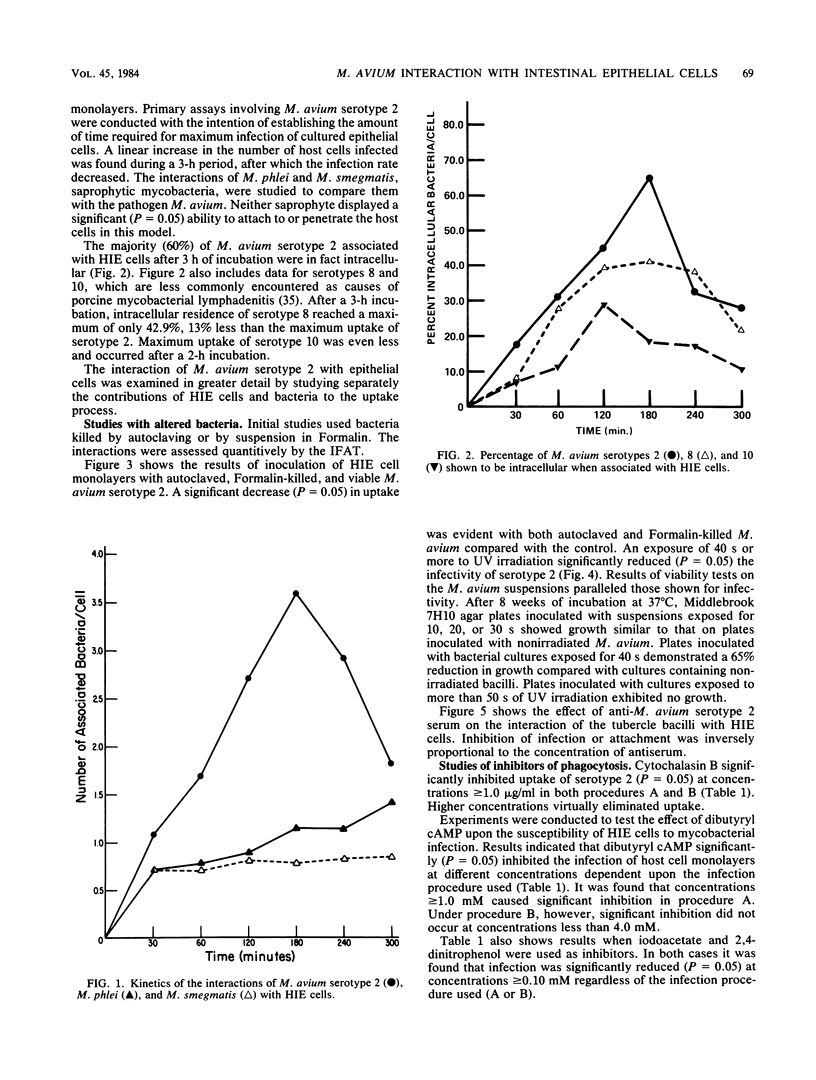
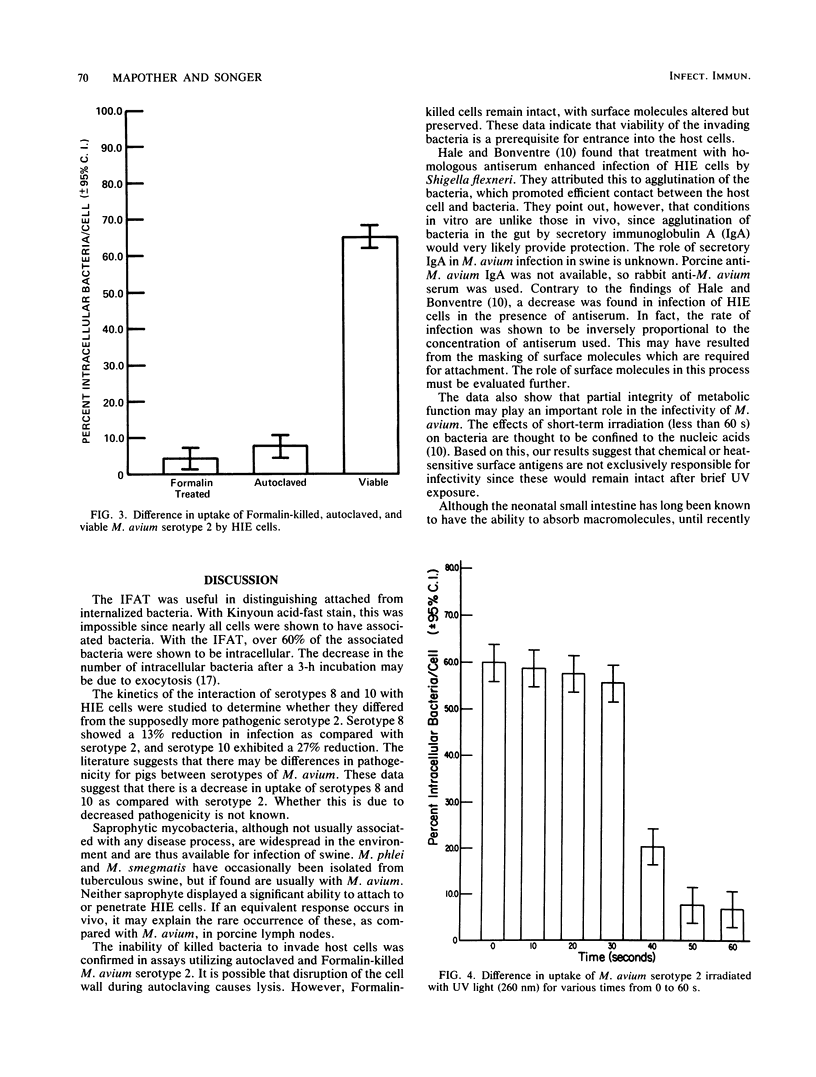
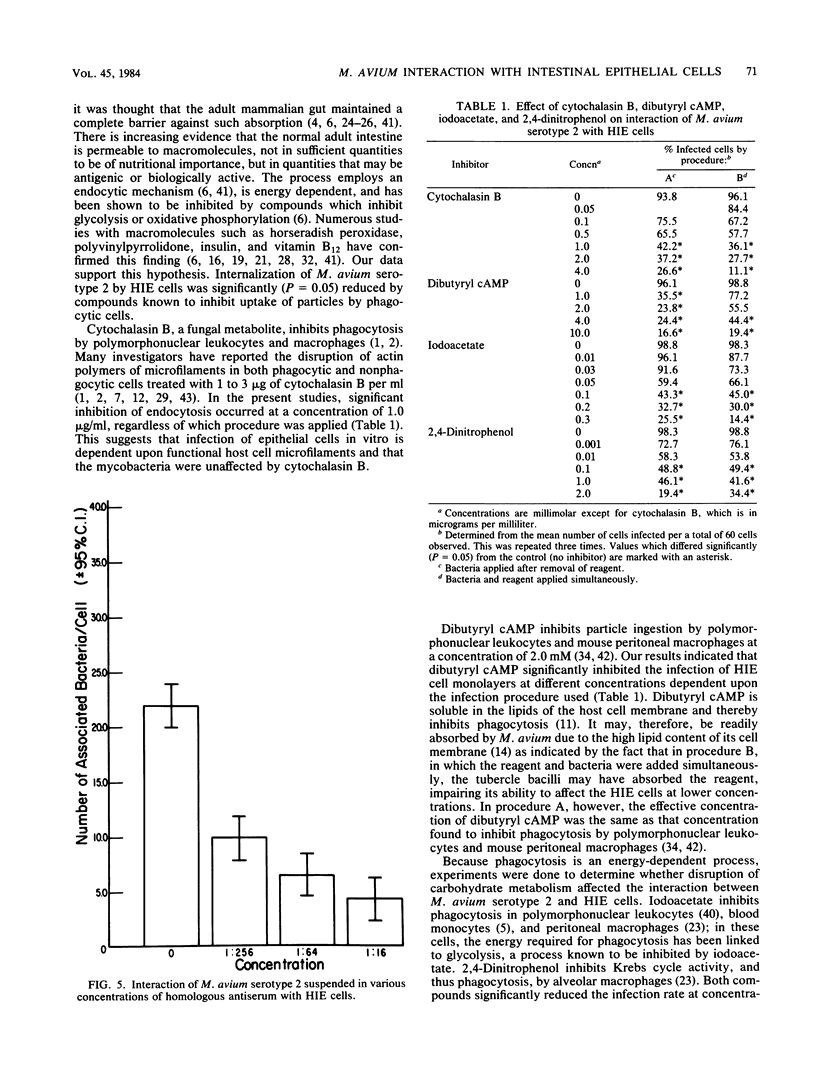
Abstract
Human intestinal epithelial cell monolayers were inoculated with cultures of Mycobacterium avium serotype 2, 8, or 10 that were viable, autoclaved, Formalin killed, exposed to UV light, or suspended in anti-M. avium serotype 2 serum. The effects of four reagents known to block phagocytosis or endocytosis (cytochalasin B, dibutyryl cyclic adenosine monophosphate, iodoacetate, and 2,4-dinitrophenol) on the bacteria-cell interaction were also studied. The maximum uptake of pathogenic M. avium by human intestinal epithelial cells occurred after 2 to 3 h of incubation. Serotype 2 was taken up in greater quantity than serotype 8 or 10. Saprophytic mycobacteria did not attach to or penetrate the host cells. The data showed that viable mycobacteria are ingested by host cells, whereas dead organisms are not. Components of the bacterial cells are partially, but not solely, responsible for the phagocytosis of M. avium serotype 2 by human intestinal epithelial cells. Furthermore, uptake of M. avium by human intestinal epithelial cells was suppressed by reagents which inhibit uptake by known phagocytic cells, suggesting that the mechanism of uptake is an endocytic process induced by virulent mycobacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Davies P., De Petris S. Role of contractile microfilaments in macrophage movement and endocytosis. Nat New Biol. 1971 Aug 4;232(31):153–155. doi: 10.1038/newbio232153a0. [DOI] [PubMed] [Google Scholar]
- Axline S. G., Reaven E. P. Inhibition of phagocytosis and plasma membrane mobility of the cultivated macrophage by cytochalasin B. Role of subplasmalemmal microfilaments. J Cell Biol. 1974 Sep;62(3):647–659. doi: 10.1083/jcb.62.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelsen J. D. Economics of the avian TB problem in swine. J Am Vet Med Assoc. 1974 Feb 1;164(3):307–308. [PubMed] [Google Scholar]
- Bockman D. E., Cooper M. D. Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer's patches. An electron microscopic study. Am J Anat. 1973 Apr;136(4):455–477. doi: 10.1002/aja.1001360406. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Lehrer R. I. Phagocytosis by human monocytes. Blood. 1968 Sep;32(3):423–435. [PubMed] [Google Scholar]
- Cooper M., Teichberg S., Lifshitz F. Alterations in rat jejunal permeability to a macromolecular tracer during a hyperosmotic load. Lab Invest. 1978 Apr;38(4):447–454. [PubMed] [Google Scholar]
- Davies P., Fox R. I., Polyzonis M., Allison A. C., Haswell A. D. The inhibition of phagocytosis and facilitation of exocytosis in rabbit polymorphonuclear leukocytes by cytochalasin B. Lab Invest. 1973 Jan;28(1):16–22. [PubMed] [Google Scholar]
- Hale T. L., Bonventre P. F. Shigella infection of Henle intestinal epithelial cells: role of the bacterium. Infect Immun. 1979 Jun;24(3):879–886. doi: 10.1128/iai.24.3.879-886.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Morris R. E., Bonventre P. F. Shigella infection of henle intestinal epithelial cells: role of the host cell. Infect Immun. 1979 Jun;24(3):887–894. doi: 10.1128/iai.24.3.887-894.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. H., Stossel T. P. Interactions of actin, myosin, and an actin-binding protein of rabbit pulmonary macrophages. III. Effects of cytochalasin B. J Cell Biol. 1976 Oct;71(1):295–303. doi: 10.1083/jcb.71.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihlström E. Infection of HeLa cells with Salmonella typhimurium 395 MS and MR10 bacteria. Infect Immun. 1977 Aug;17(2):290–295. doi: 10.1128/iai.17.2.290-295.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingham J. G., Whorwell P. J., Loehry C. A. Small intestinal permeability. 1. Effects of ischaemia and exposure to acetyl salicylate. Gut. 1976 May;17(5):354–361. doi: 10.1136/gut.17.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecce J. G. Glucose milliequivalents eaten by the neonatal pig and cessation of intestinal absorption of large molecules (closure). J Nutr. 1966 Nov;90(3):240–244. doi: 10.1093/jn/90.3.240. [DOI] [PubMed] [Google Scholar]
- Lesslie I. W. Correlation of biological potency of human and bovine tuberculin PPDs in guinea-pigs, cattle and man. J Biol Stand. 1976;4(1):39–42. doi: 10.1016/0092-1157(76)90037-8. [DOI] [PubMed] [Google Scholar]
- Murata H., Namioka S. The duration of colostral immunoglobulin uptake by the epithelium of the small intestine of neonatal piglets. J Comp Pathol. 1977 Jul;87(3):431–439. doi: 10.1016/0021-9975(77)90032-9. [DOI] [PubMed] [Google Scholar]
- OREN R., FARNHAM A. E., SAITO K., MILOFSKY E., KARNOVSKY M. L. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963 Jun;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. L., Jones A. L. Epithelial cell specialization within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974 Feb;66(2):189–203. [PubMed] [Google Scholar]
- Ray J. A., Mallmann V. H., Mallmann W. L., Morrill C. C. Pathologic and bacteriologic features and hypersensitivity of pigs given Mycobacterium bovis, Mycobacterium avium, or group 3 Mycobacteria. Am J Vet Res. 1972 Jul;33(7):1333–1345. [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Snellen J. E., Savage D. C. Freeze-fracture study of the filamentous, segmented microorganism attached to the murine small bowel. J Bacteriol. 1978 Jun;134(3):1099–1107. doi: 10.1128/jb.134.3.1099-1107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songer J. G., Bicknell E. J., Thoen C. O. Epidemiological investigation of swine tuberculosis in Arizona. Can J Comp Med. 1980 Apr;44(2):115–120. [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Hartwig J., Vaughan M. Quantitative studies of phagocytosis by polymorphonuclear leukocytes: use of emulsions to measure the initial rate of phagocytosis. J Clin Invest. 1972 Mar;51(3):615–624. doi: 10.1172/JCI106851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoen C. O., Jarnagin J. L., Richards W. D. Isolation and identification of mycobacteria from porcine tissues: a three-year summary. Am J Vet Res. 1975 Sep;36(9):1383–1386. [PubMed] [Google Scholar]
- Thoen C. O., Johnson D. W., Himes E. M., Menke S. B., Muscoplat C. C. Experimentally induced Mycobacterium avium serotype 8 infection in swine. Am J Vet Res. 1976 Feb;37(2):177–181. [PubMed] [Google Scholar]
- Thomas D. W., Hill J. C., Tyeryar F. J., Jr Interaction of gonococci with phagocytic leukocytes from men and mice. Infect Immun. 1973 Jul;8(1):98–104. doi: 10.1128/iai.8.1.98-104.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welscher H. D., Cruchaud A. The relationship between phagocytosis, release of lysosomal enzymes and 3', 5' cyclic adenosine monophosphate in mouse macrophages. Adv Exp Med Biol. 1976;66:705–710. doi: 10.1007/978-1-4613-4355-4_109. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Effects of cytochalasin B on polymorphonuclear leucocyte locomotion, phagocytosis and glycolysis. Exp Cell Res. 1972 Aug;73(2):383–393. doi: 10.1016/0014-4827(72)90062-6. [DOI] [PubMed] [Google Scholar]


