditory change detection. MMN peak latency, peak amplitude, and mean amplitude across the 100- to 250-ms poststimulus latency range did not differ significantly between groups (eg, MMN at Fz, high-risk vs control: peak latency 172 ± 27 ms vs 191 ± 36 ms, t = 1.3, P > 0.22; peak amplitude -4.3 ± 1.4 μV vs -5.3 ± 2.0 μV, t = 1.3, P > 0.22; mean amplitude, -2.4 ± 0.8 μV vs -2.5 ± 1.1 μV, t = 0.2, P > 0.82). Unpublished data from subjects participating in an earlier study from our laboratory.64
Moreover, a recent meta-analysis of MMN in schizophrenia108 reports that the effect size of patient-control group differences in the MMN elicited by a change in tone frequency as well as in tone duration shows significant positive correlations with illness duration (both r > 0.67, P = 0.05). Although all studies included were limited by utilizing cross-sectional, and not longitudinal, designs, these observations raise the possibility that MMN impairments in schizophrenia do not reflect trait and premorbid deficits but develop over time and index postonset progressive cognitive and cortical deterioration in the illness.107,108 Indeed, preliminary longitudinal follow-up data suggest that MMN, at least elicited by tone frequency–deviant stimuli and at the overall group level of analysis, is not impaired at first hospitalization for schizophrenia but declines significantly during the early course of the illness, paralleling MRI-based measures of perionset progressive gray matter loss within the left auditory cortex of these patients.138
Alternatively, incorporating the possibility that MMN partially reflects also trait and premorbid features of schizophrenia,65,123 MMN is impaired at illness onset and indexes progressive pathology but only in a subgroup of recently ill schizophrenia patients who have preexisting neurocognitive deficits and/or go on to have a less favorable outcome and a chronic course of illness.65
Conclusion
Chronically ill schizophrenia patients seem to exhibit not only high-level attention–dependent cognitive processing deficits, as indexed by P300 abnormalities, but also preattentive deficits in auditory discrimination at the initial level of the auditory cortex, as manifested by MMN abnormalities. Because MMN abnormalities observed in schizophrenia appear to be sensitive to premorbid cognitive status65 and family history risk status,123 as well as being much more dominant in chronically ill patients than in recently ill or first-episode patients,63,65,107,108 these abnormalities have been hypothesized to reflect both premorbid or trait characteristics of schizophrenia and postonset progressive illness pathology in brain regions mediating auditory perception and language processing. On this view, the MMN could offer a unique clinical tool that helps to develop and monitor therapeutic interventions aimed at halting, delaying, or even preventing putative progressive cortical and cognitive deterioration in schizophrenia.
Several critical issues, however, remain to be addressed before any conclusions about the MMN in schizophrenia can be drawn with confidence. As already indicated above, longitudinal prospective studies are required to determine whether the severity of MMN abnormalities truly increases across the preonset, recent-onset, and chronic stages of schizophrenia. Longitudinal follow-up MRI data suggest that temporal lobe cortical structures involved in MMN generation, as well as frontal and other brain regions, do show ongoing progressive volume reductions during the early stages of schizophrenia, initiating prior to or around the initial manifestation of full-blown psychotic symptoms.139–141
Additionally, several studies have reported that MMN potentials recorded at the frontal, but not at the temporal, scalp electrode locations are impaired in chronic schizophrenia patients.124,125 These observations have been interpreted as indicating that the hypothesized frontal generating sources of MMN involved in behavioral orienting, rather than the preattentive sensory mismatch detectors in auditory cortex, are compromised in schizophrenia. Alternatively, the dissociation of MMN at frontal vs temporal sites could reflect impaired coactivation or functional “disconnection” of the temporal and frontal cortical regions involved in auditory change detection and orienting.125 To disentangle possible temporal and frontal contributions to MMN abnormalities in schizophrenia, detailed topographic studies are required that record the MMN at many scalp electrodes and utilize advanced signal spatial enhancing methods and anatomical MRI-based information from each study participant in an effort to correct the distortion due to volume conduction of MMN signals through the skull and scalp.2–7,9,10,142
GBR as a Pathophysiological Marker
Event-related neuronal oscillations in the gamma-band frequency range (GBRs) are hypothesized to be fundamental to normal brain function and cognition12,13,15,143–149 and to their disruption in schizophrenia.150–152 Accordingly, the study of GBRs holds promise of unraveling a basic pathophysiological mechanism mediating widespread cognitive dysfunction in schizophrenia. Moreover, research into their underlying neuronal generating mechanisms could lead to pathophysiologically based treatment interventions in schizophrenia,152,153 even if GBRs merely index a “final common pathway”154 or set of molecular and cellular alterations that are genetically and etiologically heterogeneous and downstream consequences of the primary pathogenic process in the illness.133,155
Basic Studies
Recent basic cognitive neuroscience studies suggest that gamma oscillations and their enhancement and phase synchronization during information processing play a key role in a wide variety of cognitive and brain functions in animals and humans.12,13,15,143–149,153 GBRs have been observed during various types of information processing across sensory modalities and across species and at multiple levels of spatial analysis, from microscopic (eg, single unit) to macroscopic (eg, scalp EEG) measurements. According to Galambos,156 3 types of GBRs may be distinguished: firstly, the steady-state–evoked GBR to repetitive stimulation at different frequencies; secondly, the transient-evoked GBR that is phase locked to the onset of a transient stimulus; and lastly, the induced GBR that is not phase locked to stimulus onset. It is generally assumed that evoked GBRs primarily index sensory processing and reflect cortical responses due to changes in afferent activity, whereas induced GBRs are cognitive in nature and generated by changes in functional connectivity within neuronal networks.9,15,157 While stimulus-evoked GBRs are linked to sensory processing, they can be modulated by top-down attentional influences.158,159 Little is as yet known about the genetics of GBRs. A recent study reports significant associations of human auditory-evoked and induced GBRs with genetic polymorphisms of the dopamine receptor D4 (DRD4) and dopamine transporter (DAT1) but not with COMT polymorphism.160
Currently dominant theories hypothesize that stimulus-induced GBRs reflect the dynamic integration or temporal “binding” of spatially distinct neuronal activities within and between brain regions to enable the emergence of coherent perception, thinking, and action.12,15,143–149 According to this view, induced GBRs reflect the operation of a fundamental brain integrative mechanism that counterbalances the distributed anatomical and functional organization of brain activity and are involved in a wide range of cognitive functions, such as sensory discrimination, perception, selective attention, working memory, sensory-motor integration, and motor control. It has been suggested that, while the fast gamma oscillations seem to be most clearly involved in neural synchrony, they ultimately have to be understood in the context of the slower, lower frequencies (eg, beta, alpha, theta) as different frequencies could dynamically interact with each other (eg, gamma-to-beta transitions, multifrequency synchrony) and could reveal different dimensions or aspects of the hypothesized brain integration processes.13,149,161
The neuroanatomy and neurophysiology of GBRs are complex and not yet fully understood. Animal and human studies indicate that GBRs are locally generated in many areas of the brain,12,148,149,153,156–159 including the hippocampus and cerebral cortex, suggesting that these neuronal responses are not unitary brain events associated with a specific sensory or cognitive process, such as temporal binding, but involve a wide variety of anatomically and functionally distinct types of GBRs.13 The cellular mechanisms of GBRs are believed to involve networks of gamma-aminobutyric acid (GABA)ergic interneurons that are driven both by phasic synaptic excitation and inhibition and by electrical coupling between interneuron dendrites and between pyramidal cell axons.153,162,163 Synchronization of gamma activity within such networks of inhibitory interneurons is thought to propagate downstream in cortical microcircuits synchronizing pyramidal cell firing and enabling coherent cortical information processing. The combination of GABAergic synaptic and electrical coupling could represent a general mechanism for the synchronization of neuronal population activity in a variety of brain regions and structures.
Clinical Studies
Several studies have shown that schizophrenia patients display reduced power and phase synchronization of steady-state auditory-evoked GBRs to clicks, tone pips, or amplitude-modulated tones presented at 40 Hz but not at lower rates of stimulation.164–167 A slower buildup of steady-state visual-evoked potential amplitudes following stimulus onset has also been reported in schizophrenia,168 as well as a significant relationship between the latency of the steady-state visual-evoked potential and auditory hallucinations in schizophrenia patients.169 Another study found no significant overall patient-control group differences but reported that steady-state auditory-evoked GBRs are markedly enhanced in schizophrenia patients taking new generation or atypical antipsychotic medications as compared with patients on conventional or typical antipsychotics.170 Results have been mixed as to whether the deficits observed in schizophrenia patients are also present in persons with schizotypal personality traits.165,170
Additionally, early auditory transient-evoked GBRs, which typically are maximal over the centrofrontal scalp and emerge within the first 50 ms following stimulus onset, have been reported to be reduced in power in schizophrenia patients relative to healthy control subjects,171 but other studies detected no significant deficits in early auditory-evoked GBRs in schizophrenia patients.172,173 Also, we recently observed little or no significant abnormalities in the latency, power, and degree of intertrial phase locking of early auditory transient-evoked GBRs to task-irrelevant pure tones in high-risk, recent-onset, and chronic schizophrenia patients, nor were abnormalities observed in the simultaneously occurring auditory-evoked P50, N100, and P200 potentials of the broadband ERPs (O. van der Stelt and A. Belger, unpublished data).
Several studies have found that the integrity of stimulus-evoked GBRs in schizophrenia patients varies according to the nature and severity of their current clinical symptoms. Reduced auditory-evoked GBRs have been noted in nonparanoid, but not in paranoid, schizophrenia subtypes.174 Similarly, increased levels of clinical negative symptoms in schizophrenia patients seem to be associated with diminished auditory-evoked GBRs, whereas increased positive symptoms are associated with enlarged auditory-evoked GBRs.159,175 Moreover, exceptionally large gamma-band rhythms have been measured simultaneously during the occurrence of somatic hallucinations in a schizophrenia patient.176
Furthermore, it has been demonstrated that patients with schizophrenia exhibit impaired phase locking and phase coherence, along with normal power, of early visual transient-evoked GBRs over the occipital scalp during the perception of visual Gestalt patterns,177 suggesting that the temporal synchronization of stimulus-evoked gamma oscillations within the visual cortex is disrupted in schizophrenia. A subsequent study confirmed these findings and also demonstrated that the degree of phase locking of the occipital GBR phase locked to reaction time is positively related to the severity of clinical symptoms, in particular visual hallucinations, thought disorder, and disorganization.178
A recent study observed that visual-induced GBRs, but not evoked GBRs, are disrupted in schizophrenia patients.179 Another study, however, found that visual-induced GBRs during a Gestalt perception task are not abnormal in schizophrenia patients.180 Instead, this study found that the synchronization of oscillations in the lower, beta frequency range is reduced and accompanies performance deficits in schizophrenia patients. While the basis for the discrepancy between results remains to be elucidated, these findings underline the importance of assessing both evoked and induced variants of oscillatory neuronal responses in multiple frequency bands in schizophrenia.
Finally, abnormalities of evoked and induced GBRs do not appear to be diagnostically specific to schizophrenia but can be found in several other psychiatric and neurological disorders, including attention-deficit hyperactivity disorder, autism, epilepsy, and Alzheimer's disease.152,159
Conclusion
Several studies have reported abnormalities in the enhancement and phase synchronization of GBRs during various types of sensory and cognitive information processing in schizophrenia patients. These results provide supporting evidence for the view that neural synchrony is disrupted in schizophrenia. GBR alterations in schizophrenia, however, are not invariant across studies but seem to vary as a function of stimulus and task-specific factors and patient sample characteristics,152 including the type of antipsychotic medication being used by patients170 and the nature and severity of their current clinical symptoms.159,169,174–176,178 Accordingly, although the study of GBRs in schizophrenia is relatively recent, the currently available data seem to suggest that GBR abnormalities represent a clinical state marker, rather than a trait marker, of schizophrenia.
An intriguing finding is the observed relationship in schizophrenia patients between sensory-evoked GBRs and the severity of clinical positive symptoms, particularly hallucinations.159,169,174–176,178 These data suggest that GBRs could provide important theoretical insights into the generative brain mechanisms that give rise to perceptual disturbances and hallucinations in schizophrenia. Correspondingly, it has been hypothesized that the correlations between enlarged GBRs and hallucinations in schizophrenia reflect cortical hyperexcitability and abnormally increased neural synchrony of thalamocortical networks, leading to incoherent or “underconstrained” perception and disturbed conscious experience.147,176,181
It remains to be determined whether the observed GBR abnormalities in schizophrenia reflect disrupted local or within-area temporal synchronization or large-scale or between-area synchronization because the underlying bioelectrical generating sources of these abnormalities cannot be directly inferred and visualized, but only indirectly estimated, on the basis of scalp EEG data alone. The observations that early sensory-evoked, presumably locally generated, GBRs are often preserved in schizophrenia,172–174,179 as well as the finding of reduced synchronization of beta rather than gamma oscillations in schizophrenia patients180 seem to favor the interpretation that impaired long-range functional coordination of neuronal activation is basic to the pathophysiology and cognitive dysfunction of schizophrenia. Important goals for future EEG studies are the characterization of large-scale integration of brain activity and the assessment of dynamic relationships between neuronal oscillations in different frequency bands during information processing in schizophrenia.
Additionally, the pathophysiological significance of GBR abnormalities in schizophrenia has not yet empirically been established. Basic neuroscience studies indicate that networks of GABAergic interneurons and glutamatergic pyramidal cells are critically involved in synchronizing cortical gamma activities.153,162,163 Accordingly, it may be hypothesized that scalp GBR abnormalities in schizophrenia reflect altered chemical transmission and/or electrical coupling within such oscillating interneuronal networks, in line with current theories that implicate disrupted GABA as well as glutamate/NMDA neurotransmission in the pathophysiology and cognitive dysfunction of schizophrenia.87,88,132,133,182 Yet, to substantiate the putative role of GABAergic mechanisms in GBR abnormalities in schizophrenia, clinical studies are required that demonstrate systematic effects of therapeutic treatment interventions targeted on these underlying cellular mechanisms on the production of GBRs in schizophrenia patients.
Unresolved Issues and Future Directions
The main thrust of this article is that ERPs and EROs can offer valuable biological markers of basic pathophysiological mechanisms and cognitive dysfunctions in schizophrenia, even though they may not be specific to current psychiatry's diagnosis and classification. These quantitative biological markers can provide unique information on the nature and extent of cognitive and brain dysfunction in schizophrenia. Moreover, they could play a critically helpful role in the development of effective therapeutic treatment interventions that are focused on specific pathophysiological mechanisms and cognitive dysfunctions rather than on the clinical symptoms of schizophrenia. Also, evidence is emerging that they can be useful as an intermediate phenotype24 in identifying clinically unaffected and affected vulnerability-gene carriers26,67 and, hence, facilitating the genetic dissection of complex psychiatric disorders,92 including schizophrenia and alcoholism and related disorders. Notwithstanding, several important methodological, conceptual, and interpretative issues remain to be addressed if further progress is to be made in this research field.
Initially, a researcher interested in examining ERPs and EROs in schizophrenia is confronted by numerous, partially arbitrary, choices related to experimental design and paradigm (eg, passive vs active paradigm, stimulus characteristics), data acquisition (eg, filter characteristics, reference location), data processing (eg, artifact control), data analysis (eg, Fourier-based vs wavelet analysis), data quantification, and statistical analysis (eg, univariate vs multivariate). Different choices may produce different, or worse, conflicting study findings and conclusions. Fortunately, at least for the recording of cognitive ERPs183 and multiresolution wavelet decomposition of ERPs,184 recording guidelines and analysis protocols have recently been published that may help to resolve this problem.
An additional methodological source of variance is that electrophysiological abnormalities in schizophrenia are usually not invariant across studies and patient samples but that they can be moderated by stimulus and task-specific variables (eg, stimulus properties, task difficulty, or novelty) and subject sample characteristics (eg, clinical symptom severity, medication status, smoking, and drug use), including basic personal variables as age and sex.19,152 While the variance associated with such “moderator” variables185 should be carefully controlled in patient-control group comparisons, assessing and understanding their moderating effects are also conceptually important because these variables may specify the appropriate external and internal conditions under which, or the subpopulations in which, the cognitive dysfunction and pathophysiology of schizophrenia are most reliably be expressed in a certain event-related EEG signal.
Furthermore, the anatomical substrates and functional role in brain information processing of some event-related EEG signals (eg, MMN) are fairly well known, but many scalp EEG signals (eg, P300) are complex and, thus far, not so well understood, which limits the interpretation of observed alterations in these signals in schizophrenia. Detailed topographic studies, utilizing theoretically and empirically well-founded experimental paradigms, advanced signal decomposition, spatial enhancing, and signal-modeling techniques, and MRI-based anatomical information are required if more specific anatomical, functional, and cognitive interpretations of scalp EEG abnormalities are to be made in schizophrenia research.2–7,9,10,142 One promising approach is to utilize also functional neuroimaging or magnetoencephalography data collected from the same study participants using identical or similar experimental paradigms2–6,186 in an attempt to delineate the precise brain processes and structures that underlie scalp-recorded EEG abnormalities in schizophrenia.
Additionally, it is commonly assumed that ERPs and EROs as potential endophenotypes are simpler and more amenable to genetic dissection than the complex overt phenotype associated with the clinical psychiatric disease status itself, but the database on the genetic, environmental, and epigenetic factors that mediate human event-related EEG signals is growing but is, as yet, relatively small. Also, it seems that different event-related EEG signals, such as P300, MMN, and GBR, reflect biologically and cognitively distinct brain mechanisms and, hence, could reflect distinct pathophysiological mechanisms and cognitive dysfunctions in schizophrenia, but evidence that each of the markers is also mediated by a partially distinct set of genes has only recently been reported.187
Moreover, a number of EEG abnormalities have been observed cross-sectionally in the initial and later stages of schizophrenia, but whether these abnormalities are primary and causal or merely a correlate or secondary phenomenon accompanying the clinical illness remain to be determined. For eg, if the P300 truly reflects a vulnerability marker, then psychological or pharmacological therapeutic interventions that succeed in modifying the P300 in high-risk populations should have a modifying effect on the incidence of later psychopathology in these populations. By contrast, if the P300 merely reflects a correlate or risk factor, then interventions should not make a difference in outcome. Longitudinal prospective studies are urgently needed to assess the timing, severity, and etiological validity of electrophysiological abnormalities across the subject's lifespan and illness.
Furthermore, assuming that schizophrenia is a clinically and etiologically heterogeneous disorder,37,155,188 and given the imperfection of current psychiatric diagnostic systems to capture this heterogeneity, family-based high-risk studies should incorporate large subject sample sizes and effective research strategies in which both schizophrenia patients and their unaffected biological relatives are characterized in terms of both the putative vulnerability marker and the clinical disorder because the relatives of patients without the marker may not be at high risk for developing the subtype of the disorder that is associated with the marker under investigation.37
Finally, further studies are needed that evaluate the empirical relationships among different EEG abnormalities in schizophrenia54,63 and the relationships of the individual EEG abnormalities to neuroimaging, neurocognitive, biochemical, and molecular genetic data obtained from the same subjects.59,67 Because each research method and technique has its own strengths and limitations, as well as offering a different but complementary level of description and analysis of schizophrenia pathology, studies utilizing the EEG in conjunction with other research tools will ultimately lead to a more comprehensive description and better understanding of the cognitive and brain functions that are altered in schizophrenia.
Acknowledgments
This research was supported by grant MH58251 from the National Institute of Mental Health, Bethesda, MD; grant MH64065 from the University of North Carolina Schizophrenia Research Center-National Institute of Mental Health Silvio O. Conte Center for the Neuroscience of Mental Disorders, Chapel Hill, NC; and the Foundation of Hope, Raleigh, NC. The authors gratefully acknowledge Franc Donkers and Cy Kim for contributions to data processing and manuscript preparation.
Funding for the research and the Open Access publication charges was provided by Grant MH58251 from the National Institute of Mental Health, Bethesday, MD.
References
- 1.Nunez PL. Electric Fields of the Brain: The Neurophysics of EEG. London, U.K: Oxford University Press; 1981. [Google Scholar]
- 2.Nunez PL. Neocortical Dynamics and Human EEG Rhythms. New York, NY: Oxford University Press; 1995. [Google Scholar]
- 3.Näätänen R. Attention and Brain Function. Hillsdale, NJ: Lawrence Erlbaum; 1992. [Google Scholar]
- 4.Rugg M, editor. Electrophysiology of Mind: Event-Related Brain Potentials and Cognition. Oxford, U.K: Oxford University Press; 1995. [Google Scholar]
- 5.Gevins A. The future of electroencephalography in assessing neurocognitive functioning. Electroencephalogr Clin Neurophysiol. 1998;106:165–172. doi: 10.1016/s0013-4694(97)00120-x. [DOI] [PubMed] [Google Scholar]
- 6.Hillyard SA. Event-related potentials and magnetic fields in the human brain. In: Charney D, editor. Neuropsychopharmacology: The Fifth Generation of Progress. Philadelphia, Pa: Lippincott Williams and Wilkins; 2002. pp. 427–439. [Google Scholar]
- 7.Niedermeyer E, editor. Electroencephalography: Basic Principles, Clinical Applications and Related Fields. 5th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2004. [Google Scholar]
- 8.Lindsley DB. Psychological phenomena and the electroencephalogram. Electroencephalogr Clin Neurophysiol Suppl. 1952;4:443–456. doi: 10.1016/0013-4694(52)90075-8. [DOI] [PubMed] [Google Scholar]
- 9.Pfurtscheller G. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 10.Makeig S. Mining event-related brain dynamics. Trends Cogn Sci. 2004;8:204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Başar E. EEG-Brain Dynamics. Relation Between EEG and Brain Evoked Potentials. Amsterdam, The Netherlands: Elsevier North-Holland Biomedical Press; 1980. [Google Scholar]
- 12.Başar E, editor. Induced Rhythms in the Brain. Boston, Mass: Birkhauser; 1992. [Google Scholar]
- 13.Başar E. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001;39:241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- 14.Regan D. Human Brain Electrophysiology: Evoked potentials and Evoked Magnetic Fields in Science and Medicine. Amsterdam, The Netherlands: Elsevier; 1989. [Google Scholar]
- 15.Tallon-Baudry C. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- 16.Kruglikov SY. Interplay of electroencephalogram phase and auditory-evoked neural activity. J Neurosci. 2003;23:10122–10127. doi: 10.1523/JNEUROSCI.23-31-10122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zubin J. Event-related potential and behavioral methodology in psychiatric research. In: Shagass C, editor. Brain Electrical Potentials and Psychopathology. New York, NY: Elsevier Science Publishing; 1986. pp. 1–26. [Google Scholar]
- 18.Pritchard WS. Cognitive event-related potential correlates of schizophrenia. Psychol Bull. 1986;100:43–66. [PubMed] [Google Scholar]
- 19.Friedman D. Event-related potentials in populations at genetic risk: a methodological review. In: Rohrbaugh JW, editor. Event-Related Brain Potentials: Basic Issues and Applications. Oxford, U.K: Oxford University Press; 1990. pp. 310–332. [Google Scholar]
- 20.Gershon ES. Clinical methods in psychiatric genetics. I. Robustness of genetic marker investigative strategies. Acta Psychiatr Scand. 1986;74:113–118. doi: 10.1111/j.1600-0447.1986.tb10594.x. [DOI] [PubMed] [Google Scholar]
- 21.Schuckit MA. Biological markers in alcoholism. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:191–199. doi: 10.1016/0278-5846(86)90073-4. [DOI] [PubMed] [Google Scholar]
- 22.Nurnberger JI., Jr Should a biological marker be sensitive and specific? Acta Psychiatr Scand. 1992;86:1–4. doi: 10.1111/j.1600-0447.1992.tb03217.x. [DOI] [PubMed] [Google Scholar]
- 23.Iacono WG. Identifying psychophysiological risk for psychopathology: examples from substance abuse and schizophrenia research. Psychophysiology. 1998;35:621–637. [PubMed] [Google Scholar]
- 24.Gottesman The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 25.Braff DL. Preattentional and attentional cognitive deficits as targets for treating schizophrenia. Psychopharmacology. 2004;174:75–85. doi: 10.1007/s00213-004-1848-0. [DOI] [PubMed] [Google Scholar]
- 26.Porjesz B. The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Braff DL. Deconstructing schizophrenia: an overview of the use of endophenotypes in order to understand a complex disorder. Schizophr Bull. 2007;33:21–32. doi: 10.1093/schbul/sbl049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freedman R, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nurnberger JI., Jr A risk factor strategy for investigating affective illness. Biol Psychiatry. 1983;18:903–909. [PubMed] [Google Scholar]
- 30.Sutton S. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- 31.Donchin E. Is the P300 component a manifestation of context updating? Behav Brain Sci. 1988;11:357–374. [Google Scholar]
- 32.Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol. 1992;9:456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Polich J. P300 clinical utility and control of variability. J Clin Neurophysiol. 1998;15:14–33. doi: 10.1097/00004691-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Yordanova J. A single-sweep analysis of the theta frequency band during an auditory oddball task. Psychophysiology. 1998;35:116–126. [PubMed] [Google Scholar]
- 35.van der Stelt O. Cerebral event-related potentials associated with selective attention to color: developmental changes from childhood to adulthood. Psychophysiology. 1998;35:227–239. doi: 10.1017/s0048577298961303. [DOI] [PubMed] [Google Scholar]
- 36.Polich J. Cognitive and biological determinants of P300: an integrative review. Biol Psychol. 1995;41:113–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- 37.Cloninger CR. Event-related potentials in populations at genetic risk: genetic principles and research strategies. In: Rohrbaugh JW, editor. Event-Related Brain Potentials: Basic Issues and Applications. Oxford, U.K: Oxford University Press; 1990. pp. 333–342. [Google Scholar]
- 38.Ratsma JE. Sensation seeking behavior, event-related potential P3 and dopamine D2 receptor A1 allele in adult children of alcoholics. Alcohol Clin Exp Res. 2001;25:960–967. [PubMed] [Google Scholar]
- 39.Johnson R., Jr On the neural generators of the P300 component of the event-related potential. Psychophysiology. 1993;30:90–97. doi: 10.1111/j.1469-8986.1993.tb03208.x. [DOI] [PubMed] [Google Scholar]
- 40.Knight RT. Anatomic bases of event-related potentials and their relationship to novelty detection in humans. J Clin Neurophysiol. 1998;15:3–13. doi: 10.1097/00004691-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Halgren E. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr Clin Neurophysiol. 1998;106:156–164. doi: 10.1016/s0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- 42.Frodl-Bauch T. Neurochemical substrates and neuroanatomical generators of the event-related P300. Neuropsychobiology. 1999;40:86–94. doi: 10.1159/000026603. [DOI] [PubMed] [Google Scholar]
- 43.Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–577. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- 44.Frangou S, et al. The Maudsley Family Study. II: endogenous event-related potentials in familial schizophrenia. Schizophr Res. 1997;23:45–53. doi: 10.1016/S0920-9964(96)00089-8. [DOI] [PubMed] [Google Scholar]
- 45.van Beijsterveldt CE. Individual differences in P300 amplitude: a genetic study in adolescent twins. Biol Psychol. 1998;47:97–120. doi: 10.1016/s0301-0511(97)00025-2. [DOI] [PubMed] [Google Scholar]
- 46.van Beijsterveldt CEM. Stability of genetic and environmental influences on P300 amplitude: a longitudinal study in adolescent twins. Behav Genet. 2001;31:533–543. doi: 10.1023/a:1013389226795. [DOI] [PubMed] [Google Scholar]
- 47.Weisbrod M. Genetic influence on auditory information processing in schizophrenia: P300 in monozygotic twins. Biol Psychiatry. 1999;46:721–725. doi: 10.1016/s0006-3223(99)00022-0. [DOI] [PubMed] [Google Scholar]
- 48.Begleiter H, et al. Quantitative trait loci analysis of human event-related brain potentials: P3 voltage. Electroencephalogr Clin Neurophysiol. 1998;108:244–250. doi: 10.1016/s0168-5597(98)00002-1. [DOI] [PubMed] [Google Scholar]
- 49.Almasy L, et al. Genetics of event-related brain potentials in response to a semantic priming paradigm in families with a history of alcoholism. Am J Hum Genet. 2001;68:128–135. doi: 10.1086/316936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bramon E, et al. Is the P300 wave an endophenotype for schizophrenia? A meta-analysis and a family study. Neuroimage. 2005;27:960–968. doi: 10.1016/j.neuroimage.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 51.Roth WT. Some features of the auditory evoked response in schizophrenics. Arch Gen Psychiatry. 1972;27:466–471. doi: 10.1001/archpsyc.1972.01750280034007. [DOI] [PubMed] [Google Scholar]
- 52.Blackwood DH. Changes in auditory P3 event-related potential in schizophrenia and depression. Br J Psychiatry. 1987;150:154–160. doi: 10.1192/bjp.150.2.154. [DOI] [PubMed] [Google Scholar]
- 53.Duncan CC. Event-related brain potentials: a window on information processing in schizophrenia. Schizophr Bull. 1988;14:199–203. doi: 10.1093/schbul/14.2.199. [DOI] [PubMed] [Google Scholar]
- 54.Javitt DC. Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Arch Gen Psychiatry. 1995;52:550–558. doi: 10.1001/archpsyc.1995.03950190032005. [DOI] [PubMed] [Google Scholar]
- 55.Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36:667–682. [PubMed] [Google Scholar]
- 56.Jeon Y-W. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- 57.Bramon E. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res. 2004;70:315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 58.McCarley RW. Event-related potentials in schizophrenia: their biological and clinical correlates and a new model of schizophrenic pathophysiology. Schizophr Res. 1991;4:209–231. doi: 10.1016/0920-9964(91)90034-o. [DOI] [PubMed] [Google Scholar]
- 59.McCarley RW, et al. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first episode schizophrenia. Arch Gen Psychiatry. 2002;59:321–331. doi: 10.1001/archpsyc.59.4.321. [DOI] [PubMed] [Google Scholar]
- 60.Salisbury DF, et al. First episode schizophrenic psychosis differs from first episode affective psychosis and controls in P300 amplitude over left temporal lobe. Arch Gen Psychiatry. 1998;55:173–180. doi: 10.1001/archpsyc.55.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winterer G. Event-related potentials and genetic risk for schizophrenia. Biol Psychiatry. 2001;50:407–417. doi: 10.1016/s0006-3223(01)01072-1. [DOI] [PubMed] [Google Scholar]
- 62.Jeon Y-W. P300 asymmetry in schizophrenia: a meta-analysis. Psychiatry Res. 2001;104:61–74. doi: 10.1016/s0165-1781(01)00297-9. [DOI] [PubMed] [Google Scholar]
- 63.van der Stelt O. Impaired P3 generation reflects high-level and progressive neurocognitive dysfunction in schizophrenia. Arch Gen Psychiatry. 2004;61:237–248. doi: 10.1001/archpsyc.61.3.237. [DOI] [PubMed] [Google Scholar]
- 64.van der Stelt O. Auditory P300 in high-risk, recent-onset and chronic schizophrenia. Schizophr Res. 2005;77:309–320. doi: 10.1016/j.schres.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 65.Umbricht DSG. Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biol Psychiatry. 2006;59:762–772. doi: 10.1016/j.biopsych.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 66.Bharath S. P300 in family studies of schizophrenia: review and critique. Int J Psychophysiol. 2000;38:43–54. doi: 10.1016/s0167-8760(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 67.Blackwood DHR. Schizophrenia and affective disorders-cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am J Hum Genet. 2001;69:428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simons RF. Physical anhedonia and future psychopathology: an electrocortical continuity? Psychophysiology. 1982;2:433–441. doi: 10.1111/j.1469-8986.1982.tb02500.x. [DOI] [PubMed] [Google Scholar]
- 69.Trestman RL, et al. Event-related potentials in schizotypal personality disorder. J Neuropsychiatry Clin Neurosci. 1996;8:33–40. doi: 10.1176/jnp.8.1.33. [DOI] [PubMed] [Google Scholar]
- 70.Salisbury DF. Topographic abnormalities of P3 in schizotypal personality disorder. Biol Psychiatry. 1996;40:165–172. doi: 10.1016/0006-3223(95)00373-8. [DOI] [PubMed] [Google Scholar]
- 71.Meehl PE. Schizotaxia, schizotypy, schizophrenia. Am Psychol. 1962;17:827–838. [Google Scholar]
- 72.Meehl PE. Schizotaxia revisited. Arch Gen Psychiatry. 1989;46:935–944. doi: 10.1001/archpsyc.1989.01810100077015. [DOI] [PubMed] [Google Scholar]
- 73.Lenzenweger MF. Schizotypy: an organizing framework for schizophrenia research. Curr Dir Psychol Sci. 2006;15:162–166. [Google Scholar]
- 74.McGlashan TH, et al. The PRIME North America randomized double-blind clinical trial of olanzapine versus placebo in patients at risk of being prodromally symptomatic for psychosis. I. Study rationale and design. Schizophr Res. 2003;61:7–18. doi: 10.1016/s0920-9964(02)00439-5. [DOI] [PubMed] [Google Scholar]
- 75.Squires-Wheeler E. A longitudinal study relating P3 amplitude to schizophrenia spectrum disorders and to global personality functioning. Biol Psychiatry. 1993;33:774–785. doi: 10.1016/0006-3223(93)90018-9. [DOI] [PubMed] [Google Scholar]
- 76.van der Stelt O. The P3 component of the human event-related potential as a vulnerability marker of alcoholism. Alcohol Res. 1998;3:6–9. [Google Scholar]
- 77.van der Stelt O. Visual P3 as a potential vulnerability marker of alcoholism: evidence from the Amsterdam study of children of alcoholics. Alcohol Alcohol. 1999;34:267–282. doi: 10.1093/alcalc/34.3.267. [DOI] [PubMed] [Google Scholar]
- 78.van der Stelt O. Neuroelectrical signs of selective attention to color in boys with attention-deficit hyperactivity disorder. Brain Res Cogn Brain Res. 2001;12:245–264. doi: 10.1016/s0926-6410(01)00055-6. [DOI] [PubMed] [Google Scholar]
- 79.O'Donnell BF. Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. Int J Psychophysiol. 2004;53:45–55. doi: 10.1016/j.ijpsycho.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 80.Begleiter H. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- 81.Hill SY. Eight-year longitudinal follow-up of P300 and clinical outcome in children from high-risk for alcoholism families. Biol Psychiatry. 1995;37:823–827. doi: 10.1016/0006-3223(95)00041-E. [DOI] [PubMed] [Google Scholar]
- 82.Polich J. Meta-analysis of P300 amplitude from males at risk from alcoholism. Psychol Bull. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- 83.Porjesz B. Event-related potentials and cognitive function in alcoholism. Alcohol Health Res World. 1995;19:108–112. [PMC free article] [PubMed] [Google Scholar]
- 84.van der Stelt O. P3 scalp topography to target and novel visual stimuli in children of alcoholics. Alcohol. 1998;15:119–136. doi: 10.1016/s0741-8329(97)00106-7. [DOI] [PubMed] [Google Scholar]
- 85.Berman SM. P3 in young boys as a predictor of adolescent substance use. Alcohol. 1993;10:69–76. doi: 10.1016/0741-8329(93)90055-s. [DOI] [PubMed] [Google Scholar]
- 86.Iacono WG. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Arch Gen Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- 87.Harrison PJ. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 88.Ross CA. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 89.Gallinat J, et al. Association of the G1947A COMT (Val(108/158)Met) gene polymorphism with prefrontal P300 during information processing. Biol Psychiatry. 2003;54:40–48. doi: 10.1016/s0006-3223(02)01973-x. [DOI] [PubMed] [Google Scholar]
- 90.Tsai SJ, et al. Association study of a functional catechol-O-methyltransferase-gene polymorphism and cognitive function in healthy females. Neurosci Lett. 2003;338:123–126. doi: 10.1016/s0304-3940(02)01396-4. [DOI] [PubMed] [Google Scholar]
- 91.Bramon E, et al. Is there an association between the COMT gene and P300 endophenotypes? Eur Psychiatry. 2006;21:70–73. doi: 10.1016/j.eurpsy.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 92.Dick DM, et al. Endophenotypes successfully lead to gene identification: results from the Collaborative Study on the Genetics of Alcoholism. Behav Genet. 2006;36:112–126. doi: 10.1007/s10519-005-9001-3. [DOI] [PubMed] [Google Scholar]
- 93.Owen M. Localisation of a susceptibility locus for schizophrenia on chromosome 5. Br J Psychiatry. 1990;157:123–127. doi: 10.1192/bjp.157.1.123. [DOI] [PubMed] [Google Scholar]
- 94.Kim TA, et al. NRP/B, a novel nuclear matrix protein, associates with p110RB and is involved in neuronal differentiation. J Cell Biol. 1998;141:553–566. doi: 10.1083/jcb.141.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jones KA, et al. Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: implications for human brain dynamics and cognition. Int J Psychophysiol. 2004;53:75–90. doi: 10.1016/j.ijpsycho.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 96.Wang JC, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13:1903–1911. doi: 10.1093/hmg/ddh194. [DOI] [PubMed] [Google Scholar]
- 97.Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 98.Dean B. Muscarinic receptors in schizophrenia. Curr Mol Med. 2003;3:419–426. doi: 10.2174/1566524033479654. [DOI] [PubMed] [Google Scholar]
- 99.Raedler TJ, et al. In vivo determination of muscarinic acetylcholine receptor availability in schizophrenia. Am J Psychiatry. 2003;160:118–127. doi: 10.1176/appi.ajp.160.1.118. [DOI] [PubMed] [Google Scholar]
- 100.Turetsky BI. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.DeLisi LE. Is schizophrenia a lifetime disorder of brain plasticity, growth and aging? Schizophr Res. 1997;23:119–129. doi: 10.1016/S0920-9964(96)00079-5. [DOI] [PubMed] [Google Scholar]
- 102.Woods BT. Is schizophrenia a progressive neurodevelopmental disorder? Toward a unitary pathogenetic mechanism. Am J Psychiatry. 1998;155:1661–1670. doi: 10.1176/ajp.155.12.1661. [DOI] [PubMed] [Google Scholar]
- 103.Lieberman JA. Is schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspective. Biol Psychiatry. 1999;46:729–739. doi: 10.1016/s0006-3223(99)00147-x. [DOI] [PubMed] [Google Scholar]
- 104.Shenton ME. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mathalon DH. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:148–157. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- 106.Pantelis C, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 107.Salisbury DF. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:686–694. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- 108.Umbricht D. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 109.Näätänen R. Early selective-attention effect on evoked potential reinterpreted. Acta Psychol. 1978;42:313–329. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- 110.Tiitinen H. Attentive novelty detection in humans is governed by pre-attentive sensory memory. Nature. 1994;372:90–92. doi: 10.1038/372090a0. [DOI] [PubMed] [Google Scholar]
- 111.Picton TW. Mismatch negativity: different water in the same river. Audiol Neurootol. 2000;5:111–139. doi: 10.1159/000013875. [DOI] [PubMed] [Google Scholar]
- 112.Näätänen R. The perception of speech sounds by the human brain as reflected by the mismatch negativity (MMN) and its magnetic equivalent (MMNm) Psychophysiology. 2001;38:1–21. doi: 10.1017/s0048577201000208. [DOI] [PubMed] [Google Scholar]
- 113.Woldorff MG. The effects of channel-selective attention on the mismatch negativity wave elicited by deviant tones. Psychophysiology. 1991;28:30–42. doi: 10.1111/j.1469-8986.1991.tb03384.x. [DOI] [PubMed] [Google Scholar]
- 114.Yucel G. Graded visual attention modulates brain responses evoked by task-irrelevant auditory pitch changes. J Cogn Neurosci. 2005;17:1819–1828. doi: 10.1162/089892905775008698. [DOI] [PubMed] [Google Scholar]
- 115.Alho K. Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound changes. Ear Hear. 1995;16:38–51. doi: 10.1097/00003446-199502000-00004. [DOI] [PubMed] [Google Scholar]
- 116.Javitt DC. Intracortical mechanisms of mismatch negativity dysfunction in schizophrenia. Audiol Neurootol. 2000;5:207–215. doi: 10.1159/000013882. [DOI] [PubMed] [Google Scholar]
- 117.Rinne T. Separate time behaviors of the temporal and frontal mismatch negativity sources. Neuroimage. 2000;12:14–19. doi: 10.1006/nimg.2000.0591. [DOI] [PubMed] [Google Scholar]
- 118.Kraus N. Mismatch negativity in the assessment of central auditory function. Am J Audiol. 1994;3:39–51. doi: 10.1044/1059-0889.0302.39. [DOI] [PubMed] [Google Scholar]
- 119.van der Stelt O. No electrocortical evidence of automatic mismatch dysfunction in children of alcoholics. Alcohol Clin Exp Res. 1997;21:569–575. doi: 10.1097/00000374-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 120.Cheour M. Mismatch negativity (MMN) as a tool for investigating auditory discrimination and sensory memory in infants and children. Clin Neurophysiol. 2000;111:4–16. doi: 10.1016/s1388-2457(99)00191-1. [DOI] [PubMed] [Google Scholar]
- 121.Shelley AM. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biol Psychiatry. 1991;30:1059–1062. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- 122.Javitt DC. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin Neurophysiol. 2000;111:1733–1737. doi: 10.1016/s1388-2457(00)00377-1. [DOI] [PubMed] [Google Scholar]
- 123.Michie PT. Duration mismatch negativity in biological relatives of patients with schizophrenia spectrum disorders. Biol Psychiatry. 2002;52:749–758. doi: 10.1016/s0006-3223(02)01379-3. [DOI] [PubMed] [Google Scholar]
- 124.Baldeweg T. Impairment in frontal but not temporal components of mismatch negativity in schizophrenia. Int J Psychophysiol. 2002;43:111–122. doi: 10.1016/s0167-8760(01)00183-0. [DOI] [PubMed] [Google Scholar]
- 125.Baldeweg T. Mismatch negativity potentials and cognitive impairment in schizophrenia. Schizophr Res. 2004;69:203–217. doi: 10.1016/j.schres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 126.Umbricht D, et al. How specific are deficits in mismatch negativity generation to schizophrenia? Biol Psychiatry. 2003;53:1120–1131. doi: 10.1016/s0006-3223(02)01642-6. [DOI] [PubMed] [Google Scholar]
- 127.Light GA. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry. 2005;62:127–136. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- 128.Kawakubo Y. Support for an association between mismatch negativity and social functioning in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1367–1368. doi: 10.1016/j.pnpbp.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 129.Baldeweg T. Impaired auditory frequency discrimination in dyslexia detected with mismatch evoked potentials. Ann Neurol. 1999;45:495–503. doi: 10.1002/1531-8249(199904)45:4<495::aid-ana11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 130.Pekkonen E. Mismatch negativity in aging and in Alzheimer's and Parkinson's diseases. Audiol Neurootol. 2000;5:216–224. doi: 10.1159/000013883. [DOI] [PubMed] [Google Scholar]
- 131.Umbricht D. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57:1139–1147. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- 132.Coyle JT. The GABA-glutamate connection in schizophrenia: which is the proximate cause? Biochem Pharmacol. 2004;68:1507–1514. doi: 10.1016/j.bcp.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 133.Lewis DA. Pathophysiologically based treatment interventions in schizophrenia. Nature Med. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- 134.Bramon E, et al. Mismatch negativity in schizophrenia: a family study. Schizophr Res. 2004;67:1–10. doi: 10.1016/s0920-9964(03)00132-4. [DOI] [PubMed] [Google Scholar]
- 135.Baker K. COMT Val108/158 Met modifies mismatch negativity and cognitive function in 22q11 deletion syndrome. Biol Psychiatry. 2005;58:23–31. doi: 10.1016/j.biopsych.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 136.Ahveninen J, et al. Inherited auditory-cortical dysfunction in twin pairs discordant for schizophrenia. Biol Psychiatry. 2006;60:612–620. doi: 10.1016/j.biopsych.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 137.Brockhaus-Dumke A. Impaired mismatch negativity generation in prodromal subjects and patients with schizophrenia. Schizophr Res. 2005;73:297–310. doi: 10.1016/j.schres.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 138.Salisbury DF. Pitch and duration MMN at first hospitalization and the early course of schizophrenia. In: Shtyrov Y, editor. Fourth Conference on Mismatch Negativity (MMN) and its Clinical and Scientific Applications. Cambridge, U.K: Medical Research Council, Cognition and Brain Sciences Unit; 2006. p. 49. [Google Scholar]
- 139.Thompson PM, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci USA. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kasai K, et al. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60:766–775. doi: 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Job DE. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25:1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 142.Scherg M. New concepts of brain source imaging and localization. Electroencephalogr Clin Neurophysiol Suppl. 1996;46:127–137. [PubMed] [Google Scholar]
- 143.Eckhorn R, et al. Coherent oscillations: a mechanism of feature linking in the visual cortex? Biol Cybern. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- 144.Gray CM. Oscillatory responses in cat visual cortex exhibit intercolumnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- 145.Galambos R. A 40 Hz auditory potential recorded from the human scalp. Proc Natl Acad Sci USA. 1981;78:2643–2647. doi: 10.1073/pnas.78.4.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pantev C. Human auditory evoked gamma-band magnetic fields. Proc Natl Acad Sci USA. 1991;88:8996–9000. doi: 10.1073/pnas.88.20.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Llinas R. Coherent 40-Hz oscillation characterizes dream state in humans. Proc Natl Acad Sci USA. 1993;90:2078–2081. doi: 10.1073/pnas.90.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Engel AK. Dynamics predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 149.Varela F. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 150.Green MF. Cortical oscillations and schizophrenia. Timing is of the essence. Arch Gen Psychiatry. 1999;56:1007–1008. doi: 10.1001/archpsyc.56.11.1007. [DOI] [PubMed] [Google Scholar]
- 151.Lee K-H. Synchronous gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res Brain Res Rev. 2003;41:57–78. doi: 10.1016/s0165-0173(02)00220-5. [DOI] [PubMed] [Google Scholar]
- 152.van der Stelt O. Macroscopic fast neuronal oscillations and synchrony in schizophrenia. Proc Natl Acad Sci USA. 2004;101:17567–17568. doi: 10.1073/pnas.0407688101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Whittington MA. Neuronal fast oscillations as a target site for psychoactive drugs. Pharmacol Ther. 2000;86:171–190. doi: 10.1016/s0163-7258(00)00038-3. [DOI] [PubMed] [Google Scholar]
- 154.Williamson PC. Mind, Brain, and Schizophrenia. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 155.Andreasen NC. A unitary model of schizophrenia. Bleuler's “fragmented phrene” as schizencephaly. Arch Gen Psychiatry. 1999;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- 156.Galambos R. A comparison of certain gamma band (40 Hz) brain rhythms in cat and man. In: Başar E, editor. Induced Rhythms in the Brain. Boston, Mass: Birkhauser; 1992. pp. 201–216. [Google Scholar]
- 157.Karakaş S. Early gamma response is sensory in origin: a conclusion based on cross-comparison of results from multiple experimental paradigms. Int J Psychophysiol. 1998;31:13–31. doi: 10.1016/s0167-8760(98)00030-0. [DOI] [PubMed] [Google Scholar]
- 158.Tiitinen H. Selective attention enhances the auditory 40-Hz transient response in humans. Nature. 1993;364:59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- 159.Herrmann CS. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol. 2005;116:2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 160.Demiralp T, et al. DRD4 and DAT1 polymorphisms modulate human gamma band responses. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl011. doi:10.1093/cercor/bhl011. [DOI] [PubMed] [Google Scholar]
- 161.Haenschel C. Gamma and beta frequency oscillations in response to novel auditory stimuli: a comparison of human electroencephalogram (EEG) data with in vitro models. Proc Natl Acad Sci USA. 2000;97:7645–7650. doi: 10.1073/pnas.120162397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tamás G. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3:366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- 163.Traub RD. Persistent gamma oscillations in superficial layers of rat auditory neocortex: experiment and model. J Physiol. 2005;562:3–8. doi: 10.1113/jphysiol.2004.074641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kwon JS, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brenner CA. EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am J Psychiatry. 2003;160:2238–2240. doi: 10.1176/appi.ajp.160.12.2238. [DOI] [PubMed] [Google Scholar]
- 166.Gilmore CS. Rate of stimulation affects schizophrenia-normal differences on the N1 auditory-evoked potential. Neuroreport. 2004;15:2713–2717. [PubMed] [Google Scholar]
- 167.Light GA, et al. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 168.Clementz BA. Aberrant brain dynamics in schizophrenia: delayed buildup and prolonged decay of the visual steady-state response. Brain Res Cogn Brain Res. 2004;18:121–129. doi: 10.1016/j.cogbrainres.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 169.Line P. Steady state visually evoked potential correlates of auditory hallucinations in schizophrenia. Neuroimage. 1998;8:370–376. doi: 10.1006/nimg.1998.0378. [DOI] [PubMed] [Google Scholar]
- 170.Hong L, et al. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr Res. 2004;70:293–302. doi: 10.1016/j.schres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 171.Clementz BA. The gamma band response may account for poor P50 suppression in schizophrenia. Neuroreport. 1997;8:3889–3893. doi: 10.1097/00001756-199712220-00010. [DOI] [PubMed] [Google Scholar]
- 172.Clementz BA. Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Exp Brain Res. 2001;139:377–390. doi: 10.1007/s002210100744. [DOI] [PubMed] [Google Scholar]
- 173.Gallinat J. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin Neurophysiol. 2004;115:1863–1874. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 174.Johannesen JK. Contributions of subtype and spectral frequency analyses to the study of P50 ERP amplitude and suppression in schizophrenia. Schizophr Res. 2005;78:269–284. doi: 10.1016/j.schres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 175.Lee K-H. Gamma (40 Hz) phase synchronicity and symptom dimensions in schizophrenia. Cognit Neuropsychiatry. 2003;8:57–71. doi: 10.1080/713752240. [DOI] [PubMed] [Google Scholar]
- 176.Baldeweg T. Gamma-band electroencephalographic oscillations in a patient with somatic hallucinations. Lancet. 1998;352:620–621. doi: 10.1016/S0140-6736(05)79575-1. [DOI] [PubMed] [Google Scholar]
- 177.Spencer KM. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Spencer KM, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci USA. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cho RY. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci USA. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Uhlhaas PJ, et al. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci. 2006;26:8168–8175. doi: 10.1523/JNEUROSCI.2002-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Behrendt RP. Hallucinations in schizophrenia, sensory impairment, and brain disease: a unifying model. Behav Brain Sci. 2004;27:771–787. doi: 10.1017/s0140525x04000184. [DOI] [PubMed] [Google Scholar]
- 182.Benes FM. Emerging principles of altered neural circuitry in schizophrenia. Brain Res Brain Res Rev. 2000;31:251–269. doi: 10.1016/s0165-0173(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 183.Picton TW, et al. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- 184.Quian Quiroga R. Wavelet Transform in the analysis of the frequency composition of evoked potentials. Brain Res Brain Res Protoc. 2001;8:16–24. doi: 10.1016/s1385-299x(01)00077-0. [DOI] [PubMed] [Google Scholar]
- 185.Baron RM. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 186.Heinze HJ, et al. Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature. 1994;372:543–546. doi: 10.1038/372543a0. [DOI] [PubMed] [Google Scholar]
- 187.Hall MH. Genetic overlap between P300, P50, and duration mismatch negativity. Am J Med Genet B Neuropsychiatr Genet. 2006;141:336–343. doi: 10.1002/ajmg.b.30318. [DOI] [PubMed] [Google Scholar]
- 188.Fanous AH. Genetic heterogeneity, modifier genes, and quantitative phenotypes in psychiatric illness: searching for a framework. Mol Psychiatry. 2005;10:6–13. doi: 10.1038/sj.mp.4001571. [DOI] [PubMed] [Google Scholar]
- 189.Samar VJ. Multiresolution analysis of event-related potentials by wavelet decomposition. Brain Cogn. 1995;27:398–438. doi: 10.1006/brcg.1995.1028. [DOI] [PubMed] [Google Scholar]