Abstract
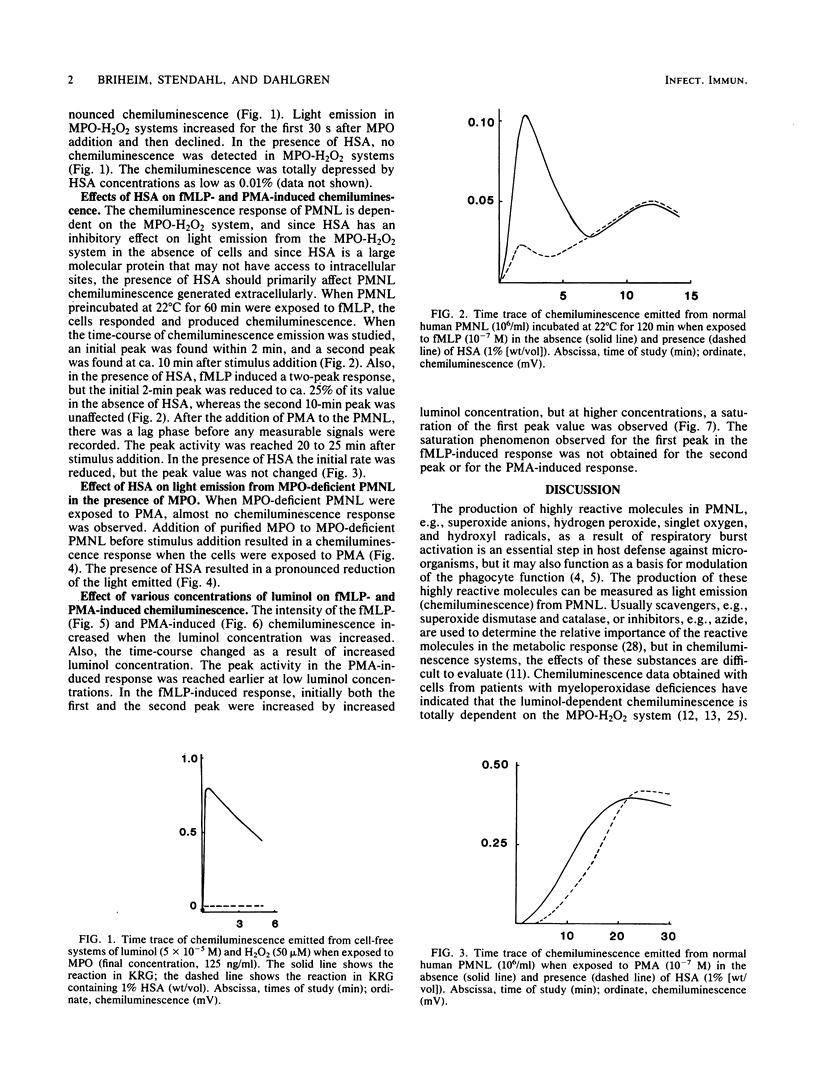
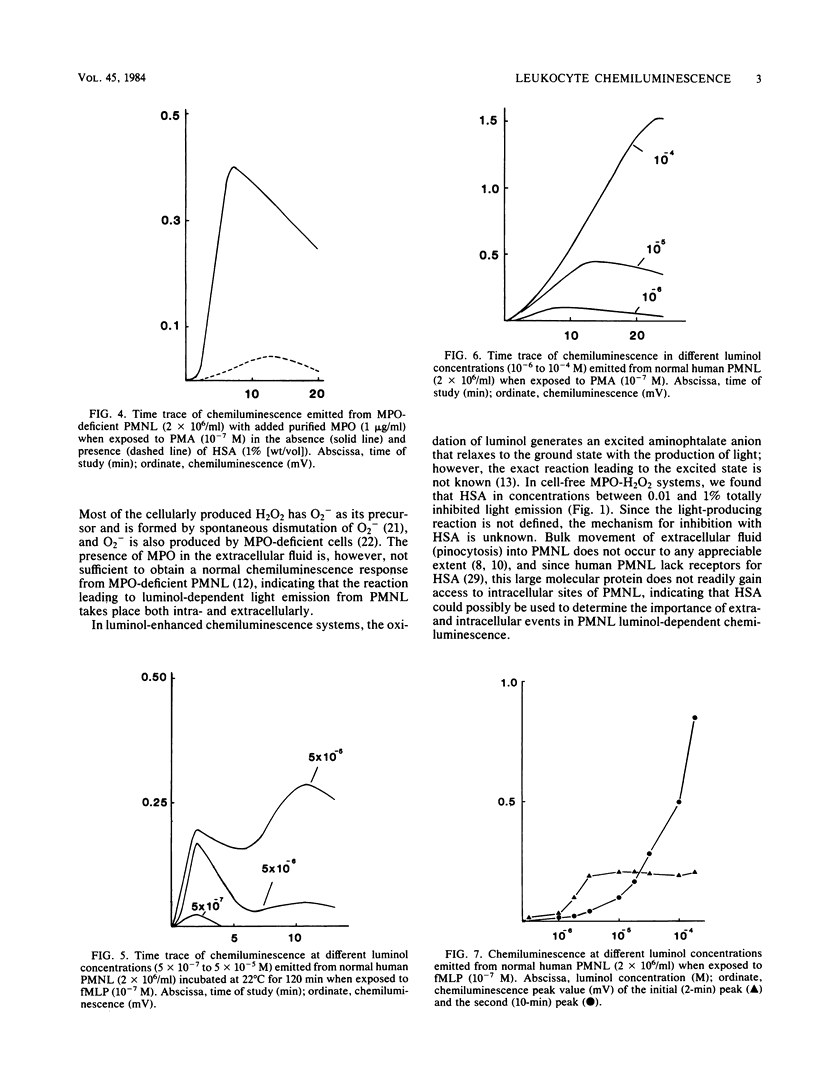
When polymorphonuclear leukocytes (PMNL) and soluble or particulate matter interact, the cells produce chemiluminescence. Luminol-dependent light emission from PMNL is linked to the myeloperoxidase (MPO)-H2O2 system. Light emission from a cell-free MPO-H2O2 system was found to be totally inhibited by human serum albumin (HSA), and since HSA is a large molecular protein that does not readily gain access to intracellular sites of PMNL, it could be used to determine the importance of extra- and intracellular events in PMNL chemiluminescence. In studies with cells from an MPO-deficient patient, we found that HSA inhibited more than 90% of extracellularly produced chemiluminescence. The chemotactic peptide formylmethionyl-leucyl-phenylalanine induced a two-peak chemiluminescence response in normal PMNL, and addition of HSA reduced the first peak, whereas the second peak was unaffected. This result indicated that the first peak was a result of extracellular reactions and the second peak was a result of intracellular reactions of the MPO-H2O2 system. Most of the phorbol myristate acetate-induced response in normal PMNL was due to intracellular events. Furthermore, chemiluminescence of intracellular origin seems to be limited not by generation of oxidative metabolites but by diffusion of luminol into the cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Andersen B. R., Amirault H. J. Important variables in granulocyte chemiluminescence. Proc Soc Exp Biol Med. 1979 Oct;162(1):139–145. doi: 10.3181/00379727-162-40633. [DOI] [PubMed] [Google Scholar]
- Anderson R., Gatner E. M., van Rensburg C. E., Grabow G., Imkamp F. M., Kok S. K., van Rensburg A. J. In vitro and in vivo effects of dapsone on neutrophil and lymphocyte functions in normal individuals and patients with lepromatous leprosy. Antimicrob Agents Chemother. 1981 Apr;19(4):495–503. doi: 10.1128/aac.19.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Boxer L. A., Allen J. M., Davis J. Autooxidation as a basis for altered function by polymorphonuclear leukocytes. Blood. 1977 Aug;50(2):327–335. [PubMed] [Google Scholar]
- Bender J. G., Van Epps D. E. Analysis of the bimodal chemiluminescence pattern stimulated in human neutrophils by chemotactic factors. Infect Immun. 1983 Sep;41(3):1062–1070. doi: 10.1128/iai.41.3.1062-1070.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. H. Studies on phagocytosis. I. Uptake of radio-iodinated (131-I) human serum albumin as a measure of the degree of phagocytosis in vitro. Exp Cell Res. 1969 Jan;54(1):42–48. doi: 10.1016/0014-4827(69)90290-0. [DOI] [PubMed] [Google Scholar]
- Dahlgren C., Magnusson K. E., Stendahl O., Sundqvist T. Modulation of polymorphonuclear leukocyte chemiluminescent response to the chemoattractant f-Met-Leu-Phe. Int Arch Allergy Appl Immunol. 1982;68(1):79–83. doi: 10.1159/000233070. [DOI] [PubMed] [Google Scholar]
- Dahlgren C., Stendahl O. Effect of in vitro preincubation of polymorphonuclear leukocytes on formylmethionyl-leucyl-phenylalanine-induced chemiluminescence. Infect Immun. 1982 Jul;37(1):34–39. doi: 10.1128/iai.37.1.34-39.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren C., Stendahl O. Role of myeloperoxidase in luminol-dependent chemiluminescence of polymorphonuclear leukocytes. Infect Immun. 1983 Feb;39(2):736–741. doi: 10.1128/iai.39.2.736-741.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren C., Tagesson C., Magnusson K. E. Modulation of the locomotion of polymorphonuclear leukocytes by a synthetic amphiphile, poly(ethyleneglycol) palmitate. Exp Cell Res. 1979 May;120(2):429–433. doi: 10.1016/0014-4827(79)90406-3. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., Long G. D., Shirley P. S., Bass D. A., Thomas M. J., Henderson F. W., Cohen M. S. Mechanism of the luminol-dependent chemiluminescence of human neutrophils. J Immunol. 1982 Oct;129(4):1589–1593. [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S. Chemiluminescence of human neutrophils induced by soluble stimuli: effect of divalent cations. Infect Immun. 1982 Jan;35(1):206–212. doi: 10.1128/iai.35.1.206-212.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glette J., Solberg C. O., Lehmann V. Factors influencing human polymorphonuclear leukocyte chemiluminescence. Acta Pathol Microbiol Immunol Scand C. 1982 Apr;90(2):91–95. doi: 10.1111/j.1699-0463.1982.tb01423.x. [DOI] [PubMed] [Google Scholar]
- Hastings M. J., Petricevic I., Williams A. J., Cole P. J., Easmon C. S. The effect of culture media on the production and measurement of luminol-dependent chemiluminescence. Br J Exp Pathol. 1982 Apr;63(2):147–153. [PMC free article] [PubMed] [Google Scholar]
- Jesaitis A. J., Naemura J. R., Painter R. G., Schmitt M., Sklar L. A., Cochrane C. G. The fate of the N-formyl-chemotactic peptide receptor in stimulated human granulocytes: subcellular fractionation studies. J Cell Biochem. 1982;20(2):177–191. doi: 10.1002/jcb.240200209. [DOI] [PubMed] [Google Scholar]
- Kato T., Wokalek H., Schöpf E., Eggert H., Ernst M., Rietschel E. T., Fischer H. Measurement of chemiluminescence in freshly drawn human blood. I. Role of granulocytes, platelets, and plasma factors in zymosan-induced chemiluminescence. Klin Wochenschr. 1981 Mar 2;59(5):203–221. doi: 10.1007/BF01476577. [DOI] [PubMed] [Google Scholar]
- Niedel J., Wilkinson S., Cuatrecasas P. Receptor-mediated uptake and degradation of 125I-chemotactic peptide by human neutrophils. J Biol Chem. 1979 Nov 10;254(21):10700–10706. [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976 Jul;58(1):50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A., Jesaitis A. J., Painter R. G., Cochrane C. G. Ligand/receptor internalization: a spectroscopic analysis and a comparison of ligand binding, cellular response, and internalization by human neutrophils. J Cell Biochem. 1982;20(2):193–202. doi: 10.1002/jcb.240200210. [DOI] [PubMed] [Google Scholar]
- Stevens P., Winston D. J., Van Dyke K. In vitro evaluation of opsonic and cellular granulocyte function by luminol-dependent chemiluminescence: utility in patients with severe neutropenia and cellular deficiency states. Infect Immun. 1978 Oct;22(1):41–51. doi: 10.1128/iai.22.1.41-51.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S. J., Zigmond S. H. Chemotactic peptide receptor modulation in polymorphonuclear leukocytes. J Cell Biol. 1980 Jun;85(3):703–711. doi: 10.1083/jcb.85.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb L. S., Keele B. B., Jr, Johnston R. B., Jr Inhibition of phagocytosis-associated chemiluminescence by superoxide dismutase. Infect Immun. 1974 Jun;9(6):1051–1056. doi: 10.1128/iai.9.6.1051-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



