Abstract
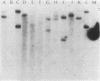
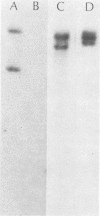
A DNA sequence specifying tetracycline resistance (Tcr) has been previously cloned from a clinical isolate of Streptococcus mutans designated U202 (J. A. Tobian and F. L. Macrina, J. Bacteriol. 152:215-222, 1982). We used this sequence as a molecular probe in studying the dissemination of Tcr among oral streptococcal species isolated from patients treated with tetracycline. Eleven strains (including S. sanguis I, S. sanguis II, S. mitis, and S. salivarius) from seven patients were examined by Southern blot analysis. Seven strains showed strong hybridization to the Tcr probe, two showed weak hybridization, and two did not display detectable hybridization. Based on previous characterization of the cloned sequence, our data suggest the dissemination of the tetM class of resistance determinants among these oral streptococci. One of the clinical S. sanguis I isolates studied was able to transfer its Tcr phenotype to other oral streptococci and to enteric streptococci in the absence of plasmid DNA. This transfer appeared to be conjugation-like on the basis of its insensitivity to DNase and its dependence on intimate cell-to-cell contact. Using the cloned Tcr sequence, we were able to study the progeny of the matings. Our data suggest that this resistance transfer element occupies a chromosomal location in streptococcal cells and that it strongly resembles the conjugative transposon Tn916 in its behavior.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burdett V., Inamine J., Rajagopalan S. Heterogeneity of tetracycline resistance determinants in Streptococcus. J Bacteriol. 1982 Mar;149(3):995–1004. doi: 10.1128/jb.149.3.995-1004.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buu-Hoï A., Horodniceanu T. Conjugative transfer of multiple antibiotic resistance markers in Streptococcus pneumoniae. J Bacteriol. 1980 Jul;143(1):313–320. doi: 10.1128/jb.143.1.313-320.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. P., Jr, Macrina F. L. Streptococcal R plasmid pIP501: endonuclease site map, resistance determinant location, and construction of novel derivatives. J Bacteriol. 1983 Jun;154(3):1347–1355. doi: 10.1128/jb.154.3.1347-1355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R. Recognition of group D streptococcal species of human origin by biochemical and physiological tests. Appl Microbiol. 1972 Jun;23(6):1131–1139. doi: 10.1128/am.23.6.1131-1139.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon R. J. Letter: Tetracycline-resistant group A streptococci. Br Med J. 1974 Sep 21;3(5933):741–741. doi: 10.1136/bmj.3.5933.741-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti J. J., Ward M. Susceptibility of Streptococcus mutans to antimicrobial agents. Antimicrob Agents Chemother. 1976 Aug;10(2):274–276. doi: 10.1128/aac.10.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A. E., Clewell D. B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal" transfer in the absence of a conjugative plasmid. J Bacteriol. 1981 Jan;145(1):494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A. E., Clewell D. B. Evidence for conjugal transfer of a Streptococcus faecalis transposon (Tn916) from a chromosomal site in the absence of plasmid DNA. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):77–80. doi: 10.1101/sqb.1981.045.01.014. [DOI] [PubMed] [Google Scholar]
- Gawron-Burke C., Clewell D. B. A transposon in Streptococcus faecalis with fertility properties. Nature. 1982 Nov 18;300(5889):281–284. doi: 10.1038/300281a0. [DOI] [PubMed] [Google Scholar]
- Hawley R. J., Lee L. N., LeBlanc D. J. Effects of tetracycline on the streptococcal flora of periodontal pockets. Antimicrob Agents Chemother. 1980 Mar;17(3):372–378. doi: 10.1128/aac.17.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Lee L. N. Characterization of two tetracycline resistance determinants in Streptococcus faecalis JH1. J Bacteriol. 1982 May;150(2):835–843. doi: 10.1128/jb.150.2.835-843.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little W. A., Thomson L. A., Bowen W. H. Antibiotic susceptibility of Streptococcus mutans: comparison of serotype profiles. Antimicrob Agents Chemother. 1979 Mar;15(3):440–443. doi: 10.1128/aac.15.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Smith M. D., Guild W. R. DNase-resistant transfer of chromosomal cat and tet insertions by filter mating in Pneumococcus. Plasmid. 1980 Jan;3(1):80–87. doi: 10.1016/s0147-619x(80)90036-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tobian J. A., Macrina F. L. Helper plasmid cloning in Streptococcus sanguis: cloning of a tetracycline resistance determinant from the Streptococcus mutans chromosome. J Bacteriol. 1982 Oct;152(1):215–222. doi: 10.1128/jb.152.1.215-222.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]