Abstract
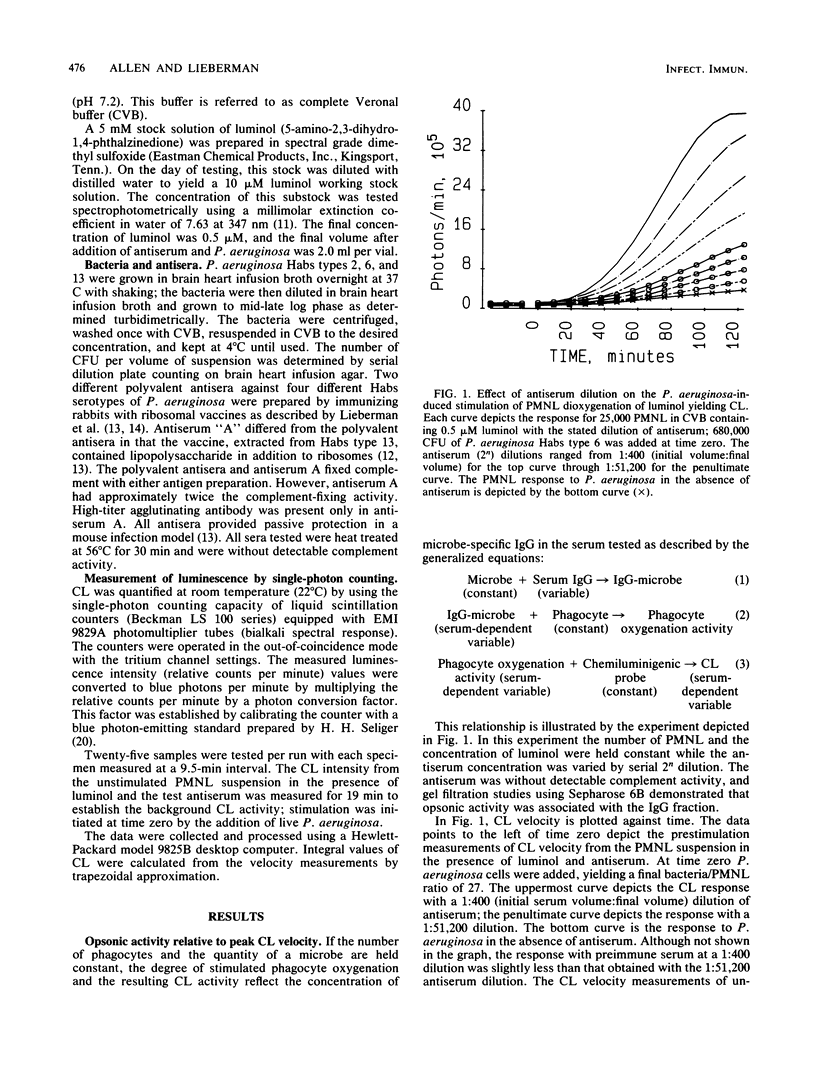
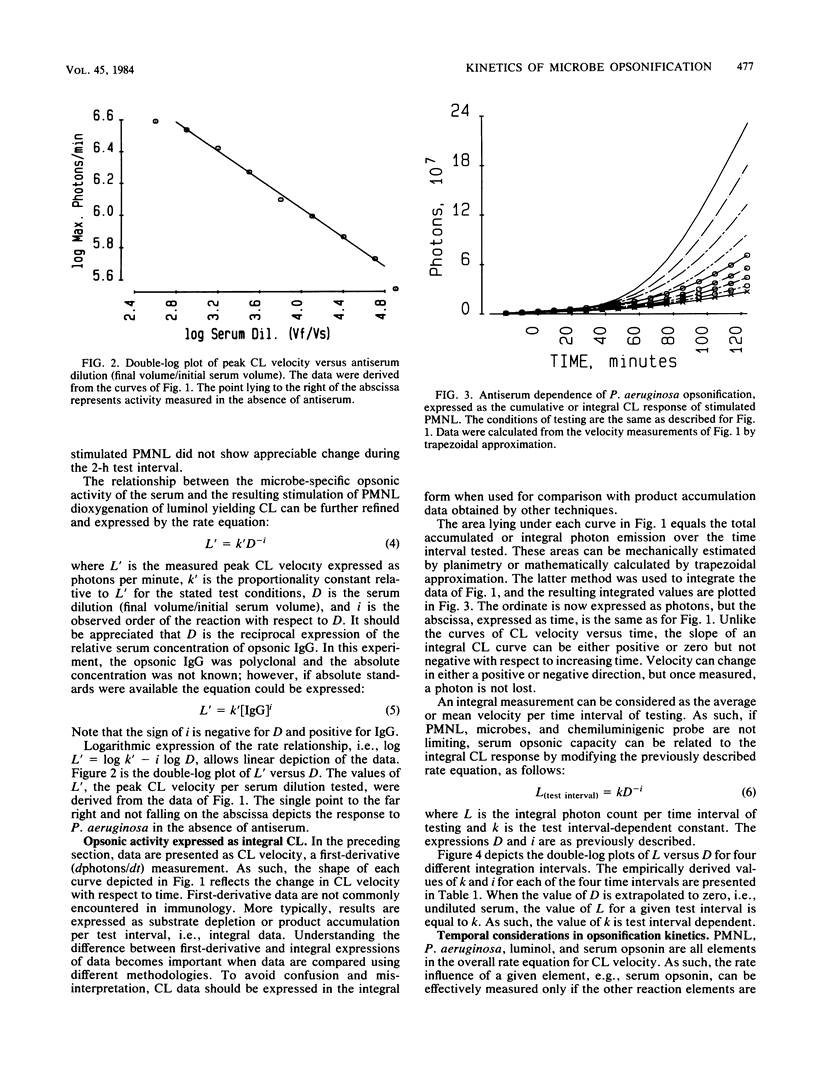
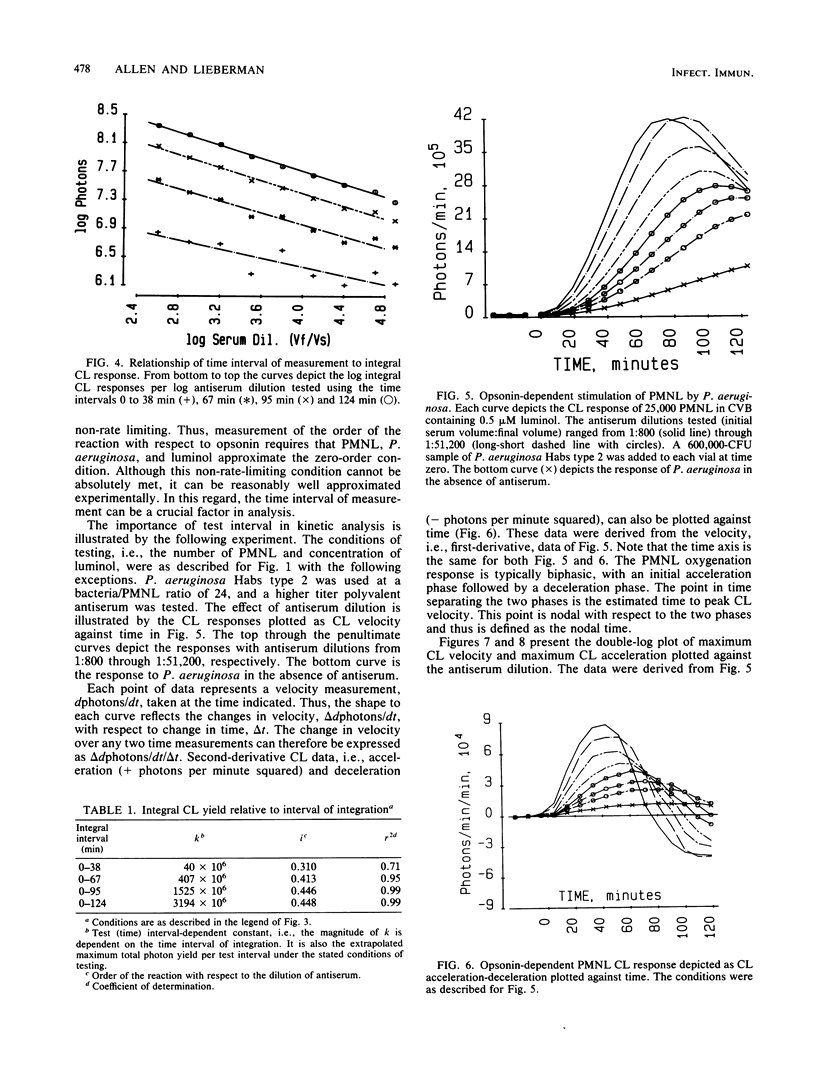
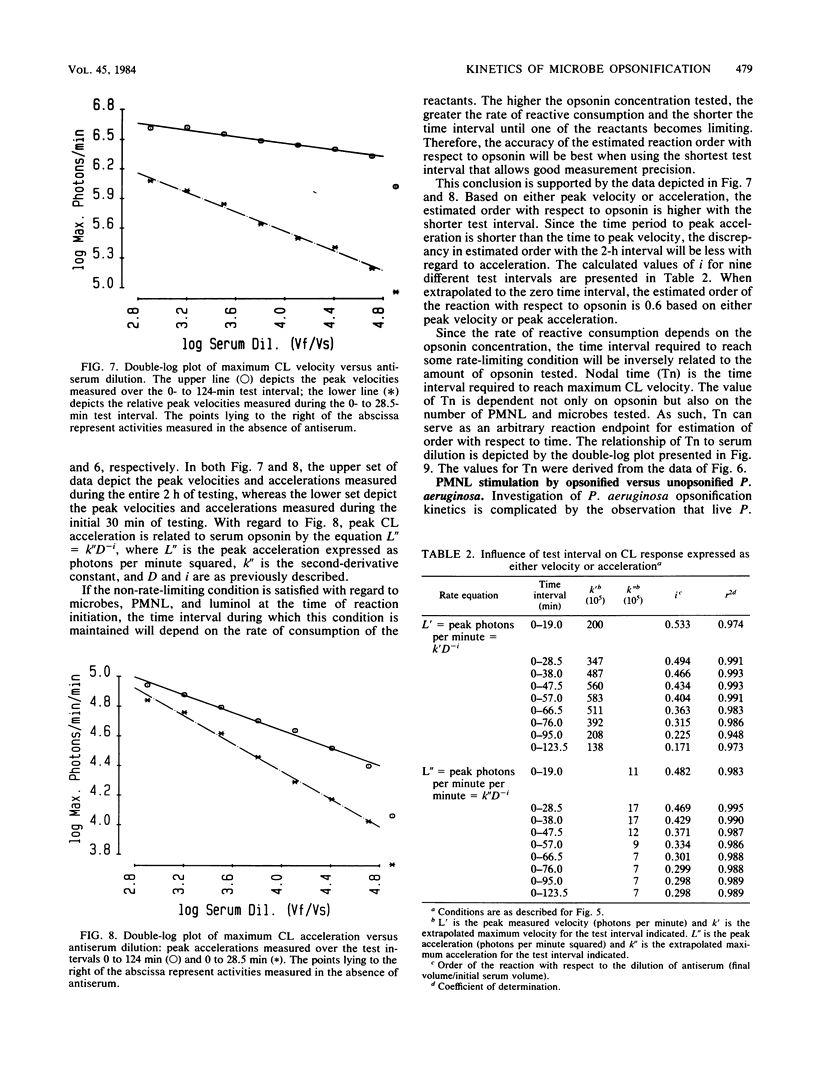
With Pseudomonas aeruginosa as the target microbes and polymorphonuclear leukocytes (PMNL) as effector phagocytes, the microbe-specific, immunoglobulin G (IgG)-dependent opsonic capacities of preimmune and immune sera were measured as the rate of stimulated PMNL dioxygenation of luminol yielding chemiluminescence (CL). When the reactants other than opsonin are present in concentrations that are not rate limiting, the information-effector relationship linking specific opsonin concentration to effector PMNL stimulation is described by the rate equation: L' = k'[IgG]i, where L' is the peak CL velocity (photons per minute), k' is the proportionality constant, [IgG] is the concentration of specific opsonin, and the exponent i is the order of the reaction with respect to opsonin. Since the specific opsonins were polyclonal IgG of unknown absolute serum concentration, the reciprocal rate expression, L' = k'D-i, was employed for data presentation; D is the serum dilution (final volume/initial serum volume), and the sign of i is changed to negative. The relationships of integral, first-derivative, and second-derivative expressions of the CL response to opsonin concentration are illustrated with experimentally obtained data. Based on peak CL velocity or peak CL acceleration measurements taken over different time intervals of testing, the estimated order with respect to opsonin is highest, and probably most accurate, using the shortest test interval allowing reasonably good precision of measurement. As an alternative temporal approach, microbe opsonification kinetics are analyzed based on nodal time (Tn) measurements. The Tn is the time point separating the acceleration and deceleration phases of the PMNL oxygenation response to stimulation and as such satisfies the criterion of a selected condition of PMNL activation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C. Evaluation of serum opsonic capacity by quantitating the initial chemiluminescent response from phagocytizing polymorphonuclear leukocytes. Infect Immun. 1977 Mar;15(3):828–833. doi: 10.1128/iai.15.3.828-833.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Pruitt B. A., Jr Humoral-phagocyte axis of immune defense in burn patients. Chemoluminigenic probing. Arch Surg. 1982 Feb;117(2):133–140. doi: 10.1001/archsurg.1982.01380260019004. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Borsos T., Rapp H. J. Complement fixation on cell surfaces by 19S and 7S antibodies. Science. 1965 Oct 22;150(3695):505–506. doi: 10.1126/science.150.3695.505. [DOI] [PubMed] [Google Scholar]
- Hemming V. G., Hall R. T., Rhodes P. G., Shigeoka A. O., Hill H. R. Assessment of group B streptococcal opsonins in human and rabbit serum by neutrophil chemiluminescence. J Clin Invest. 1976 Dec;58(6):1379–1387. doi: 10.1172/JCI108593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971 Dec;107(6):1535–1546. [PubMed] [Google Scholar]
- Lieberman M. M., McKissock D. C., Wright G. L. Passive immunization against Pseudomonas with a ribosomal vaccine-induced immune serum and immunoglobulin fractions. Infect Immun. 1979 Feb;23(2):509–521. doi: 10.1128/iai.23.2.509-521.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. M. Pseudomonas ribosomal vaccines: preparation, properties, and immunogenicity. Infect Immun. 1978 Jul;21(1):76–86. doi: 10.1128/iai.21.1.76-86.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. M., Wright G. L., Wolcott K. M., McKissock-Desoto D. C. Polyvalent antisera to Pseudomonas ribosomal vaccines: protection of mice against clinically isolated strains. Infect Immun. 1980 Aug;29(2):489–493. doi: 10.1128/iai.29.2.489-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madonna G. S., Allen R. C. Shigella sonnei phase I and phase II: susceptibility to direct serum lysis and opsonic requirements necessary for stimulation of leukocyte redox metabolism and killing. Infect Immun. 1981 Apr;32(1):153–159. doi: 10.1128/iai.32.1.153-159.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Johnston R. B., Jr Role of binding through C3b and IgG in polymorphonuclear neutrophil function: studies with trypsin-generated C3b. J Immunol. 1979 Oct;123(4):1839–1846. [PubMed] [Google Scholar]
- Rossi F., Romeo D., Patriarca P. Mechanism of phagocytosis-associated oxidative metabolism in polymorphonuclear leucocytes and macrophages. J Reticuloendothel Soc. 1972 Aug;12(2):127–149. [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Stevens P., Young L. S. Quantitative granulocyte chemiluminescence in the rapid detection of impaired opsonization of Escherichia coli. Infect Immun. 1977 Jun;16(3):796–804. doi: 10.1128/iai.16.3.796-804.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


