Abstract
Neural oscillations and their synchronization may represent a versatile signal to realize flexible communication within and between cortical areas. By now, there is extensive evidence to suggest that cognitive functions depending on coordination of distributed neural responses, such as perceptual grouping, attention-dependent stimulus selection, subsystem integration, working memory, and consciousness, are associated with synchronized oscillatory activity in the theta-, alpha-, beta-, and gamma-band, suggesting a functional mechanism of neural oscillations in cortical networks. In addition to their role in normal brain functioning, there is increasing evidence that altered oscillatory activity may be associated with certain neuropsychiatric disorders, such as schizophrenia, that involve dysfunctional cognition and behavior. In the following article, we aim to summarize the evidence on the role of neural oscillations during normal brain functioning and their relationship to cognitive processes. In the second part, we review research that has examined oscillatory activity during cognitive and behavioral tasks in schizophrenia. These studies suggest that schizophrenia involves abnormal oscillations and synchrony that are related to cognitive dysfunctions and some of the symptoms of the disorder. Perspectives for future research will be discussed in relationship to methodological issues, the utility of neural oscillations as a biomarker, and the neurodevelopmental hypothesis of schizophrenia.
Keywords: oscillations, synchrony, schizophrenia
Neural Oscillations and Synchrony
A prominent property of neural networks is their tendency to engage in oscillatory activity. Hans Berger (1873–1941) was one of the first scientists to observe the brain's rhythms which he recorded in the form of electrical activity on the scalp of healthy, awake participants.1 He also introduced the current nomenclature naming different rhythms by Greek letters. Rhythmic electroencephalographic (EEG) activity is commonly subdivided in 5 major frequency bands, the delta- (0–3 Hz), theta- (4–7 Hz), alpha- (8–12 Hz), beta- (13–30 Hz), and gamma-band (30–200 Hz). (We did not include oscillations in the delta-frequency range [0–3 Hz] in the review, as evidence on the functional role of delta oscillations is so far limited.)
Since the early discoveries of Berger and others, EEG has become a valuable tool for the study of brain functions as well as for clinical investigations of various neurological and psychiatric disorders. More recently, a method for recording the brain's magnetic activity noninvasively—magnetoencephalogram (MEG)—has been developed that has significantly improved the spatial resolution of extracranial recordings and, thereby, also the detectability of low-amplitude, high-frequency oscillations.
In the last 2 decades, the investigation of brain activity in EEG and MEG data has experienced an important paradigm shift because the focus has moved from the analysis of event-related averaging of neuronal responses (event-related potentials [ERPs]) to methods that investigate the power, the coherence, and the phase locking of nonaveraged oscillating signals. The reasons for this paradigm shift are in part technical; the access to computational power has increased, and the analysis techniques have proliferated.2 However, the most important reason for this shift is conceptual: the discovery of synchronized, oscillatory in neuronal spiking activity led to novel hypotheses about the putative functions of the phenomenon,3–5 and the testing of these hypothesis required the analysis of nonstimulus-locked internally generated temporal patterns.
Oscillations that reflect self-generated activity are referred to as induced oscillations and preclude evaluation of averaged responses and, therefore, require single-trial analyses because their latency varies from trial to trial. Typically, the latency in which induced oscillations occur is within the time window of 150–400 ms. In contrast, evoked oscillations are strictly phase locked to the onset of the stimulus and are measured by stimulus-triggered averages of responses. Evoked oscillations typically occur within a latency window of 50–150 ms and have been related to early, stimulus-driven encoding processes.
An important link between oscillations and cortical computations was the discovery of the role of oscillatory rhythms in the beta/gamma range (20–80 Hz) in establishing precise synchronization of distributed neural responses. Gray and colleagues6,7 showed that action potentials generated by cortical cells align with the oscillatory rhythm in the beta and gamma range, which has the consequence that neurons participating in the same oscillatory rhythm synchronize their discharges with very high precision. Thus, it is a central role of cortical oscillations in the beta/gamma range to enable neuronal synchronization.4,5
Self-generated oscillations and synchronization are highly dynamic phenomena and depend on numerous conditions, such as central states,4,5,8 stimulus configuration,6,7 or attention.9 The occurring strength of synchrony is closely correlated with perceptual processes such as feature binding, subsystem integration, brightness perception, and interocular rivalry.4 In addition, the strength of synchronization predicts whether an animal will give a correct response in an upcoming trial of a perceptual decision task,10 suggesting its important functional role.
Synchronization patterns between pairs of neurons have a particular topology that exhibits the properties of small-world networks. Networks with “small-world properties” are characterized by a combination of local clustering of activity and a short path length as an index of global integration. The hubs of these networks—ie, neurons synchronized most strongly with the rest of the network—exhibit most prominently their cortical functions, ie, orientation selectivity in the early visual areas, suggesting a close relation between neuronal oscillations and synchrony on the one hand and the organization of network interactions on the other.11
Possibly the most important function of synchronized, oscillatory activity is the implementation of a mechanism that can exploit the relative phase of oscillations. Recent data show a systematic relation between the phase offset of synchronized spiking and stimulus properties,12–14 suggesting that information is encoded in the relative firing times of the discharges of distributed neurons.
As the empirical data indicate, cells that are excited more strongly tend to fire earlier than those excited less strongly.14 This offers a particularly efficient mechanism for encoding stimulus-related information, which can be either redundant or complementary to that already provided by the rate responses. Redundant information would provide more precision to the encoding process, and complementarity would provide information additional to that coded by firing rates. Either way, an important advantage of such a temporal coding scheme is the processing speed with which participating neuronal structures may conduct computations.
The information about a neuron's firing rate can be assessed accurately only after observing this neuron's activity for some time (eg, 100 ms or more). In contrast, the information about the phase of an action potential is present instantaneously (ie, within a single cycle of a beta/gamma oscillation) and, hence, can be, at least in principle, also extracted within such a short period of time. Such accurate and readily available information can enhance numerous cognitive processes, ranging from object recognition to decision making.
The mechanisms underlying the generation of these spatiotemporal patterns associated with beta/gamma oscillations are likely to be similar to those responsible for the phase precession of pyramidal cell firing in the hippocampus relative to theta oscillations.15,16 Cortical neurons not only generate such precise temporal information but also appear highly capable of reading out the same information when receiving inputs from earlier processing stages. The possible readout mechanisms have been discussed most commonly in the context of the varying spike latencies of the sensory inputs.16–18
In addition to the high-frequency oscillations in the beta- and gamma-band, oscillatory rhythms in the theta- and alpha-band also play an important role in cortical computations. Alpha activity (8–12 Hz) has been not only associated with an inhibitory function19 but also with the long-distance coordination of gamma oscillations,20 and theta activity has been proposed to support large-scale integration of subsystems serving the formation and recall of memories.21–23 In general, there is a correlation between the distance over which synchronization is observed and the frequency of the synchronized oscillations. Short-distance synchronization tends to occur at higher frequencies (gamma-band) than long-distance synchronization, which often manifests itself not only in the beta- but also in the theta- (4–8 Hz) and alpha- (8–12 Hz) frequency range.23,24
Thus, oscillations and their synchronization are important correlates of neuronal processing and provide valuable measures for the assessment of normal and pathological functions. (It is important to note that the remaining part of this review focuses on studies based solely on field recordings [EEG, MEG] rather than the activity of individual neurons. Consequently, it can never be directly resolved whether an increase in the power of these field recordings is due to the increase in the strength of synchrony or in the strength of oscillations.).
Theta Oscillations
Theta oscillations occurring in the frequency range of approximately 4–8 Hz represent one of the best-studied rhythms in the mammalian brain (for a review, see Buzsaki,21 Kahana et al,25 and Lengyel et al26). In mammals, theta oscillations are particularly prominent in the hippocampus but occur also in extrahippocampal regions, such as the ento- and the perirhinal cortex, the prefrontal, somatosensory, and visual cortex, and superior colliculus.27,28 In the hippocampus, theta oscillations are generated by an interplay of glutamatergic and gamma-aminobutyric acidergic (GABAergic) neurons.29,30 In addition, GABAergic inputs are modulated by cholinergic inputs from the septum that possibly acts as a pacemaker for theta activity.31
Studies in rodents revealed a close relationship between the occurrence of theta oscillations in the hippocampus, locomotion, and the place-specific firing of hippocampal pyramidal cells (place cells).15 These place cells have spatially selective fields and discharge when the animal is at the location corresponding to the receptive place fields.32 In a seminal study, O'Keefe and Recce15 reported that the phase of the place cell firing relative to the ongoing theta oscillations advances gradually as the rat passes through the cell's place field. This phenomenon, called phase precession, has been interpreted as a mechanism to increase the precision of spatial coding and to bind assemblies of place cells for the representation of movement trajectories.21,16
Spatial navigation, a process requiring evaluation and temporary storage of relations, is only one of the numerous functions of the hippocampus. A large body of evidence indicates that the special ability of the hippocampus to process relations is used in a much wider context and plays a crucial role in the formation and recall of episodic and declarative memory.33,34
Accordingly, theta oscillations have also been assigned functions in memory-related processes. (1) Manipulations that eliminate theta activity in the hippocampus produce deficits in spatial and nonspatial working memory (WM) tasks in rodents (for a review, see Gevins et al35); (2) theta oscillations correlate with WM load35,36 and link neural assemblies in frontal and parietal cortices during the maintenance of items in WM22; (3) significant increases in theta power during encoding of information predict subsequent recall of information37; and (4) in human EEG data, a theta component that is largest over midline frontal regions (frontal midline theta) is enhanced in tasks involving WM and requiring focused attention.35,36
An involvement of theta activity in memory processes is also supported by the evidence that rhythmic stimulation in this frequency range (theta burst stimulation) is particularly effective in inducing long-term potentiation (LTP) and that LTP induction is highly sensitive to the phase between stimulation relative to the ongoing theta rhythm. Stimulation at the depolarizing peak of the theta cycle favors LTP while stimulation in the through causes depotentiation. Thus, both in vitro and in vivo data suggest that theta oscillations act as a windowing mechanism determining the threshold and polarity of synaptic modifications.38–40
Alpha Oscillations
Alpha oscillations, the frequency of which is centered around 10 Hz, were the first rhythm to be discovered by Berger in 1924.1 Alpha activity is very prominent in the thalamus and can be sustained by isolated thalamic networks,41 which led to the assumption that cortical alpha is driven by thalamic peacemakers (see Basar et al42 for a review). However, da Silva et al43 demonstrated that cortical alpha results from synergistic interactions within thalamo-cortical-thalamic reentrant networks (see Sauseng et al44 for a review). In addition, alpha oscillations have also been recorded in subcortical areas, such as the hippocampus and the reticular formation.42
It is well established that alpha rhythms result from reciprocal interactions between excitatory and inhibitory neurons whereby the synchronization is in addition stabilized by gap junctions among inhibitory interneurons.45,46 The susceptibility of these networks to engage in alpha rhythms is in turn modulated by cholinergic and serotonergic mechanisms and by glutamatergic afferents acting via metabotropic receptors.47
Alpha activity is most prominent over occipital cortex when the eyes are closed and subjects are in a relaxed state and has therefore been regarded as reflecting cortical idling.48 Typically, opening the eyes results in an alpha blockade which has been linked to active stimulus processing.44,49 It is currently under debate, whether alpha oscillatory activity is related to functional inhibition of task-irrelevant processing44 or whether it is a direct and essential constituent of the active network (for a review, see Palva and Palva20). Results from studies investigating visuospatial attention50–52 and WM,44,53,54 especially when capacity limits are reached,44 have been interpreted in favor of the inhibition hypothesis.
In contrast, evidence for an involvement of alpha activity in information processing has been provided by studies on mental imagery,55 conscious somatosensory perception,56 and WM tasks.20 Furthermore, there is an evidence for a relationship between long-range coherence in the alpha-frequency band and perceptual and cross-modal binding.54,57,58
Several lines of research indicate a relationship between baseline alpha activity and the expression of ERPs. Low prestimulus alpha amplitudes are associated with larger P1 responses in a visual discrimination task and enhance good performance. By contrast, high prestimulus alpha is associated with enhanced P3 activity and enhanced retention in WM tasks.44 The underlying assumption is that the sensory stimulus induces phase resetting of ongoing rhythmic activity in each trial and that averaging these phase-coherent rhythms produces the ERP.59,60 Another possibility is that the ERP reflects a transient response to the stimulus that is superimposed on the background EEG. The current literature suggests that these 2 mechanisms coexist (for a review, see Sauseng et al44).
Nevertheless, it is still a technical challenge to disentangle the ERP response from ongoing oscillations.61 The available methods do not allow to distinguish conclusively between phase resetting and transient activity. Phase-coherent activity specifically in the lower frequency bands has been taken as evidence for the former model. However, the contrary may be true as well because transient bursts of neural activity may likewise result in similar phase-coherent activity. Thus, many of their physical characteristics may be overlapping and should not be used as criteria for the presence of either kind of activity (for a discussion, see Yeung et al61).
Beta Oscillations
Frequencies between 12 and 30 Hz are termed beta activity. Beta oscillations occur in all cortical areas and numerous subcortical structures including nonspecific thalamic nuclei, the hippocampus, the basal ganglia, and olfactory bulb.
Generation of beta-band oscillations has been linked to neurotransmitter systems including metabotropic glutamate as well as N-methyl D-aspartate (NMDA) receptors and GABAa receptor activity.62,63 Of particular clinical relevance is the dopaminergic modulation of beta oscillations in the basal ganglia, the subthalamic nucleus, and the motor cortex.64 In Parkinson disease, reduced dopaminergic control leads to the instability to desynchronize these beta oscillations that prevail in the rest condition and need to be replaced by gamma oscillations in order to enable the initiation of voluntary movements.65
This central beta oscillation has been related to the rolandic mu rhythm that is generated by the sensorimotor cortex and is most prominent at rest and attenuated or abolished by moving or observing biological movements.66 There is evidence for enhanced synchronized beta-band activity prior to movements in sensorimotor cortex influencing descending motor commands to contralateral hand muscles.67–69 Such corticomuscular coherence is the strongest during steady hold periods after movement but is abolished during the actual movement.68
In addition, beta activity has been implicated in a variety of cognitive tasks, such as learning in the mammalian olfactory bulb,70 novelty detection in the auditory system,71 sensory gating,72,73 and reward evaluation.74 It has been suggested that the common denominator of beta oscillations is to highlight a stimulus as novel or salient that warrants further attention.72
Furthermore, beta-band activity is involved in large-scale coordination of distributed neural activity. Kopell and colleagues29 showed that beta oscillations support more effectively coherence over large distances than gamma oscillations because their synchronization is less susceptible to long conduction delays. Consistent with this hypothesis, Tallon-Baudry and colleagues75 observed increased oscillatory activity in the beta-frequency range between 2 sites in inferior temporal cortex during the maintenance period in a WM task. Further evidence for a relationship between long-range synchronization and beta-band activity was reported by Schnitzler and Gross.76 The authors investigated the neural networks underlying attention control in visual processing in the attentional blink task. The results revealed that communication within the fronto-parieto-temporal attentional network proceeds via transient long-range phase synchronization in the beta-band.
Gamma Oscillations
Rhythms >30 Hz are addressed as gamma oscillations and occur in virtually all brain structures, including the olfactory bulb and the retina. These oscillations cover a broad frequency band ranging up to 200 Hz. Gamma-band oscillations were first recorded by Adrian and Matthews48 from the olfactory bulb following odor stimulation and have been later observed in the visual cortex following visual stimulation.
Generation of gamma-band activity is critically dependent upon several neurotransmitter systems. The networks of chemically and electrically coupled GABAergic neurons play a pivotal role in the primary generation of high-frequency oscillations and local synchronization77–81 while more far-reaching glutamatergic connections appear to control their strength, duration, and long-range synchronization.79 Cholinergic modulation via muscarinic receptors plays a crucial role both in the fast, state-dependent facilitation of gamma oscillations and associated response synchronization as well as in the control of use-dependent long-term modification of cortical dynamics that favor synchronization of responses in the gamma-frequency range.82,83
First indications for a functional role of gamma-band oscillations for information processing in cortical networks have been obtained in studies investigating the relations between stimulus-induced synchronization of gamma oscillations and feature binding in cat primary visual cortex (V1) by Gray and Singer7 (see figure 1). This relationship was then confirmed in a series of studies with EEG and MEG that found robust support for the role of gamma oscillations in the grouping of stimulus elements into coherent object representations.84,85
Fig. 1.
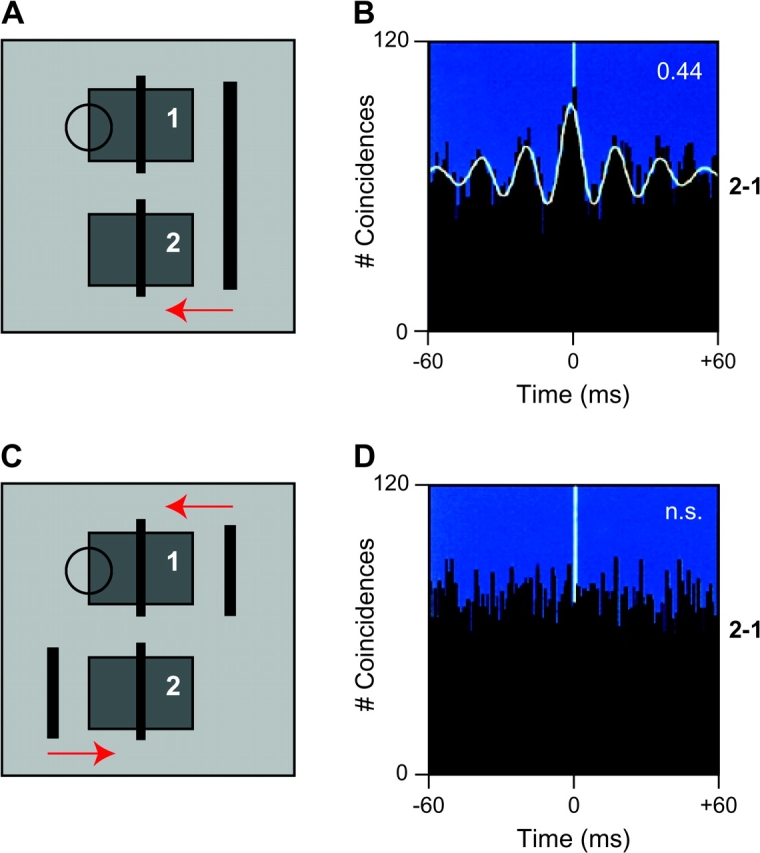
Synchronous Gamma Oscillations and Perceptual Binding. Responses are recorded for a pair of multiunit (MUA) activity with nonoverlapping receptive fields in primary visual cortex of an anesthetized cat. In the 2 different visual stimulation conditions, the stimuli consisted either of a single long moving bar (A) or of 2 small bars moving in different directions (C). In both conditions, the receptive fields were stimulated near optimally and simultaneously, and consequently, no changes in neurons' firing rates were observed (results not shown). In contrast, neuronal synchronization between the 2 MUAs changed strongly, as estimated by the cross-correlation histograms (CCHs) in (B) and (D). The high center peak and the good fit of a Gabor function in (B) indicate that the stimulus in (A) evoked strong neuronal synchrony, while the flat CCH in (D) indicates lack of synchronization between the responses to stimuli in (C).
Further research has found a close relationship between attention, oscillations, and synchrony in the gamma-band range. Fries and colleagues86 recorded neurons in cortical area V4 while macaque monkeys attended to behaviorally relevant stimuli and ignored distracters. Neurons activated by the attended stimulus showed increased gamma synchronization (35–90 Hz) compared with neurons at nearby V4 sites activated by distracters. Consistent evidence for the relationship between attention and gamma-band activity both during visual and auditory perception has been also found in human EEG and MEG recordings.87–89 Taken together, these findings suggest that gamma-band oscillations have a general computational role in dynamically selecting neurons that communicate information about sensory inputs effectively.
Gamma oscillations have been also implicated in higher cognitive functions, such as memory. Tallon-Baudry and colleagues90 examined EEG-activity during a visual short-term memory task. Induced gamma-band activity was observed during the delay over frontal and parietal electrodes, indicating that gamma oscillations are involved in the maintenance of information in WM. Additional evidence for the role of gamma oscillations during WM has been obtained from intracranial recordings91 as well as from studies that examined the relationship between auditory WM and gamma oscillations in MEG data.92 Finally, gamma oscillations may also be involved in long-term memory because there is a relationship between the amount of gamma-band activity during the encoding of information and the subsequent recall.37,93,94
Recent data suggest that synchronized gamma-band activity may also be related to consciousness. A large body of evidence suggest that consciousness has to be understood as a function of numerous interacting systems that require a mechanism that transiently synchronizes a number of widely distributed neural assemblies.95 Melloni et al96 examined EEG data in response to the processing of visible and invisible words in a delayed matching to sample task. Both perceived and nonperceived words caused a similar increase of local (gamma) oscillations in the EEG, but only perceived words induced a transient long-distance synchronization of gamma oscillations across widely separated regions of the brain.
In addition to the role of gamma-band oscillations in perceptual organization, attention, memory, and consciousness, gamma-band activity has also been related to other cognitive phenomena, including language processing and motor coordination (for comprehensive reviews, see Fries et al4 and Singer5).
As has been discussed for theta oscillations, gamma oscillations are also involved in the gating of synaptic plasticity.82 The mechanisms are similar, but because of the high frequency of gamma oscillations, the temporal resolution of the gating process is much higher and capable of distinguishing temporal offsets between pre- and postsynaptic activity in the range of milliseconds.
Neural Oscillations in Cortical Networks
In summary, cortical networks have a strong tendency to engage in oscillatory activity at multiple frequency bands. These oscillations provide a temporal frame for the timing of discharges and an option to use precise temporal resolutions for the encoding of information. The special case of synchrony is likely to be exploited for the definition of relatedness both in information processing and learning. In addition, synchronization seems to be used to increase the salience of signals, to facilitate their propagation across sparsely connected networks, and to assure selective routing. Moreover, systematic phase shifts between the discharges of individual neurons and population oscillations appear to be exploited for cortical computations. However, numerous questions concerning the functional roles and definition of oscillations are still unanswered.
One question relates to the validity of the current system of assigning oscillations into distinct frequencies. For example, theta oscillations in the rodent hippocampus cover a wide range of frequencies that can vary with the behavioral state of the animal. Similarly, it is unclear whether oscillations in the beta- and gamma-band should be considered as strictly distinct rhythms or whether there is continuum between both frequencies that may relate to a common underlying physiological mechanism.
Moreover, it is conceivable that subbands within theta, alpha, beta, and gamma oscillations may serve differential mechanisms. Gamma oscillations cover a wide frequency range from 30 to 200 Hz, possibly containing different subclasses of rhythms. Vidal et al,89 eg, recently reported gamma-band activity in 2 frequency bands in MEG-recordings during perceptual grouping, suggesting that grouping is associated with high (70–120 Hz) and attentional focusing with lower (44–66 Hz) gamma-band oscillations.
Another important conundrum is the interaction between oscillations of different frequencies. A characteristic of oscillations in cortical networks is that multiple frequency bands coexist, and there is evidence that interactions between oscillations at different frequencies are used for the encoding of nested relations (for a review, see Jensen and Colgin93).
Cross-frequency coupling has been observed between theta and gamma-rhythms during various cognitive tasks and in several brain areas. Specifically, modulation of gamma oscillations through the phase of the theta rhythm is associated with visual perception97 and WM.98 Lisman99 proposed that the coupling between these 2 rhythms may represent a more general coding scheme for information processing.
In addition to the coupling between the phase of a particular frequency and the power of an oscillation, different principles of cross-frequency interaction are conceivable.93 For example, phase-to-phase interactions have been observed between several frequency bands by Palva et al.100 The authors tested cross-frequency coupling (n:m phase synchrony) in MEG data through examining phase synchronization between oscillations during a mental arithmetic task. Significant interactions between alpha, beta, and gamma oscillations were observed that increased with task load and with pronounced interaction between gamma- and alpha-band oscillations (table 1).
Table 1.
Neural Oscillations in Cortical Networks
| Theta (4–7 Hz) | Alpha (8–12 Hz) | Beta (13–30 Hz) | Gamma (30–200 Hz) | |
| Anatomy | Hippocampus, prefrontal cortex, sensory cortex | Thalamus, hippocampus, reticular formation, sensory cortex, motor cortex | All cortical structures, subthalamic nucleus, hippocampus, basal ganglia, olfactory bulb | All brain structures, retina, olfactory bulb |
| Neurotransmitters | GABA, glutamate, acetylcholine | Glutamate, acetylcholine, serotonin | Glutamate, GABA, dopamine | GABA, glutamate, acetylcholine |
| Function | Memory, synaptic plasticity, top-down control, long-range synchronization | Inhibition, attention, consciousness, top-down control, long-range synchronization | Sensory gating, attention, perception, motor control, long-range synchronization | Perception, attention, memory, consciousness, synaptic plasticity, motor control |
Note: GABA, gamma-aminobutyric acid.
Neural Oscillations and Schizophrenia
Further insights into neural oscillations and their relationship to cognitive processes may be gained by correlations between abnormal brain states, such as in schizophrenia, that are accompanied by cognitive abnormalities as well as by changes in neurotransmitter systems that are involved in the generation of oscillations and their synchronization.
Until recently, research into the role of aberrant oscillatory activity in the pathophysiology of schizophrenia has focused largely on the investigation of resting state EEG data. This research has produced consistent evidence for abnormalities in several frequency bands (for a review, see Boutros et al101). However, these data allow only limited insights into the relationship between dysfunctional cognitive processes and impaired oscillatory activity in schizophrenia. With the advent of advanced time-frequency analyses tools (for a review, see Le Van Quyen and Bragin2 and Roach and Mathalon102), recent research has begun to examine task-related oscillatory activity in patients with schizophrenia. These data have revealed novel insights into the neural mechanisms underlying cognitive dysfunctions that may have important implications for the pathophysiology of schizophrenia.
Theta Oscillations
As reviewed above, theta oscillations have been implicated in episodic and WM and in the top-down control of cognitive functions. Schizophrenia is associated with impairments in all these domains, suggesting the possibility of abnormal theta activity. Preliminary evidence suggests that this is the case in patients with schizophrenia. Schmiedt et al103 reported abnormal evoked theta activity during WM and cognitive control, ie, the ability to adjust strategies flexibly in accordance with one's intentions and goals. WM was examined in an N-back task in 10 patients and 10 healthy participants. In addition, the authors manipulated cognitive control by monitoring simple actions during WM performance. In controls, theta activity was particularly prominent over frontal electrodes in the high cognitive control condition and increased with WM load. In contrast, patients with schizophrenia did not show an increase in evoked theta activity in either the difficult high control condition or with increased WM load.
Ford et al104 investigated the role of long-range synchronization of theta oscillations between frontal and temporal lobes in the context of auditory hallucinations. EEG data were recorded in 2 conditions: Participants listened either to their own played back speech or were instructed to talk aloud. In normal participants and patients with schizophrenia without hallucinations, talking was associated with an increase in theta coherence between left frontal and temporal electrodes relative to the listening condition. In patients with schizophrenia with auditory hallucinations, this modulation was absent. The finding of increased theta coherence in controls and patients without hallucinations may reflect the action of a corollary discharge mechanism whereby frontal brain structures prepare temporal lobe areas for speech production. The lack of such a corollary signal has been proposed as a pathophysiological mechanism for auditory hallucinations.105
Alpha Oscillations
Neural oscillations in the alpha-band have been investigated predominantly in the resting EEG of patients with schizophrenia as well as in relation to ERP generation (see below). There is consistent evidence that patients with schizophrenia are characterized by diminished alpha power in the resting state EEG.106–108 The diminished alpha activity in the resting EEG is difficult to interpret. It could be due to abnormal functions of the alpha generators, but because of the marked state dependence of alpha oscillations, it could also result from tonically enhanced arousal and/or neuroleptic treatment.
Beta Oscillations
Oscillations in the beta-frequency range have been associated with long-range synchronization between neuronal assemblies in the context of polymodal sensory processing, sensory-motor coordination, the maintenance of limb positions, and WM. Uhlhaas et al109 examined the extent of long-range synchronization deficits in 19 chronic patients with schizophrenia and 19 healthy controls during the perception of Mooney faces (see figure 2). Mooney faces consist of degraded pictures of human faces where all shades of gray are removed, leaving only black and white contours. Perception of Mooney faces requires the grouping of the fragmentary parts into coherent images. EEG data were analyzed for induced spectral power and phase synchronization in the beta- and gamma-frequency range. Patients with schizophrenia exhibited a deficit in the perception of Mooney faces and reduced phase synchrony in the beta-band (20-30 Hz) while the power of induced gamma-band oscillations was in the normal range, suggesting a differential impairment in long-range synchronization during perceptual organization.
Fig. 2.
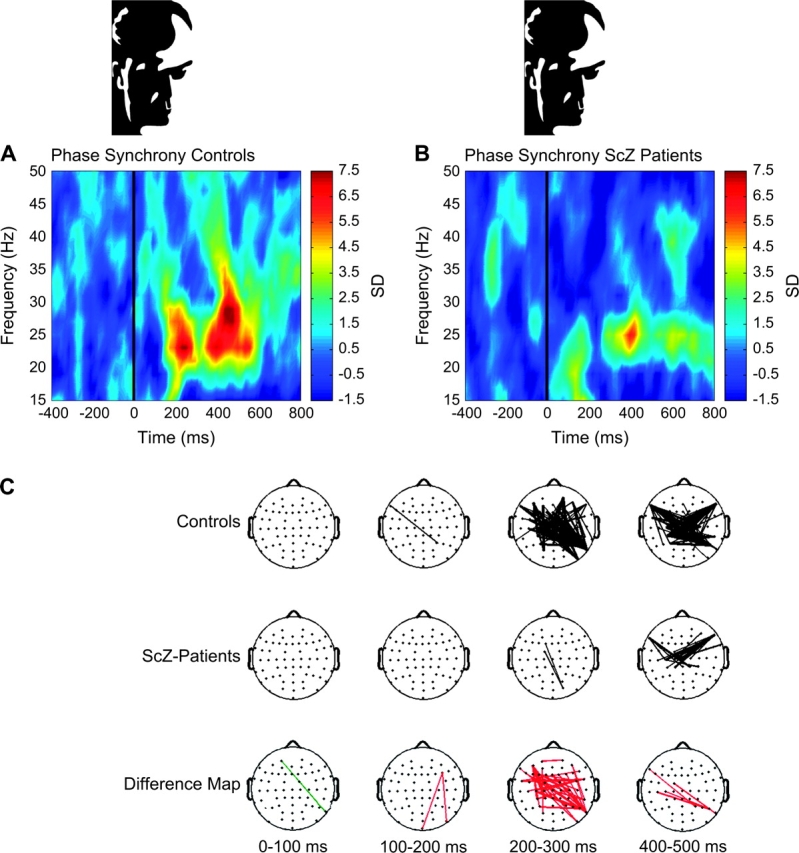
Neural Synchrony During Gestalt Perception in Schizophrenia. Group average of phase synchrony for all electrodes and for correct trials during a Gestalt perception task in controls (A). Phase synchrony during Gestalt perception in controls exhibited 2 maxima over an average frequency range of 20–30 Hz (A). The increase in phase synchrony between 200 and 300 ms has been related to the construction of coherent object representations,84 whereas the second peak indexes the preparation and execution of the motor response. In patients with schizophrenia (B), the onset of the first peak in the face condition was delayed and occurred between 350 and 400 ms in the frequency range of 20–25 Hz. In addition, a second, weaker peak was found around 600 ms. Compared with controls, the reduction in phase synchrony in patients with schizophrenia in the frequency range of 20–30 Hz was significant (frequency range: 20–30 Hz, time interval: 200–280 ms, t(36) = 2.96, P = .005). (C) This shows the topography of phase synchrony between 20 and 30 Hz. Synchrony between pairs of electrodes is indicated by connecting lines, which were drawn only if the synchrony value is beyond a 2-tailed probability of P < .0005. Differences between groups are displayed in the bottom row. Red lines indicate a decrease in synchrony in schizophrenia patients compared with controls. Green lines indicate increase in synchrony for patients with schizophrenia relative to controls. The decrease in phase synchrony between 200 and 300 ms indexes a deficit in the long-range synchronization during Gestalt perception in schizophrenia. Adapted with permission from Uhlhaas et al.109 Copyright 2006 Society for Neuroscience.
Ford et al110 examined EEG activity within 150 ms preceding speech onset compared with prelistening and related this to the ERP activity following speech or listening. Results showed that beta intertrial coherence (ITC) was larger for prespeech compared with prelistening. The ERP component N1 was suppressed during talking compared with listening in controls but not in patients and here especially in those with a history of auditory hallucinations. Furthermore, there was a strong correlation between ITC and N1 suppression in controls but not in patients. The authors suggested that the ITC reflects the action of a forward model system that dampens auditory responsiveness to self-generated speech.
Krishnan et al111 probed the ability of cortical networks to generate and maintain oscillatory activity with a steady-state visual evoked potentials (SSVEPs) paradigm in chronic patients with schizophrenia. SSVEP represents a basic neural response to a temporally modulated stimulus to which it is synchronized in frequency and phase. The authors presented flicker at 7 different frequencies of stimulation (4, 8, 17, 20, 23, 30, and 40 Hz). Results showed that patients with schizophrenia showed significantly reduced SSVEPs at 17, 23, and at 30 Hz of stimulation relative to controls. Contrary to earlier findings,112,113 SSVEP deficits were not present in the lower (theta, alpha) frequency ranges.
Dysfunctions in SSEVPs in several frequency bands are consistent with pervasive dysfunctions in visual processing in schizophrenia that have been found at the phenomenological, physiological, and structural level (for a review, see Uhlhaas and Silverstein114 and Uhlhaas and Mishara115)
Gamma Oscillations
A series of studies has examined gamma-band activity in patients with schizophrenia, providing consistent evidence for the presence of abnormal gamma-band oscillations. Kwon et al116 employed an auditory steady-state paradigm in 15 chronic patients with schizophrenia and 15 control participants to examine evoked gamma-band activity. Patients with schizophrenia showed reduced EEG power at 40 Hz but not at lower frequencies of stimulation. In addition, gamma-band oscillations in patients with schizophrenia were characterized by a delayed onset of phase synchronization and delayed desynchronization to click trains at 40 Hz.
Reduced power and synchronization of gamma oscillations evoked by steady-state stimulation have now been confirmed in several studies.117 These abnormalities were not confined to patients with a chronic disease history but were also found in first-degree relatives,118 in adolescent patients with a psychotic disorder,119 and first-episode patients with schizophrenia.120 This dysfunctional evoked gamma-band activity is not an artifact of medication because similar deficits have been found in unmedicated, first-episode patients with schizophrenia tested with an oddball paradigm in a late latency range of 220–350 ms.121
Several studies have also reported abnormalities in induced gamma-band activity during auditory stimulation. Haig et al122 examined induced spectral power during an auditory oddball paradigm in 35 medicated schizophrenics and 35 age- and gender-matched normal controls. For target trials, patients showed a significant decrease in poststimulus gamma oscillations in the left hemisphere and over frontal sites and an increase in the right hemisphere and over parietooccipital sites. In the nontarget trials (at a different latency), patients with schizophrenia showed a widespread decrease in gamma power. In subsequent studies,123,124 the authors also examined phase synchrony between electrode sites in first-episode and chronic patients with schizophrenia using the same paradigm. These studies revealed reduced gamma-band phase synchronization in both first-episode and chronic patients.
Impaired gamma-band activity has also been demonstrated during visual processing. Spencer and colleagues125 analyzed EEG data during an illusory square task in chronic patients and healthy controls for evoked activity and phase synchrony. Chronic patients showed reduced evoked activity for the early gamma-band response as well as reductions in long-range synchronization in the 37- to 44-Hz frequency range. In a second article,126 analyses of the evoked response revealed that gamma-band activity was phase locked to the reaction times in healthy participants. In contrast, in patients with schizophrenia evoked activity in the gamma-band was significantly reduced and was replaced by an increase in beta-frequency activity during the motor response.
Further studies have confirmed the presence of abnormal gamma-band activity during visual stimulation.127–129 Wynn et al129 examined evoked gamma-band oscillations during a backward masking paradigm in 32 patients with schizophrenia and 15 controls. Evoked gamma-band activity was significantly reduced in the right hemisphere for masked targets, whereas the oscillatory response to unmasked targets was intact. In an earlier article,128 induced gamma-band activity was also found to be impaired during visual masking.
Abnormal gamma-band activity has also been related to disturbed corollary modulation of sensory processes. Ford et al110 tested the hypothesis that impaired generation of efference copies (corollary discharges) might be related to impaired neural synchrony in patients with schizophrenia. EEG data were collected while participants performed a simple button press task. In controls, there was an increase in gamma-band phase locking approximately 100 ms prior to the motor response that was significantly reduced in patients with schizophrenia.
In addition to studies that have found abnormalities in gamma-band oscillations during sensory processing, there is also evidence suggesting a relation between abnormal gamma-band activity and impairments in higher cognitive processes, such as executive processes and WM (for a review, see Haenschel et al130). Cho et al131 examined the relationship between executive processes and gamma-band oscillations in a cognitive control paradigm that required participants to inhibit a prepotent response. Patients with schizophrenia were characterized by a significant decrease of induced gamma-band power over frontal electrodes sites relative to controls during this cognitive control task. This impairment was significantly correlated with their reduced performance.
Impaired gamma-band activity has also been reported in a WM task132 during the performance of mental arithmetic133 and during the Wisconsin Card Sorting Test134 providing further support for a relation between abnormal gamma-band oscillations and the disturbance of higher cognitive processes in schizophrenia.
Finally, there is evidence that abnormal gamma-band activity correlates with the severity and nature of psychopathological syndromes. Lee et al135 demonstrated that enhanced phase synchrony in responses to targets in an oddball paradigm was positively correlated with increased positive symptoms while negative symptoms were associated with a decrease in gamma-band activity in a sample of chronic patients with schizophrenia. In contrast, Bucci et al136 observed that induced gamma power and coherence were relatively preserved in patients with primary negative symptoms. Finally, Spencer et al125 noted a relationship between the frequency of phase locking in the beta-frequency range and the severity of core symptoms of schizophrenia, suggesting that lower frequencies of the evoked oscillations are associated with more severe symptomatology.
Neural Oscillations, ERPs, and Cortical Noise
In addition to the analysis of task-related oscillations, several studies have examined the potential relationship between ERPs and oscillatory activity in schizophrenia. As pointed out above, recent experimental and theoretical evidence suggests that ERPs are partially generated by a reorganization of ongoing oscillations in the EEG.61 Thus, the core assumption is that the background oscillations undergo a phase reset that contributes to the evoked components in response to a stimulus. Accordingly, impaired neural oscillations in schizophrenia could not only account for deficits in cognitive processes but could also explain some of the ERP abnormalities.
Jansen et al137 used an auditory sensory gating paradigm to measure N100 and P200 ERP components and their relationship to phase synchronization in the 2- to 12-Hz frequency range. Results showed that patients with schizophrenia were characterized by reduced activity between 4 and 8 Hz for responses to the first stimulus. Furthermore, whereas N100 and P200 amplitudes correlated with phase synchronization in controls, this relationship was not present in patients with schizophrenia. Supporting evidence for a link between ERP components during sensory gating and reduced theta/alpha phase-synchronization was recently reported by Brockhaus-Dumke and colleagues.138
There is also evidence for a relation between abnormalities in high-frequency oscillation and altered ERPs in schizophrenia.73,139,140 Hong et al73 measured evoked gamma and beta activity following paired auditory clicks. Results showed that the reduced evoked beta-band amplitude to the first click contributed to the lack of P50 suppression to the second click in patients with schizophrenia. Johannesen et al140 subdivided the patients according to the sensory gating inventory into a group with either large or normal perceptual disturbances. Results indicated that patients with large perceptual disturbances showed smaller P50 amplitudes and weaker gamma-band attenuation.
In summary, the current evidence thus suggests that reductions in gamma and beta activity contribute to P50 impairments, whereas reductions in theta and alpha activity contribute to N100 and P200 impairments.
Abnormal oscillatory activity has been hypothesized as a source of noise impeding the function of prefrontal circuits in schizophrenia. To examine this hypothesis, Winterer et al141 measured the increase in response variability of the event-related oscillatory activity in an auditory oddball paradigm in patients with schizophrenia (N = 65), their unaffected siblings (N = 115), and healthy subjects. The results showed enhanced broadband, nonstimulus-locked activity (0.5–45.5 Hz) over frontal and central electrodes in patients with schizophrenia and in unaffected siblings. Furthermore, the extent of noise was negatively correlated to the performance in an N-back WM task. According to Winterer et al, this abnormal high noise level in prefrontal circuits is due to a lack of stimulus-induced phase resetting of ongoing oscillations (table 2).
Table 2.
Neural Oscillations and Schizophrenia
| Theta (4–7 Hz) | Alpha (8–12 Hz) | Beta (13–30 Hz) | Gamma (30–200 Hz) | |
| Executive processes | Working memory, cognitive control, prefrontal noise | Prefrontal noise | Prefrontal noise | Cognitive control, working memory, mental arithmetic prefrontal noise |
| Sensory processes | Sensory gating, corollary discharge | Sensory gating, steady-state visual evoked potentials | Perceptual binding, sensory gating, corollary discharge, steady-state visual evoked potentials | Perceptual binding, auditory steady-state evoked potentials, corollary discharge, backward masking, auditory perception |
| Symptoms | Positive symptoms | — | Positive and negative symptoms | Positive and negative symptoms disorganization |
| Endophenotype | Prefrontal noise | Prefrontal noise | Prefrontal noise | Auditory steady-state evoked potentials |
| Effect in first episode | Auditory steady-state evoked potentials, auditory perception |
Neural Oscillations, Cognition, and Schizophrenia
The data reviewed so far suggest that oscillations in the theta-, alpha-, beta-, and gamma-frequency ranges are crucially involved in a wide range of cognitive functions. Although investigations of task-related oscillations and synchrony in pathological conditions have a short history, there is growing evidence for a close relation between psychopathology and abnormal brain dynamics. Thus, further research on the role of oscillations and synchrony in the pathophysiology of schizophrenia is warranted.
Current theories of schizophrenia have emphasized that core aspects of the pathophysiology arise from a disconnection syndrome between and within cortical areas of the brain.142–144 Deficits in neural oscillations may represent the functional correlate of disconnectivity in cortical networks and thus underlie the characteristic fragmentation of mind and behavior in schizophrenia. Functional disconnection between neural assemblies may furthermore reflect anatomical disconnectivity, such as white matter anomalies (see Kubicki et al145 for a review). Studies involving lesions146 and developmental manipulations147 indicate that gamma-band activity and its synchronization are at least in part mediated by corticocortical connections that not only link reciprocally the cells situated in the same cortical area but also the cells distributed across different areas and even across the 2 hemispheres.
Although much of the available evidence emphasizes abnormalities of oscillations in the gamma-frequency range, abnormal task-related oscillatory activity has also been observed in theta-, alpha-, and beta-bands, suggesting that schizophrenia is associated with a widespread deficit in the generation and synchronization of rhythmic activity. Overall, the evidence suggests that schizophrenia is associated with a reduction of oscillatory activity which has been derived from amplitude measures of spectral activity in EEG and MEG studies. The amplitude of these signals represents neural activity that arises from the synchronous and periodic discharges of thousands or millions of neurons and the associated synaptic events and increases with the number of synchronously active neurons and with the precision of synchrony.
Thus, several factors could underlie the reduction of neural oscillations in schizophrenia. Reduced amplitudes could reflect reduced numbers of participating neurons (for a review, see Glantz et al148), reduced synaptic connectivity,149 and/or reduced synchrony. The latter could be the result of abnormalities in the rhythm-generating networks of inhibitory interneurons150 or deficits in the pathways mediating long-distance synchrony such as disturbances in glutamatergic transmission151 and/or abnormalities in the myelination or topology of long-distance corticocortical or cortico-thalamo-cortical connections.
However, other factors may also contribute to reduced amplitude of oscillations in schizophrenia. Neural oscillations and their synchronization are critically dependent upon central states of the nervous systems, such as the level of arousal and attention.8,9 Accordingly, alterations in these state variables could also influence task-related neural oscillations, but the influence of these factors has so far not been systematically examined.
A more direct measure of synchrony than spectral power is the degree of phase locking between oscillations because it is independent of signal amplitudes.152 Phase locking can be computed for single electrodes if responses are time locked to stimuli or between electrodes providing a measure of local- and long-range synchronization, respectively (see Roach et al102). Several studies that have employed synchrony measures have found reliable deficits in both local- and long-range synchronization in patients with schizophrenia110,125,143 with some studies, suggesting that phase-locking measures provide a more sensitive measure toward alterations in oscillatory activity than power estimates.143 These findings highlight the possible importance of precise synchronization of oscillatory activity in schizophrenia as a crucial pathophysiological mechanism rather than the overall amount of oscillatory activity.
Neural Oscillations and Schizophrenia: A Perspective
While the available evidence provides already robust support for the involvement of altered oscillatory activity in the pathophysiology of schizophrenia, we would like to raise a number of issues that we consider critical for further progress in this field of research.
The search for the pathophysiological mechanisms underlying the signs and symptoms of schizophrenia has implicated a large number of biological mechanisms, yet the precise causes of this devastating disorder have remained elusive. This is in part due to the heterogeneity of the disorder that is likely to involve multiple biological pathways. Another problem is that our understanding of the mechanisms through which cortical networks accomplish complex cognitive and executive processes is still at the very beginning.
The current interest in the relationship between neural oscillations and the pathophysiology of schizophrenia is based on the evidence that the putative functions of oscillations support those cognitive and executive functions that are most affected in schizophrenia. These functions have in common that they require large-scale integration of subsystems. Because the neuronal mechanisms responsible for the generation of oscillatory activity and its synchronization have been studied extensively both in vivo and in vitro, it is now possible to start focused search for abnormalities in these mechanisms (see Roopun et al153) and to explore new avenues for the treatment of this disorder (see Gonzalez-Burgos and Lewis154). Eventually, because of their quantifiability, abnormalities in oscillatory activity might become a valuable diagnostic marker and then also serve as endophenotype for genetic studies.
While these prospects are encouraging, we believe that several issues need to be considered that may be critical in the search for the electrographic correlations of schizophrenia.
Methodology
In contrast to conventional analyses of ERPs, eg, there are currently no general guidelines and standards regarding the analyses of task-related oscillations and their synchronization. Analyses of oscillations are dependent upon several parameters, such as the choice of size of the width of the analysis windows, the assessment of pre-task baseline activity, the methods to assess spectral densities, phase locking, and coherence. Last but not least, even details such as the definition of reference electrodes in EEG recordings need to be standardized to guarantee replicability and comparison of results.
In addition to standardization of analyses routines, new methods that allow more precise measurements of oscillations and their synchronization are crucial. Measurement of synchronized, oscillatory activity in scalp EEG and MEG data is contaminated by volume conduction and muscle artifacts that can mimic neural synchrony. One solution is the transformation of EEG and MEG data into source space.155,156 Besides more precise estimation of synchronous, oscillatory activity, this technique has the additional advantage of substantially improving the spatial resolution of EEG and MEG measurements. Several techniques have been developed to achieve this goal. They allow the measurement of synchronization between sources that have yielded new insights into the relationship between synchronous, oscillatory activity and cognitive processes.76,156
The majority of studies on schizophrenia have so far concentrated on high-frequency oscillations but because of the interdependence of high- and low-frequency activity, the latter deserve equal attention, as well as the relations between the different frequency bands. As discussed above, theta oscillations are crucially involved in learning, memory, and executive processes, and there is extensive evidence that these processes reflect core domains of pathology in schizophrenia.
Oscillations as a Biomarker for Disturbed Network Activity in Schizophrenia
The search for biomarkers that provide an objective and quantifiable index of network activity is an important target of schizophrenia research.157 Biomarkers may potentially provide an objective measure toward the diagnosis, detection, and therapeutic response of a clinical syndrome. Moreover, identification of specific biological pathways will be crucial toward the development of targeted, pharmacological intervention that adjusts altered neural oscillations in neuropsychiatric disorders.
Neural oscillations may be particularly suited for this purpose because much is already known about the underlying neurotransmitter systems involved in the generation of oscillations. Manipulation of neurotransmitter systems in in vitro experiments (see Roopun et al153), eg, allow a direct test of the involvement of specific receptor systems in the generation of abnormal rhythmic activity that provide critical tests for pathophysiological mechanisms in schizophrenia.
However, there are concerns regarding the specificity of altered neural oscillations that may reduce their potential utility as a biomarker for schizophrenia. Abnormal oscillations are associated with a wide range of neuropsychiatric syndromes, such as autism, Alzheimer disease, and epilepsy,143 suggesting that abnormal rhythmic activity may represent a core feature of neuropsychiatric disorders that involve changes in cognitive functions. The overlap between abnormal neural oscillations in schizophrenia and other clinical syndromes is consistent with the fact that numerous putative biomarkers in schizophrenia have also been found to be abnormal in other psychiatric syndromes.157 Although overlap between existing nosological entities is plausible, there are distinct differences between Alzheimer disease, schizophrenia, and autism, with respect to the developmental profile, their phenomenology, and the associated cognitive dysfunctions.
In order to identify the similarities and differences between the abnormal patterns of rhythmic activity associated with schizophrenia and other major psychiatric syndromes, we suggest that future studies should compare data from schizophrenia patients with other clinical populations. While it is conceivable that the present methods still lack the sensitivity to detect subtle differences in neural oscillations between different syndromes, we believe that novel methodological approaches adapted from the field of nonlinear dynamics, such as the analyses of cross-frequency interactions and advanced source-analyses techniques, may achieve this goal.
Neurodevelopment, Neural Oscillations, and Schizophrenia
There is consensus in the literature that schizophrenia is likely the result from an abnormal development of cortical networks, possibly involving an early pre- or perinatal insult associated with later bifurcations of maturational events that lead to the emergence of psychosis during the transition from adolescence to adulthood.141,158
It is conceivable that abnormal oscillations and deficient synchrony are not solely the consequence of developmental disturbances but are also one of the causes of abnormal development of neuronal networks. Oscillations and the concomitant generation of synchronized neuronal activity play a crucial role in the activity-dependent self-organization of developing networks. The development and maturation of cortical networks critically depend on neuronal activity, whereby synchronized oscillatory activity plays an important role in the stabilization and pruning of connections.159 It is to be expected that these essential developmental processes become impaired when rhythm- and synchrony-generating mechanisms are dysfunctional. We face a classical vicious circle.
Imprecise synchrony leads to imprecise connectivity that can only support imprecise temporal dynamics. It is thus conceivable that the marked increases of beta and gamma synchronization that normally occurs toward the end of adolescence cannot be achieved. This would agree with the fact that schizophrenia manifests itself preferentially toward the end of adolescence, when the temporal precision of synchronous high-frequency oscillatory activity normally reaches adult levels.
Abnormal oscillations can in principle affect all stages of brain development. During prenatal and early postnatal development, the cerebral cortex exhibits self-generated synchronized oscillatory activity that is believed to be essential for the shaping of cortical circuits (for a review, see Khazipov and Luhmann160). Accordingly, abnormal neural oscillations during early development may be one critical factor that leads to abnormal cortical networks that in turn lead to subtle changes in cognitive and motor functions that have been reported in children who are at risk for the development of schizophrenia.161
Furthermore, there is emerging evidence that neural oscillations undergo substantial changes during normal adolescent development that may be related to the emergence of psychotic symptoms. In a recent study, we were able to show that well-synchronized, oscillatory activity in the theta-, beta-, and gamma-band emerges relatively late during the transition from adolescence to adulthood,162 suggesting that late adolescence is a critical development period during which the temporal patterning of brain activity reaches adult levels. These data are consistent with late maturational changes in signaling systems that are involved in the generation of neural oscillations, such as GABAergic interneurons137 and NMDA-dopamine interactions.163 Moreover, in this developmental period, one also observes changes in myelination patterns and the ratio between gray and white matter, suggesting ongoing reorganization of circuitry.164
Because of the evidence that synchronous oscillatory activity plays a crucial role in the selection and pruning of developing cortical connections, further research is required in animal models and in at-risk subjects that closely examine the putative contribution of abnormal development of neural synchrony in schizophrenia. The hypothesis needs to be examined that early pre- or postnatal developmental errors initiate a vicious circle that jeopardizes the self-organizing developmental process required for the expression of normal connectivity patterns. The assumption is that genetic or epigenetic disturbances of the mechanisms responsible for the generation of temporally structured activity patterns (oscillations in the different frequency bands and their synchronization) impede the activity-dependent specification of developing circuitry which in turn leads to abnormal temporal patterns. One possibility is that this vicious developmental circle leads to a decompensation of the system once developmental processes come to an end and can no longer compensate through self-organizing processes for the accumulation of connection errors.
Summary
The review has highlighted that neural networks tend to engage in synchronized oscillatory activity in the theta-, alpha-, beta-, and gamma-frequency ranges that support a wide range of cognitive functions. Specifically, neural oscillations facilitate synchronization which may in turn serve as tag of relatedness for the formation of distributed assemblies that underlie coherent action and cognition. Moreover, an oscillatory patterning of activity allows the exploitation of phase space for the encoding of information.
As the data indicate schizophrenia appears to be associated with abnormal oscillatory activity in a wide range of frequencies and with disturbed large-scale synchronization of oscillations. Moreover, these are consistently associated with core cognitive dysfunctions and symptoms of the disorder, suggesting a close, perhaps even causal, relation. If so, this has important implications for the understanding of pathophysiological mechanisms and diagnosis and, hopefully, also for the treatment of the disorder.
In order to increase the utility of neural oscillations and synchrony as a biomarker in schizophrenia, standardization and further development of EEG and MEG analysis techniques are essential. Comparison with other neuropsychiatric syndromes might furthermore identify commonalities and differences in biological pathways that will be crucial for the advancement of targeted, pharmacological interventions. Finally, the neurodevelopmental hypothesis of schizophrenia is consistent with the involvement and possible dysfunction of neural oscillations in early development of cortical circuits and with the delayed manifestation of the disorder in late adolescence.
Funding
The Max Planck Society; the Bundesministerium für Bildung und Forschung (01GWS055).
References
- 1.Berger H. Ueber das Electrocephalogramm des Menschen. Arch Psychiatr Nervenkr. 1929;87:527–570. [Google Scholar]
- 2.Le Van Quyen M, Bragin A. Analysis of dynamic brain oscillations: methodological advances. Trends Neurosci. 2007;30:365–373. doi: 10.1016/j.tins.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 4.Fries P, Nikolić D, Singer W. The gamma cycle. Trends Neurosci. 2007;30:309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. 111–125. [DOI] [PubMed] [Google Scholar]
- 6.Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- 7.Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herculano-Houzel S, Munk MH, Neuenschwander S, Singer W. Precisely synchronized oscillatory firing patterns require electroencephalographic activation. J Neurosci. 1999;19:3992–4010. doi: 10.1523/JNEUROSCI.19-10-03992.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Womelsdorf T, Fries P. The role of neuronal synchronization in selective attention. Curr Opin Neurobiol. 2007;17:154–160. doi: 10.1016/j.conb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Kreiter AK, Singer W. Stimulus-dependent synchronization of neuronal responses in the visual cortex of the awake macaque monkey. J Neurosci. 1996;16:2381–2396. doi: 10.1523/JNEUROSCI.16-07-02381.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu S, Huang D, Singer W, Nikolić D. A small world of neuronal synchrony. Cereb Cortex. doi: 10.1093/cercor/bhn047. doi:10.1093;cercor/bhn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider G, Havenith MN, Nikolić D. Spatiotemporal structure in large neuronal networks detected from cross-correlation. Neural Comput. 2006;18:2387–2413. doi: 10.1162/neco.2006.18.10.2387. [DOI] [PubMed] [Google Scholar]
- 13.Nikolić D. Non-parametric detection of temporal order across pairwise measurements of time delays. J Comput Neurosci. 2007;22:5–19. doi: 10.1007/s10827-006-9441-7. [DOI] [PubMed] [Google Scholar]
- 14.Konig P, Engel AK, Roelfsema PR, Singer W. How precise is neuronal synchronization? Neural Comput. 1995;7:469–485. doi: 10.1162/neco.1995.7.3.469. [DOI] [PubMed] [Google Scholar]
- 15.O'Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 16.Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- 17.Hopfield JJ, Brody CD. What is a moment? Transient synchrony as a collective mechanism for spatiotemporal integration. Proc Natl Acad Sci USA. 2001;98:1282–1287. doi: 10.1073/pnas.031567098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VanRullen R, Guyonneau R, Thorpe SJ. Spike times make sense. Trends Neurosci. 2005;28:1–4. doi: 10.1016/j.tins.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007;30:150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Buzsaki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- 22.Sarnthein J, Petsche H, Rappelsberger P, Shaw GL, von Stein A. Synchronization between prefrontal and posterior association cortex during human working memory. Proc Natl Acad Sci USA. 1998;95:7, 092–7096. doi: 10.1073/pnas.95.12.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Stein A, Chiang C, Konig P. Top-down processing mediated by interareal synchronization. Proc Natl Acad Sci USA. 2000;97:14748–14753. doi: 10.1073/pnas.97.26.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahana MJ, Seelig D, Madsen JR. Theta returns. Curr Opin Neurobiol. 2001;11:739–744. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- 26.Lengyel M, Huhn Z, Erdi P. Computational theories on the function of theta oscillations. Biol Cybern. 2005;92:393–408. doi: 10.1007/s00422-005-0567-x. [DOI] [PubMed] [Google Scholar]
- 27.Raghavachari S, Lisman JE, Tully M, Madsen JR, Bromfield EB, Kahana MJ. Theta oscillations in human cortex during a working-memory task: evidence for local generators. J Neurophysiol. 2006;95:1630–1638. doi: 10.1152/jn.00409.2005. [DOI] [PubMed] [Google Scholar]
- 28.Tsujimoto T, Shimazu H, Isomura Y. Direct recording of theta oscillations in primate prefrontal and anterior cingulate cortices. J Neurophysiol. 2006;95:2987–3000. doi: 10.1152/jn.00730.2005. [DOI] [PubMed] [Google Scholar]
- 29.White JA, Banks MI, Pearce RA, Kopell NJ. Networks of interneurons with fast and slow gamma-aminobutyric acid type A (GABAA) kinetics provide substrate for mixed gamma-theta rhythm. Proc Natl Acad Sci USA. 2000;97:8128–8133. doi: 10.1073/pnas.100124097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung LS, Shen B. GABAB receptor blockade enhances theta and gamma rhythms in the hippocampus of behaving rats. Hippocampus. 2007;17:281–291. doi: 10.1002/hipo.20267. [DOI] [PubMed] [Google Scholar]
- 31.Stewart M, Fox SE. Do septal neurons pace the hippocampal theta rhythm? Trends Neurosci. 1990;13:163–168. doi: 10.1016/0166-2236(90)90040-h. [DOI] [PubMed] [Google Scholar]
- 32.O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 33.Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 34.Vertes RP. Hippocampal theta rhythm: a tag for short-term memory. Hippocampus. 2005;15:923–935. doi: 10.1002/hipo.20118. [DOI] [PubMed] [Google Scholar]
- 35.Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- 36.Gundel A, Wilson GF. Topographical changes in the ongoing EEG related to the difficulty of mental tasks. Brain Topogr. 1992;5:17–25. doi: 10.1007/BF01129966. [DOI] [PubMed] [Google Scholar]
- 37.Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci. 2003;23:10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huerta PT, Lisman JE. Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature. 1993;364:723–725. doi: 10.1038/364723a0. [DOI] [PubMed] [Google Scholar]
- 39.Holscher C, McGlinchey L, Anwyl R, Rowan MJ. HFS-induced long-term potentiation and LFS-induced depotentiation in area CA1 of the hippocampus are not good models for learning. Psychopharmacology. 1997;130:174–182. doi: 10.1007/s002130050226. [DOI] [PubMed] [Google Scholar]
- 40.Pavlides C, Greenstein YJ, Grudman M, Winson J. Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta-rhythm. Brain Res. 1988;439:383–387. doi: 10.1016/0006-8993(88)91499-0. [DOI] [PubMed] [Google Scholar]
- 41.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 42.Basar E, Schurmann M, Basar-Eroglu C, Karakas S. Alpha oscillations in brain functioning: an integrative theory. Int J Psychophysiol. 1997;26:5–29. doi: 10.1016/s0167-8760(97)00753-8. [DOI] [PubMed] [Google Scholar]
- 43.da Silva FH, Schrijer CF, van Leeuwen WS. Organization of thalamic and cortical alpha rhythms: spectra and coherences. Electroencephalogr Clin Neurophysiol. 1973;35:627–639. doi: 10.1016/0013-4694(73)90216-2. [DOI] [PubMed] [Google Scholar]
- 44.Sauseng P, Klimesch W, Gruber WR, Hanslmayr S, Freunberger R, Doppelmayr M. Are event-related potential components generated by phase resetting of brain oscillations? A critical discussion. Neuroscience. 2007;146:1435–1444. doi: 10.1016/j.neuroscience.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 45.Lorincz ML, Crunelli V, Hughes SW. Cellular dynamics of cholinergically induced alpha (8-13 Hz) rhythms in sensory thalamic nuclei in vitro. J Neurosci. 2008;28:660–671. doi: 10.1523/JNEUROSCI.4468-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hughes SW, Lorincz M, Cope DW, et al. Synchronized oscillations at alpha and theta frequencies in the lateral geniculate nucleus. Neuron. 2004;42:253–268. doi: 10.1016/s0896-6273(04)00191-6. [DOI] [PubMed] [Google Scholar]
- 47.Millson DS, Haworth SJ, Rushton A, Wilkinson D, Hobson S, Harry J. The effects of a 5-HT2 receptor antagonist (ICI 169,369) on changes in waking EEG, pupillary responses and state of arousal in human volunteers. Br J Clin Pharmacol. 1991;32:447–454. doi: 10.1111/j.1365-2125.1991.tb03929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adrian ED, Matthews BH. The Berger rhythm: potential changes from the occipital lobes in man. Brain Res Rev. 1934;57:355–385. [Google Scholar]
- 49.Andrew C, Pfurtscheller G. Event-related coherence as a tool for studying dynamic interaction of brain regions. Electroencephalogr Clin Neurophysiol. 1996;98:144–148. doi: 10.1016/0013-4694(95)00228-6. [DOI] [PubMed] [Google Scholar]
- 50.Kelly SP, Lalor EC, Reilly RB, Foxe JJ. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol. 2006;95:3844–3851. doi: 10.1152/jn.01234.2005. [DOI] [PubMed] [Google Scholar]
- 51.Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J Neurosci. 2000;20:RC63. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;15:1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- 54.Jokisch D, Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci. 2007;27:3244–3251. doi: 10.1523/JNEUROSCI.5399-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooper NR, Croft RJ, Dominey SJ, Burgess AP, Gruzelier JH. Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implications for idling and inhibition hypotheses. Int J Psychophysiol. 2003;47:65–74. doi: 10.1016/s0167-8760(02)00107-1. [DOI] [PubMed] [Google Scholar]
- 56.Palva S, Linkenkaer-Hansen K, Naatanen R, Palva JM. Early neural correlates of conscious somatosensory perception. J Neurosci. 2005;25:5248–5258. doi: 10.1523/JNEUROSCI.0141-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mima T, Oluwatimilehin T, Hiraoka T, Hallett M. Transient interhemispheric neuronal synchrony correlates with object recognition. J Neurosci. 2001;21:3942–3948. doi: 10.1523/JNEUROSCI.21-11-03942.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hummel F, Gerloff C. Larger interregional synchrony is associated with greater behavioral success in a complex sensory integration task in humans. Cereb Cortex. 2005;15:670–678. doi: 10.1093/cercor/bhh170. [DOI] [PubMed] [Google Scholar]
- 59.Sayers BM, Beagley HA, Henshall WR. The mechansim of auditory evoked EEG responses. Nature. 1974;247:481–483. doi: 10.1038/247481a0. [DOI] [PubMed] [Google Scholar]
- 60.Makeig S, Westerfield M, Jung TP, et al. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- 61.Yeung N, Bogacz R, Holroyd CB, Cohen JD. Detection of synchronized oscillations in the electroencephalogram: an evaluation of methods. Psychophysiology. 2004;41:822–832. doi: 10.1111/j.1469-8986.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- 62.Traub RD, Bibbig A, LeBeau FEN, Buhl EH, Whittington MA. Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annu Rev Neurosci. 2004;27:247–278. doi: 10.1146/annurev.neuro.27.070203.144303. [DOI] [PubMed] [Google Scholar]
- 63.Yamawaki N, Stanford IM, Hall SD, Woodhall GL. Pharmacologically induced and stimulus evoked rhythmic neuronal oscillatory activity in the primary motor cortex in vitro. Neuroscience. 2008;151:386–395. doi: 10.1016/j.neuroscience.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 64.Sharott A, Magill PJ, Harnack D, Kupsch A, Meissner W, Brown P. Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur J Neurosci. 2005;21:1413–1422. doi: 10.1111/j.1460-9568.2005.03973.x. [DOI] [PubMed] [Google Scholar]
- 65.Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J Neurosci. 2001;21:1033–1038. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Niedermeyer E. The normal EEG of the waking adult. In: Niedermeyer E, Lopez da Silva F, editors. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. 5th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2005. pp. 167–192. [Google Scholar]
- 67.Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci USA. 1992;89:5670–5674. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kilner JM, Baker SN, Salenius S, Hari R, Lemon RN. Human cortical muscle coherence is directly related to specific motor parameters. J Neurosci. 2000;20:8838–8845. doi: 10.1523/JNEUROSCI.20-23-08838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roelfsema PR, Engel AK, Konig P, Singer W. Visuomotor integration is associated with zero time-lag synchronization among cortical areas. Nature. 1997;385:157–161. doi: 10.1038/385157a0. [DOI] [PubMed] [Google Scholar]
- 70.Martin C, Gervais R, Hugues E, Messaoudi B, Ravel N. Learning modulation of odor-induced oscillatory responses in the rat olfactory bulb: a correlate of odor recognition? J Neurosci. 2004;24:389–397. doi: 10.1523/JNEUROSCI.3433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haenschel C, Baldeweg T, Croft RJ, Whittington M, Gruzelier J. Gamma and beta frequency oscillations in response to novel auditory stimuli: a comparison of human electroencephalogram (EEG) data with in vitro models. Proc Natl Acad Sci USA. 2000;97:7, 645–7650. doi: 10.1073/pnas.120162397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kisley MA, Cornwell ZM. Gamma and beta neural activity evoked during a sensory gating paradigm: effects of auditory, somatosensory and cross-modal stimulation. Clin Neurophysiol. 2006;117:2549–2563. doi: 10.1016/j.clinph.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hong LE, Buchanan RW, Thaker GK, Shepard PD, Summerfelt A. Beta (∼16 Hz) frequency neural oscillations mediate auditory sensory gating in humans. Psychophysiology. 2008;45:197–204. doi: 10.1111/j.1469-8986.2007.00624.x. [DOI] [PubMed] [Google Scholar]
- 74.Marco-Pallares J, Cucurell D, Cunillera T, et al. Human oscillatory activity associated to reward processing in a gambling task. Neuropsychologia. 2008;46:241–248. doi: 10.1016/j.neuropsychologia.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 75.Tallon-Baudry C, Mandon S, Freiwald WA, Kreiter AK. Oscillatory synchrony in the monkey temporal lobe correlates with performance in a visual short-term memory task. Cereb Cortex. 2004;14:713–720. doi: 10.1093/cercor/bhh031. [DOI] [PubMed] [Google Scholar]
- 76.Schnitzler A, Gross J. Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci. 2005;6:285–296. doi: 10.1038/nrn1650. [DOI] [PubMed] [Google Scholar]
- 77.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 78.Draguhn A, Traub RD, Schmitz D, Jefferys JG. Electrical coupling underlies high-frequency oscillations in the hippocampus in vitro. Nature. 1998;394:189–192. doi: 10.1038/28184. [DOI] [PubMed] [Google Scholar]
- 79.Traub RD, Bibbig A, LeBeau FE, Buhl EH, Whittington MA. Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annu Rev Neurosci. 2004;27:247–278. doi: 10.1146/annurev.neuro.27.070203.144303. [DOI] [PubMed] [Google Scholar]
- 80.Fukuda T, Kosaka T, Singer W, Galuske RA. Gap junctions among dendrites of cortical GABAergic neurons establish a dense and widespread intercolumnar network. J Neurosci. 2006;26:3434–3443. doi: 10.1523/JNEUROSCI.4076-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nase G, Singer W, Monyer H, Engel AK. Features of neuronal synchrony in mouse visual cortex. J Neurophysiol. 2003;90:1115–1123. doi: 10.1152/jn.00480.2002. [DOI] [PubMed] [Google Scholar]
- 82.Wespatat V, Tennigkeit F, Singer W. Phase sensitivity of synaptic modifications in oscillating cells of rat visual cortex. J Neurosci. 2004;24:9067–9075. doi: 10.1523/JNEUROSCI.2221-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rodriguez R, Kallenbach U, Singer W, Munk MH. Short- and long-term effects of cholinergic modulation on gamma oscillations and response synchronization in the visual cortex. J Neurosci. 2004;24:10369–10378. doi: 10.1523/JNEUROSCI.1839-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ. Perception's shadow: long-distance synchronization of human brain activity. Nature. 1999;397:430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- 85.Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- 86.Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 87.Herrmann CS, Munk MH, Engel AK. Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn Sci. 2004;8:347–355. doi: 10.1016/j.tics.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 88.Tiitinen H, Sinkkonen J, Reinikainen K, Alho K, Lavikainen J, Naatanen R. Selective attention enhances the auditory 40-Hz transient response in humans. Nature. 1993;364:59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- 89.Vidal JR, Chaumon M, O'Regan JK, Tallon-Baudry C. Visual grouping and the focusing of attention induce gamma-band oscillations at different frequencies in human magnetoencephalogram signals. J Cogn Neurosci. 2006;18:1850–1862. doi: 10.1162/jocn.2006.18.11.1850. [DOI] [PubMed] [Google Scholar]
- 90.Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci. 1998;18:4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Howard MW, Rizzuto DS, Caplan JB, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 92.Kaiser J, Lutzenberger W. Cortical oscillatory activity and the dynamics of auditory memory processing. Rev Neurosci. 2005;16:239–254. doi: 10.1515/revneuro.2005.16.3.239. [DOI] [PubMed] [Google Scholar]
- 93.Jensen O, Colgin LL. Cross-frequency coupling between neuronal oscillations. Trends Cogn Sci. 2007;11:267–269. doi: 10.1016/j.tics.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 94.Gruber T, Tsivilis D, Montaldi D, Muller MM. Induced gamma band responses: an early marker of memory encoding and retrieval. Neuroreport. 2004;15:1837–1841. doi: 10.1097/01.wnr.0000137077.26010.12. [DOI] [PubMed] [Google Scholar]
- 95.Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci. 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- 96.Melloni L, Molina C, Pena M, Torres D, Singer W, Rodriguez E. Synchronization of neural activity across cortical areas correlates with conscious perception. J Neurosci. 2007;27:2858–2865. doi: 10.1523/JNEUROSCI.4623-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Demiralp T, Bayraktaroglu Z, Lenz D, et al. Gamma amplitudes are coupled to theta phase in human EEG during visual perception. Int J Psychophysiol. 2007;64:24–30. doi: 10.1016/j.ijpsycho.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 98.Canolty RT, Edwards E, Dalal SS, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lisman J. The theta/gamma discrete phase code occurring during the hippocampal phase precession may be a more general brain coding scheme. Hippocampus. 2005;15:913–922. doi: 10.1002/hipo.20121. [DOI] [PubMed] [Google Scholar]
- 100.Palva JM, Palva S, Kaila K. Phase synchrony among neuronal oscillations in the human cortex. J Neurosci. 2005;25:3962–3972. doi: 10.1523/JNEUROSCI.4250-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boutros NN, Arfken C, Galderisi S, Warrick J, Pratt G, Iacono W. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophr Res. 2007;99:225–237. doi: 10.1016/j.schres.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roach D, Mathalon DH. Calculating synchrony: across regions, across time. Schizophr Bull. In press. [Google Scholar]
- 103.Schmiedt C, Brand A, Hildebrandt H, Basar-Eroglu C. Event-related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Brain Res Cogn Brain Res. 2005;25:936–947. doi: 10.1016/j.cogbrainres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 104.Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51:485–492. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- 105.Ford JM, Mathalon DH. Corollary discharge dysfunction in schizophrenia: can it explain auditory hallucinations? Int J Psychophysiol. 2005;58:179–189. doi: 10.1016/j.ijpsycho.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 106.Sponheim SR, Clementz BA, Iacono WG, Beiser M. Resting EEG in first-episode and chronic schizophrenia. Psychophysiology. 1994;31:37–43. doi: 10.1111/j.1469-8986.1994.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 107.Itil TM, Saletu B, Davis S. EEG findings in chronic schizophrenics based on digital computer period analysis and analog power spectra. Biol Psychiatry. 1972;5:1–13. [PubMed] [Google Scholar]
- 108.Iacono WG. Bilateral electrodermal habituation-dishabituation and resting EEG in remitted schizophrenics. J Nerv Ment Dis. 1982;170:91–101. doi: 10.1097/00005053-198202000-00005. [DOI] [PubMed] [Google Scholar]
- 109.Uhlhaas PJ, Linden DE, Singer W, et al. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci. 2006;26:8168–8175. doi: 10.1523/JNEUROSCI.2002-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ford JM, Roach BJ, Faustman WO, Mathalon DH. Synch before you speak: auditory hallucinations in schizophrenia. Am J Psychiatry. 2007;164:458–466. doi: 10.1176/ajp.2007.164.3.458. [DOI] [PubMed] [Google Scholar]
- 111.Krishnan GP, Vohs JL, Hetrick WP, et al. Steady state visual evoked potential abnormalities in schizophrenia. Clin Neurophysiol. 2005;116:614–624. doi: 10.1016/j.clinph.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 112.Rice DM, Potkin SG, Jin Y, et al. EEG alpha photic driving abnormalities in chronic schizophrenia. Psychiatry Res. 1989;30:313–324. doi: 10.1016/0165-1781(89)90022-x. [DOI] [PubMed] [Google Scholar]
- 113.Jin Y, Potkin SG, Sandman CA, Bunney WE., Jr Electroencephalographic photic driving in patients with schizophrenia and depression. Biol Psychiatry. 1997;41:496–499. doi: 10.1016/S0006-3223(96)00473-8. [DOI] [PubMed] [Google Scholar]
- 114.Uhlhaas PJ, Silverstein SM. Perceptual organization in schizophrenia spectrum disorders: empirical research and theoretical implications. Psychol Bull. 2005;131:618–632. doi: 10.1037/0033-2909.131.4.618. [DOI] [PubMed] [Google Scholar]
- 115.Uhlhaas PJ, Mishara AL. Perceptual anomalies in schizophrenia: integrating phenomenology and cognitive neuroscience. Schizophr Bull. 2007;33:142–156. doi: 10.1093/schbul/sbl047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kwon JS, O'Donnell BF, Wallenstein GV, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Light GA, Hsu JL, Hsieh MH, et al. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 118.Hong LE, Summerfelt A, McMahon R, et al. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr Res. 2004;70:293–302. doi: 10.1016/j.schres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 119.Wilson TW, Hernandez OO, Asherin RM, Teale PD, Reite ML, Rojas DC. Cortical gamma generators suggest abnormal auditory circuitry in early-onset psychosis. Cereb Cortex. 2007;18:371–378. doi: 10.1093/cercor/bhm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. doi: 10.1016/j.biopsych.2008.02.021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gallinat J, Winterer G, Herrmann CS, Senkowski D. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin Neurophysiol. 2004;115:1863–1874. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 122.Haig AR, Gordon E, De Pascalis V, Meares RA, Bahramali H, Harris A. Gamma activity in schizophrenia: evidence of impaired network binding? Clin Neurophysiol. 2000;111:1461–1468. doi: 10.1016/s1388-2457(00)00347-3. [DOI] [PubMed] [Google Scholar]
- 123.Slewa-Younan S, Gordon E, Harris AW, et al. Sex differences in functional connectivity in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2004;161:1595–1602. doi: 10.1176/appi.ajp.161.9.1595. [DOI] [PubMed] [Google Scholar]
- 124.Symond MP, Harris AW, Gordon E, Williams LM. “Gamma synchrony” in first-episode schizophrenia: a disorder of temporal connectivity? Am J Psychiatry. 2005;162:459–465. doi: 10.1176/appi.ajp.162.3.459. [DOI] [PubMed] [Google Scholar]
- 125.Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Spencer KM, Nestor PG, Perlmutter R, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci USA. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Spencer KM, Niznikiewicz MA, Shenton ME, McCarley RW. Sensory-evoked gamma oscillations in chronic schizophrenia. Biol Psychiatry. 2007;63:744–747. doi: 10.1016/j.biopsych.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Green MF, Mintz J, Salveson D, et al. Visual masking as a probe for abnormal gamma range activity in schizophrenia. Biol Psychiatry. 2003;53:1113–1119. doi: 10.1016/s0006-3223(02)01813-9. [DOI] [PubMed] [Google Scholar]
- 129.Wynn JK, Light GA, Breitmeyer B, Nuechterlein KH, Green MF. Event-related gamma activity in schizophrenia patients during a visual backward-masking task. Am J Psychiatry. 2005;162:2330–2336. doi: 10.1176/appi.ajp.162.12.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Haenschel C, Uhlhaas PJ, Singer W. Synchronous oscillatory activity and working memory in schizophrenia. Pharmacopsychiatry. 2007;40:S54–S61. [Google Scholar]
- 131.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci USA. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Basar-Eroglu C, Brand A, Hildebrandt H, Karolina Kedzior K, Mathes B, Schmiedt C. Working memory related gamma oscillations in schizophrenia patients. Int J Psychophysiol. 2007;64:39–45. doi: 10.1016/j.ijpsycho.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 133.Kissler J, Muller MM, Fehr T, Rockstroh B, Elbert T. MEG gamma band activity in schizophrenia patients and healthy subjects in a mental arithmetic task and at rest. Clin Neurophysiol. 2000;111:2079–2087. doi: 10.1016/s1388-2457(00)00425-9. [DOI] [PubMed] [Google Scholar]
- 134.Gonzalez-Hernandez JA, Cedeno I, Pita-Alcorta C, Galan L, Aubert E, Figueredo-Rodriguez P. Induced oscillations and the distributed cortical sources during the Wisconsin card sorting test performance in schizophrenic patients: new clues to neural connectivity. Int J Psychophysiol. 2003;48:11–24. doi: 10.1016/s0167-8760(03)00019-9. [DOI] [PubMed] [Google Scholar]
- 135.Lee KH, Williams LM, Haig A, Gordon E. “Gamma (40 Hz) phase synchronicity” and symptom dimensions in schizophrenia. Cognit Neuropsychiatry. 2003;8:57–71. doi: 10.1080/713752240. [DOI] [PubMed] [Google Scholar]
- 136.Bucci P, Mucci A, Merlotti E, Volpe U, Galderisi S. Induced gamma activity and event-related coherence in schizophrenia. Clin EEG Neurosci. 2007;38:96–104. doi: 10.1177/155005940703800212. [DOI] [PubMed] [Google Scholar]
- 137.Jansen BH, Hegde A, Boutros NN. Contribution of different EEG frequencies to auditory evoked potential abnormalities in schizophrenia. Clin Neurophysiol. 2004;115:523–533. doi: 10.1016/j.clinph.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 138.Brockhaus-Dumke A, Mueller R, Faigle U, Klosterkoetter J. Sensory gating revisited: relation between brain oscillations and auditory evoked potentials in schizophrenia. Schizophr Res. 2008;99:238–249. doi: 10.1016/j.schres.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 139.Clementz BA, Blumenfeld LD, Cobb S. The gamma band response may account for poor P50 suppression in schizophrenia. Neuroreport. 1997;8:3889–3893. doi: 10.1097/00001756-199712220-00010. [DOI] [PubMed] [Google Scholar]
- 140.Johannesen JK, Bodkins M, O'Donnell BF, Shekhar A, Hetrick WP. Perceptual anomalies in schizophrenia co-occur with selective impairments in the gamma frequency component of midlatency auditory ERPs. J Abnorm Psychol. 2008;117:106–118. doi: 10.1037/0021-843X.117.1.106. [DOI] [PubMed] [Google Scholar]
- 141.Winterer G, Coppola R, Goldberg TE, et al. Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. Am J Psychiatry. 161:490–500. doi: 10.1176/appi.ajp.161.3.490. [DOI] [PubMed] [Google Scholar]
- 142.Phillips WA, Silverstein SM. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behav Brain Sci. 2003;26:65–82. doi: 10.1017/s0140525x03000025. discussion 137. [DOI] [PubMed] [Google Scholar]
- 143.Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 144.Friston KJ. Schizophrenia and the disconnection hypothesis. Acta Psychiatr Scand Suppl. 1999;395:68–79. doi: 10.1111/j.1600-0447.1999.tb05985.x. [DOI] [PubMed] [Google Scholar]
- 145.Kubicki M, McCarley R, Westin CF, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Engel AK, Konig P, Kreiter AK, Singer W. Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science. 1991;252:1177–1179. doi: 10.1126/science.252.5009.1177. [DOI] [PubMed] [Google Scholar]
- 147.Lowel S, Singer W. Selection of intrinsic horizontal connections in the visual cortex by correlated neuronal activity. Science. 1992;255:209–212. doi: 10.1126/science.1372754. [DOI] [PubMed] [Google Scholar]
- 148.Glantz LA, Gilmore JH, Lieberman JA, Jarskog LF. Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophr Res. 2006;81:47–63. doi: 10.1016/j.schres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 149.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 150.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 151.Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40:881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 152.Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Roopun AK, Cunningham MO, Racca C, Alter K, Traub RD, Whittington MA. Region-specific effects of ketamine on cortical gamma and beta2 frequency population rhythms. Schizophr Bull. doi: 10.1093/schbul/sbn059. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. doi: 10.1093/schbul/sbn070. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mosher JC, Lewis PS, Leahy RM. Multiple dipole modeling and localization from spatio-temporal MEG data. IEEE Trans Biomed Eng. 1992;39:541–557. doi: 10.1109/10.141192. [DOI] [PubMed] [Google Scholar]
- 156.Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topogr. 2004;16:233–238. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- 157.Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7:68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J. 1987;295:681–682. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Singer W. Development and plasticity of cortical processing architectures. Science. 1995;270:758–764. doi: 10.1126/science.270.5237.758. [DOI] [PubMed] [Google Scholar]
- 160.Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 2006;29:414–418. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 161.Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994;20:441–451. doi: 10.1093/schbul/20.3.441. [DOI] [PubMed] [Google Scholar]
- 162.Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. Development of task-related neural synchrony reflects maturation and restructuring of cortical networks in humans. Soc Neurosci. 2008:11. doi: 10.1073/pnas.0900390106. [abstract 791] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tseng KY, Lewis BL, Lipska BK, O'Donnell P. Post-pubertal disruption of medial prefrontal cortical dopamine-glutamate interactions in a developmental animal model of schizophrenia. Biol Psychiatry. 2007;62:730–738. doi: 10.1016/j.biopsych.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


