Abstract
The interaction between the T-cell receptor (TCR) and its peptide–major histocompatibility complex (pepMHC) ligand plays a critical role in determining the activity and specificity of the T cell. The binding properties associated with these interactions have now been studied in many systems, providing a framework for a mechanistic understanding of the initial events that govern T-cell function. There have been various other reviews that have described the structural and biochemical features of TCR : pepMHC interactions. Here we provide an overview of four areas that directly impact our understanding of T-cell function, as viewed from the perspective of the TCR : pepMHC interaction: (1) relationships between T-cell activity and TCR : pepMHC binding parameters, (2) TCR affinity, avidity and clustering, (3) influence of coreceptors on pepMHC binding by TCRs and T-cell activity, and (4) impact of TCR binding affinity on antigenic peptide specificity.
Keywords: agonists, antagonists, binding affinity, coreceptors, dissociation rate, major histocompatibility complex, peptide specificity, peptide–major histocompatibility complex, serial triggering, T-cell receptor, T-cell receptor clustering
Introduction
Studies of the interactions between antibodies and antigens, which have been studied for decades, have been made easier by virtue of the fact that antibodies are expressed as secreted molecules at relatively high concentrations. The antigen-specific molecule on T cells, the T-cell receptor (TCR), has been considerably more difficult to express in soluble form, making the analogous studies more difficult.1 Nevertheless, various strategies have been used to measure the intrinsic binding properties of TCRs for their antigens, complexes of peptides and a product of the major histocompatibility complex (MHC). Early studies revealed that TCRs have relatively low affinities for their peptide–MHC (pepMHC) ligands, similar to the affinities found for antibodies from primary responses. This feature has also made studies of TCR : pepMHC binding properties more difficult (i.e. higher concentrations of soluble TCR are required to measure lower affinity interactions). Another property of T-cell function that distinguishes the system from antibodies is the fact that in their normal context, TCRs are expressed as multichain membrane protein complexes on cells that express other proteins (namely, coreceptors CD4 and CD8) that are also intimately involved in the initial recognition process. Despite these complexities, the impact of TCR : pepMHC binding properties on T-cell activity and specificity has been revealed by the work of many laboratories, using various approaches. Here we review these findings, and describe some of the questions that remain to be addressed.
Relationships between T-cell activity and TCR : pepMHC binding parameters
In its simplest, reversible form, the interaction of TCR and pepMHC can be described by the reaction:
involving an association rate (kon) and a dissociation rate (koff). The half-life of the interaction (t1/2) is derived from the dissociation rate**. At equilibrium, a binding constant (KD = 1/KA) can be determined for the interaction using standard Scatchard approaches (or the kinetic parameters,† KD = koff/kon), KD = [TCR][pepMHC]/[TCR : pepMHC].
In a typical SPR experiment, with immobilized pepMHC, when the concentration of unbound pepMHC equals the concentration of bound pepMHC (i.e. half maximal resonance units in an SPR titration with increasing TCR), the KD is equal to the concentration of free, unbound TCR.‡
Using primarily, but not exclusively, SPR-based approaches, wild-type TCR affinities (KD values) have been shown to be in the range of 1–100 µm,2 characterized by slow association rates and intermediate dissociation rates (Table 1). There has been considerable debate as to which parameter of the TCR : pepMHC interaction, KD or half-life, determines the activation status of the T cell. The issue is important because there are fundamental signalling models (see below) that are predicated on which parameter is important. This question has been especially difficult to address because in most cases, the KD value is proportional to the t1/2. In addition, agonists that span the range of affinities and t1/2 values have been reported (Table 1 and Fig. 1a), complicating further any simple correlation between activity and these parameters.
Table 1.
Binding measurements for wild-type T-cell receptors
| TCR | Peptide/MHC | kon (per m second) | koff (per second) | t1/2 (seconds) | KD (μm) | Activity | References |
|---|---|---|---|---|---|---|---|
| CT26 | AH1(A5)/Ld | 58 000 | 0·11 | 6·3 | 1·9 | Agonist | 3 |
| P14 | gp33/Db | 400 000 | 0·975 | 0·7 | 2·4 | Agonist | 4 |
| 2C | p2Ca/Ld | 8300 | 0·027 | 25·7 | 3·3 | Agonist | 5 |
| 2C | QL9/Ld | 6350 | 0·025 | 26·8 | 3·9 | Agonist | 5 |
| CT26 | AH1/Ld | 61 000 | 0·35 | 2·0 | 5·7 | Agonist | 3 |
| OT-1 | OVA/Kb | 3720 | 0·022 | 31·5 | 5·9 | Agonist | 6 |
| OT-1 | OVA(G4)/Kb | 900 | 0·009 | 77 | 10 | Weak agonist | 7 |
| CT26 | AH1(A7)/Ld | 16 000 | 0·28 | 2·4 | 18 | Weak agonist | 3 |
| 2C | SIY/Kb | 22 000 | 0·464 | 1·5 | 27·4 | Agonist | 8 |
| AHIII 12.2 | p1058/Db | 6610 | 0·538 | 1·2 | 81·4 | Weak agonist | 9 |
| 2C | dEV8/Kb | 2200 | 0·185 | 3·7 | 84·1 | Antagonist | 5 |
| B7 | Tax/HLA-A2 | 96 000 | 0·13 | 5·2 | 1·2 | Agonist | 10 |
| A6 | Tax/HLA-A2 | 49 000 | 0·11 | 6·1 | 1·9 | Agonist | 10 |
| G10 | HIVgagSLY/HLA-A2 | 330 000 | 0·06 | 11·2 | 2·2 | Agonist | 11 |
| GRB | Flu/HLA-B27 | 39 000 | 0·09 | 7·4 | 3 | 12 | |
| JM22 | Flu/HLA-A2 | 31 000 | 0·16 | 4·2 | 5·2 | 13 | |
| G10 | HIVgagSLF/HLA-A2 | 340 000 | 0·16 | 4·2 | 5·2 | Agonist | 11 |
| CMV | pp65/HLA-A2 | 70 000 | 0·44 | 1·5 | 6·3 | 14 | |
| gp100 | gp100/HLA-A2 | 31 000 | 0·23 | 2·9 | 7 | 12 | |
| AHIII 12.2 | p1049/HLA-A2 | 26 200 | 0·295 | 2·3 | 11·3 | Agonist | 9 |
| LC13 | FLR(A)/HLA-B8 | 35 800 | 0·42 | 1·7 | 12·5 | Agonist | 15 |
| AM3 | EBV/HLA-A24 | 7300 | 0·21 | 3·2 | 28 | 12 | |
| 1G4 | NY-ESO-1/HLA-A2 | 40 000 | 0·128 | 6·4 | 32 | Agonist | 16 |
| TEL | tel/HLA-A2 | 3500 | 0·14 | 4·8 | 40 | 12 | |
| LC13 | FLR(F)/HLA-B8 | 2620 | 0·35 | 2·0 | 132 | Antagonist | 15 |
| 172.10 | MBP1-11[4Y]/I-Au | 37 200 | 0·219 | 3·1 | 5·9 | Agonist | 17 |
| 3.L2 | Hb/I-Ek | 5557 | 0·06 | 10·8 | 12 | Agonist | 18 |
| 1934.4 | MBP1-11[4Y]/I-Au | 5130 | 0·16 | 4·2 | 31 | Weak agonist | 17 |
| 2B4 | MCC/I-Ek | 633 | 0·057 | 11·7 | 90 | Agonist | 2 |
| MAW 13 | M-HSP/HLA-DR3 | 4000 | 0·12 | 5·6 | 30 | 12 | |
| AH1.23 | C-HSP/HLA-DR4 | 4400 | 0·16 | 4·2 | 36 | 12 | |
| 1A12 | MBP/HLA-DR2 | 2100 | 0·17 | 3·9 | 81 | 12 | |
| 2E11 | MBP/HLA-DR2 | 5900 | 0·73 | 0·9 | 123 | 12 |
KD, equilibrium binding constant; kon, association rate; koff, dissociation rate; MHC, major histocompatibility complex; t1/2, half-life of the interaction; TCR, T-cell receptor.
Measurements performed at 25°.
Figure 1.
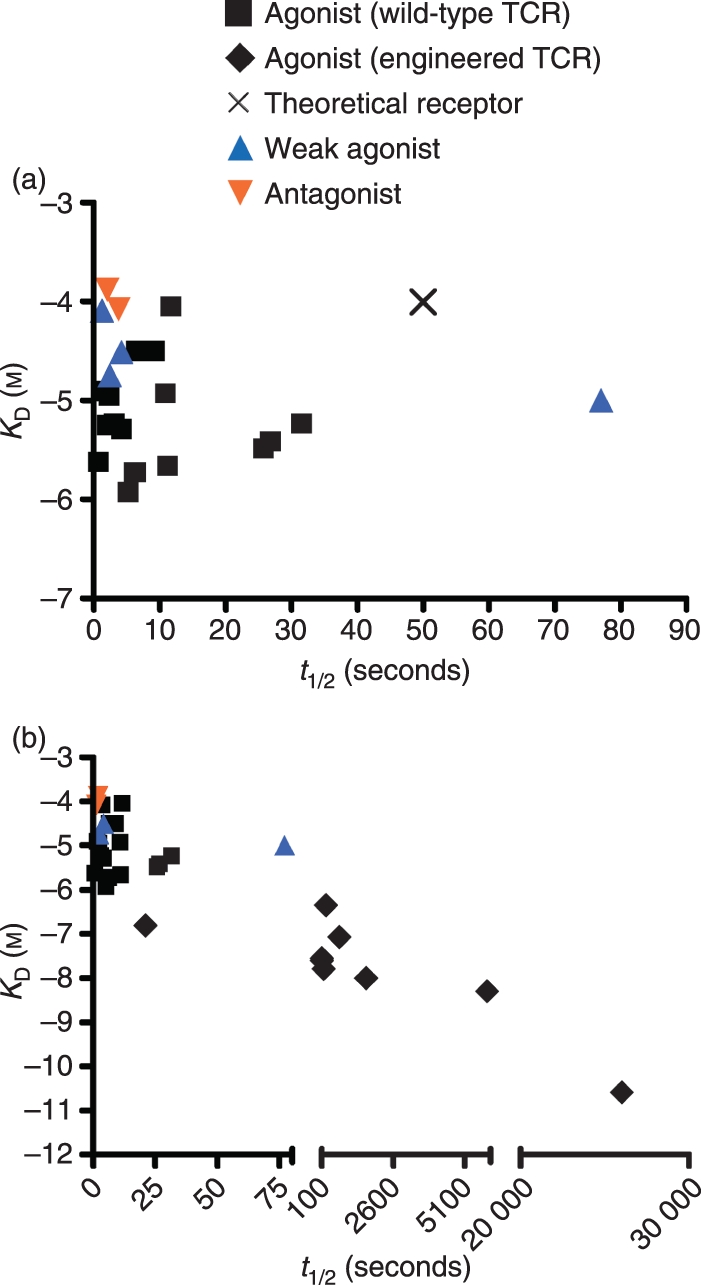
Relationships among T-cell activity, T-cell receptor (TCR) equilibrium binding constants, and TCR dissociation kinetics. (a) Equilibrium binding constants KD (affinity) and half-life (t1/2) measurements for wild-type TCRs were plotted using published values shown in Table 1. Theoretical receptor (X) represents the binding parameters of a TCR that, if available, would provide additional insight into the mechanisms of T-cell activation as described in the text. (b) Equilibrium binding constants KD (affinity) and half-life (t1/2) measurements for TCRs engineered for high affinity, from published values shown in Table 2, were added to the data in (a). The type of T-cell activity (agonist, weak agonist, antagonist) mediated by each TCR is indicated by the symbols.
The critical nature of the dissociation rate was an inherent feature of the kinetic proofreading and discrimination models first suggested over a decade ago. Those interactions with sufficiently long dissociation rates would allow for completion of intracellular signalling cascades that result in T-cell activation.19,20 A hierarchy of early T-cell signalling events, including the phosphorylation state of the CD3 subunits and intracellular calcium release, have been associated with a range of defined agonist, antagonist and null ligands.20,21 Many of these studies have concluded that the occupancy of the TCR, as correlated with the dissociation rate, determined the magnitude of the T-cell response. However, one study using the class I restricted OT-1 TCR suggested that these kinetic models may be oversimplified, as they observed that high-occupancy ligands (slow dissociation) initiated different T-cell activation kinetics (e.g. see t1/2‘outlier’ that is a weak agonist, blue triangle in Fig. 1a).7
Another observation consistent with the possible importance of dissociation rates involves the serial triggering hypothesis, in which a single pepMHC can bind to and trigger up to 200 individual TCRs.22 A tenet of this hypothesis is that not only did the interaction need to be sufficiently long to complete proximal signalling, but the dissociation rates needed to be sufficiently short to allow multiple TCRs the opportunity to bind to the same pepMHC. This led to the prediction that there should be an ‘optimal dwell time’ between the TCR and pepMHC, where a Gaussian distribution around t1/2 values explains T-cell activation; those complexes with too rapid, or too prolonged, dissociation rates would lie outside the optimal range, resulting in reduced activity.23,24 Studies by Kalergis et al. using a wild-type TCR provided evidence in favour of the model,23,24 but they relied on binding measurements with pepMHC tetramers (see below), rather than monomeric measurements assessed by SPR. Accordingly, it is not possible to compare their binding parameters relative to other systems shown in Table 1.
Other results appear to be inconsistent with the notion of an ‘optimal dwell time’. For example, TCRs have been engineered to have dissociation rates that are 100-fold to 1000-fold slower than wild-type TCRs, yet T cells transduced with these TCRs were fully able to be activated.25–27Table 2 shows a list of the binding parameters of these TCRs, which include mouse TCR mutants directed against the class I complexes SIY/Kb and QL9/Ld, mouse TCR mutants directed against the class II complex Hb/I-Ek, and human TCR mutants against the class I complexes NY-ESO-1/HLA-A2 and Tax/HLA-A2. The affinities of these high-affinity TCR : pepMHC interactions ranged from 30 nm to 26 pm, and the t1/2 values were as long as 425 min, yet they have shown strong agonist activity (Fig. 1b).
Table 2.
Binding measurements for engineered, high-affinity T-cell receptors
| TCR | Peptide/MHC | kon (per m per second) | koff (per second) | t1/2 (seconds) | KD (nm) | Activity | References |
|---|---|---|---|---|---|---|---|
| 2Cm6 | QL9/Ld | 42 000 | 0·0004 | 1650 | 10 | Agonist | 28 |
| 2Cm67 | SIY/Kb | 277 000 | 0·0044 | 158 | 16 | Agonist | 29 |
| 2Cm33 | SIY/Kb | 235 000 | 0·0066 | 105 | 28 | Agonist | 29 |
| 2Cm13 | QL9/Ld | 357 000 | 0·04 | 21 | 154 | Agonist | 8 |
| 1G4(c58/c61) | NY-ESO-1/HLA-A2 | 570 000 | 0·000027 | 26 000 | 0·026 | Agonist | 16 |
| A6(c134) | Tax/HLA-A2 | 80 000 | 0·0002 | 3120 | 2·5 | Agonist | 26 |
| 1G4(c5/c100) | NY-ESO-1/HLA-A2 | 39 000 | 0·000197 | 5880 | 5 | Agonist | 16 |
| 1G4(c10/c1) | NY-ESO-1/HLA-A2 | 16 500 | 0·00138 | 720 | 84 | Agonist | 16 |
| 1G4(c12/c2) | NY-ESO-1/HLA-A2 | 9000 | 0·004 | 240 | 450 | Agonist | 16 |
| 3.L2 m15 | Hb/I-Ek | 281 000 | 0·0068 | 104 | 25 | Agonist | 27 |
KD, binding constant; kon, association rate; koff, dissociation rate; MHC, major histocompatibility complex; t1/2, half-life of the interaction; TCR, T-cell receptor.
Measurements performed at 25°.
What about the role of affinity versus t1/2 values (i.e. put another way, do on-rates impact T-cell activity)? Several TCR : pepMHC interactions with known KD and t1/2 parameters (Table 1; TCRs OT-1, LC13 and P14) have provided evidence that on-rates influence activity (i.e. KD is important). As indicated above, in the OT-1 TCR system, a variant of the OVA peptide (G4) has a long t1/2, but its slower on-rate appears to be associated with the weak agonist activity of OVA(G4)/Kb (e.g. blue triangle in Fig. 1a).7 In another study, the LC13 TCR had affinities of 12·5 and 132 µm for two pepMHC ligands, a difference that was almost exclusively the result of on-rates.15 These ligands showed agonist activity in the case of the 12·5 µm interaction, and antagonist activity in the case of the 132 µm interaction, suggesting that the on-rate accounted for the difference in activities. More recently, activity mediated by the P14 TCR against gp33 peptide variants has also been shown to be influenced by the on-rate of the reaction.30 A caveat of these studies is that different peptides were used such that it is formally possible that T-cell activity could be influenced by MHC binding, not only TCR binding. In addition, in the latter studies, the dissociation kinetics were relatively fast (t1/2 = 0·3–2 seconds), in a range that is difficult to measure using SPR methodology.
While there appear to be several convincing cases where quite fast dissociation rates can still yield strong agonist activity (in apparent contradiction to dissociation rate models), there remain unanswered questions. Experimentally, it would be most decisive if one could identify additional TCR : pepMHC interactions with long dissociation rates (t1/2 > 50 seconds) but very slow on-rates, such that the affinities were at the low end of the range (e.g. > 100 µm). The dissociation rate model would predict that T cells expressing these TCRs would be fully active (e.g. see X in Fig. 1a). Another caveat of TCR studies is that most binding measurements have been made at 25°, whereas activity is measured at 37°. Kinetic constants at 37° are very difficult to measure because rates are generally faster and so become even less precise. Development of improved techniques, such as stop-flow fluorescence-based approaches, for kinetic measurements in the millisecond to second range would be useful. Finally, there is some evidence that TCR conformational plasticity, as measured by thermodynamic properties of TCR : pepMHC interactions including entropic binding penalties17 or heat capacity,31 influences T-cell activity (reviewed in ref. 32). It remains to be seen whether such effects will further complicate the efforts to dissect the importance of various binding parameters.
TCR affinity, avidity and clustering
Although much speculation revolves around the specific binding parameters which delineate a productive TCR : pepMHC interaction from a non-productive or antagonist interaction, for most T cells the binding parameters of the TCR are unknown. TCR binding has often been estimated using soluble pepMHC tetramers. MHC tetramer technology was introduced in 1996,33 and refers to biotinylated pepMHC complexes linked to streptavidin, which has four biotin binding sites. Multivalency of the pepMHC complexes allows binding to be detected through avidity, even though monovalent affinity may be inadequate to allow detectable binding by current methods.34,35 Fluorescent labelling of streptavidin allows easy monitoring of MHC tetramer-bound T-cell populations by flow cytometry. These oligomeric class I or class II pepMHC complexes have been used for identifying, tracking, isolating and characterizing antigen-specific CD8+ and CD4+ T-cell populations (reviewed in refs 36,37).
In addition to their qualitative uses for detection of TCR binding, MHC tetramers have been used as a proxy for defined affinity measurements, using tetramer staining titrations, cell surface-bound tetramer off-rates, and, occasionally, tetramer binding to TCRs in surface plasmon resonance.34,35,38,39 One should not confuse results obtained through these assays with actual monomeric TCR affinities or kinetic rates, as many other factors influence these measurements. To illustrate the impact of several factors, Fig. 2 shows simulated effects of different variables on MHC tetramer binding to (Fig. 2a–c) and dissociation from (Fig. 2d–f) the surface of a T cell, using a simplified multivalent binding model that has been used previously to describe streptavidin oligomer binding to T cells.40,41 Variations of this model have been applied to T-cell binding and triggering.41–44§
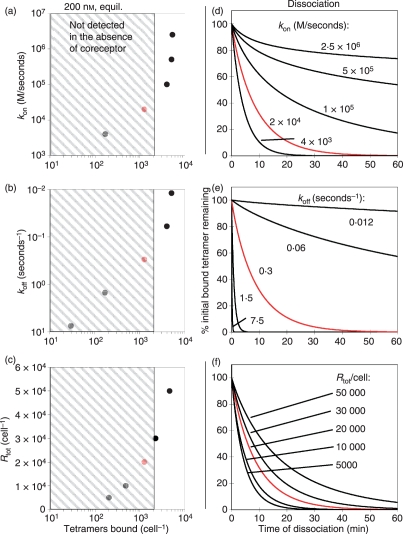
Figure 2.
Simulated effect of T-cell receptor : peptide–major histocompatibility complex (TCR:pepMHC) association kinetics, dissociation kinetics, or TCR surface levels on pepMHC tetramer binding. Using a simplified multivalent binding model, simulated values and curves were generated to predict (a–c) number of pepMHC tetramers bound per T cell or (d–f) dissociation rates of bound tetramers from a T cell. The varied TCR parameters included the kon (a,d), the koff (b,e), and the Rtot [total number of TCRs per T cell (c,f)]. In each panel, the fixed parameters correspond to the 2C TCR binding to SIY/Kb (kon = 20 000/m/second, koff∼0·3/second, Rtot∼20 000 per cell) and the simulated result for 2C TCR is shown in red. In panels (a–c) the equilibrium tetramer staining was simulated using 200 nm pepMHC tetramer, and the grey hatched boxes encompass the region below 2000 molecules per cell, a ‘threshold’ below which there may be no binding detectable by flow cytometry (i.e. detected as ‘no staining’ above the contol). Panels (d–f) show simulated tetramer dissociation rates predicted by varying the indicated parameters. The potential contribution of CD8 to binding is not assessed.
Simulated effects of binding parameters, as well as overall receptor levels, are shown, using approximate values for the CD8-dependent clone 2C TCR binding to SIY/Kb in each case. Equilibrium tetramer staining is reduced by a slower monomeric on-rate (Fig. 2a), a faster monomeric off-rate (Fig. 2b), or reduced TCR surface levels (as might be seen after T-cell stimulation or in TCR transduction approaches, Fig. 2c). Similarly, tetramer dissociation rates from the surface of T cells are slowed most dramatically by slower monomeric off-rates (Fig. 2e), but are also significantly impacted by increased on-rates (Fig. 2d) or higher TCR levels (Fig. 2f). The variability follows predictable trends for each individual parameter, but variations in multiple parameters between different TCR : pepMHC pairs would make correlations between the tetramer-staining properties and monomer-binding properties difficult and imprecise.
Further complications in analysing quantitative tetramer binding data arise from the non-standard distribution of oligomeric states in fluorescent MHC tetramer preparations, the effects of TCR density and T-cell activation state,45 and the potential interactions of MHC tetramers with coreceptors CD4 or CD8.44 More recently, the term ‘functional avidity’ has been used to describe tetramer-based binding results, which combines these considerations into a single term. While the multimeric measurements do little to deconvolute the questions surrounding the relevant TCR binding parameters required to trigger T-cell activation, they have nevertheless led to important insights. For example, Palmer and colleagues showed that a sharp threshold of KD,tet = 55–90 nm (KD,tet ∼ 576 nm, t1/2,tet < 12 seconds, measured with minimized contribution from CD8 binding) reliably delineates positive and negative thymic selection in CD8 T cells.38,46 In addition, Savage et al.47 showed an increase in overall functional avidity between polyclonal T cells in a secondary versus a primary antigen response. The use of MHC tetramers to compare the functional avidity of T cells with different activities therefore has significant potential.
While the ability of multivalent pepMHC multimers to bind to cell surface TCRs is only indirectly related to the activation potential of a T cell, many studies have shown that multivalent clustering of TCRs is necessary for T-cell signalling.48–51 Although T cells are not thought to undergo affinity maturation in their antigen receptors, at least to the same extent as antibodies, evidence has suggested that T-cell populations can exhibit increased sensitivity to antigen via avidity-based mechanisms. Naïve versus effector or memory cells with the same TCR exhibit different abilities to bind soluble MHC reagents and to respond to antigen.42,45,52 It has been determined that the TCR assembles with a single αβ hetrodimer per signalling complex, giving an overall stoichiometry of αβδεγεζζ.53 However, the state(s) of the TCR on the surface of T cells has been difficult to determine experimentally, and models including multiple binding pairs per assembled signalling complex have been proposed.54–56 Schamel et al.56 argue that monovalent TCR complexes coexist with multivalent, cholesterol-dependent TCR complexes that are preferentially triggered at low antigen concentration. They estimate that the preformed, static TCRs form clusters that contain approximately 2 to ≥ 20 TCR-αβ pairs per group; these may be precursors to early activation-linked TCR structures called microclusters (approximately 50 complexes or more).57–60
In addition to static clustering, T cells may regulate sensitivity by altering the mobility of cell surface receptors in the membrane. Glycosylation differences corresponding to differences in T-cell activation state have been identified, and reduction of glycosylation through pretreatment of cells with neuraminidase52,61 or inhibition of intracellular glycosylation enzymes62,63 leads to increased receptor clustering, hypersensitivity and even some loss of specificity. Upon antigen recognition, T cells have been shown to cluster their TCRs and other adhesion and costimulatory molecules into an immunological synapse, which increases the local concentration of TCRs and pepMHC for binding.64,65 Although the function of the immunological synapse has been controversial,66 and does not seem to be required for signalling to occur,67,68 it may function to ‘tune’ the immune response; amplifying responses to weak stimuli, and down-modulating responses to very strong stimuli.69–71
Interestingly, while T-cell activation requires a minimal binding threshold and TCR clustering, attempts to quantify the number of agonist peptide complexes on an antigen-presenting cell required to elicit a T-cell response have found that the number is vanishingly small – as low as a single agonist for a response to be recorded, and numbers in the order of 3–25 complexes to induce full effector responses.68,72,73 These findings prompted studies of the contribution of endogenous, or non-agonist pepMHC complexes in T-cell recognition.74 Despite having unmeasurable binding affinities for the relevant TCR, large numbers of endogenous pepMHC complexes have been found in mature immunological synapses,72 and addition of some null complexes amplifies a response to agonist complexes alone.74 Recent studies with CD4 T cells have shown that TCR triggering can be induced by selected pairs of soluble ‘heterodimers’ consisting of a single agonist pepMHC complex covalently linked to a weaker binding complex,75 indicating that in a system where avidity is crucial, even very low affinity interactions play an important role in supporting activation.
Influence of coreceptors on pepMHC binding by TCRs and T-cell activity
MHC ligands can be engaged not only by the TCR, but also by the coreceptor CD4 (class II MHC) or CD8 (class I MHC). These additional binding interactions further complicate the measurement of TCR affinity on the surface of T cells. The two coreceptors have some similarities but also seemingly different roles and effects on binding and activation.
The CD4 coreceptor binds to class II MHC with a weak KD (150–200 µm) and rapid kinetics. Intracellularly, CD4 associates with the p56lck kinase, which is important in T-cell triggering. CD4 seems to have little influence on MHC tetramer binding, and clusters with kinetics different from CD3ζ during activation.60 In one system, inhibition of CD4 binding raised the minimum number of agonist pepMHC complexes required for activation from between 1 and 10 up to ∼ 25; however, activation appeared to be identical beyond this threshold.72 A structure of human CD4 bound to a murine class II MHC revealed that the binding site of CD4 lies on the relatively invariant α2–β2 domain of MHC II, and that CD4 protrudes at a significant angle.76 The angle has led many to doubt that the CD4 bound to a given pepMHC would be in a position to interact with a TCR bound to the same pepMHC. The ‘pseudodimer’ model of CD4 involvement72,75 proposes that CD4 associates with a TCR that is bound to an agonist pepMHC, positioning p56lck to efficiently phosphorylate adjacent TCRs that are bound to either agonist or endogenous pepMHC (reviewed in refs. 77,78). This suggests that CD4 contributes to T-cell recognition by enhancing TCR cross-linking and increasing the local concentration of p56lck, rather than by contributing direct binding energy to the interaction of TCR with MHC.
CD8 exists on the T-cell surface as either αα homodimers or αβ heterodimers. The binding affinity of αα and αβ dimers to MHC I has been measured to be in the range of 10–200 µm, with differences that are in part the result of the allele of the class I MHC.79–83 Like CD4, CD8 binds with rapid kinetics including a koff of ∼ 18/second.84 Unlike CD4, however, CD8 seems to be capable of enhancing the on-rate and off-rate of tetramer binding,44,85 and many T-cell clones require the full participation of CD8 for MHC tetramer staining or activation (reviewed in ref. 83). A mutation in the MHC heavy chain that interferes with CD8 binding (D227K68,86–88) or the presence of a CD8-blocking antibody89 impairs MHC binding and recognition by the T cell. The TCRs with low binding affinities, as seen with most wild-type responses, require CD8 for activity and are referred to as CD8-dependent.
Some CD8-independent TCRs have been identified through allogeneic or xenogeneic stimulation9,90,91 or TCR engineering.16,28 These receptors have significantly higher affinities than CD8-dependent TCRs (4 µm–26 pm) but the precise binding threshold that makes a TCR CD8-independent is not yet defined. Laugel et al.85 described CD8-independent MHC-binding or T-cell activation with TCRs having KD values of 35 or 200 µm, respectively. In contrast, a lower KD threshold for CD8-independence of approximately 3 µm has been proposed based on the 2C T-cell system.25 The often studied TCR OT-1 is CD8-dependent and has a KD value of ∼ 6 µm for OVA/Kb6,92). It remains to be seen how CD8 synergizes with the TCR to achieve enhanced pepMHC binding, and whether the magnitude of the enhanced binding depends on the properties of the TCR. Unlike pepMHC class II ligands, it has been proposed that self pepMHC class I ligands do not operate to enhance T-cell signalling through interactions with the TCR, but operate exclusively through the participation of CD8.93
Impact of TCR binding affinity on antigenic peptide specificity
The T-cell antigenic (peptide) specificity is influenced by peptide binding to the MHC product and/or by peptide binding to the TCR (as a pepMHC complex). In the case of binding to the MHC, specificity is perceived as a loss of T-cell activity when the peptide binding to MHC is reduced. For example, if a viral peptide were mutated at an MHC anchor residue and was no longer efficiently presented, a peptide-specific T cell may lose the ability to recognize the virus-infected cell. In this case, the T cell appears to be highly specific for the wild-type peptide at this amino acid position, but the T-cell ‘specificity’ is in a sense indirect, because it is a consequence of the concentration (density) of the cell surface pepMHC ligand (i.e. the affinity of the peptide for the MHC product determines the cell surface density of the pepMHC complex, and a minimum pepMHC density is necessary for the T cell to be stimulated).
Here we focus on the case of peptide specificity influenced by binding of the pepMHC complex to the TCR (reviewed in ref. 94). As described above, the outcome of this interaction (i.e. T-cell activation) is determined by a minimum threshold of TCR binding (KD or t1/2) that must be achieved to activate the T cell. Changes in the peptide sequence that lower the TCR binding affinity could potentially yield an interaction energy that is below this threshold.95 For example, if we presume that the minimum affinity necessary for CD8+ T-cell activation is 500 µm, then any changes in peptide residues that lower the affinity below a KD value of 500 µm will in essence generate an inactive peptide variant (null peptide) or possibly an antagonist (see below).
In this context, T cells that bear TCRs with low affinity may appear to be more specific than higher affinity TCRs because very minor changes in the peptide could reduce the binding energy, and consequently the affinity, resulting in a loss of T-cell activity (Fig. 3a). In contrast, higher affinity TCRs would ‘tolerate’ more significant changes in peptide structure yet remain above the activation threshold (Fig. 3b). In these cases, the T cell would be activated by a greater array of peptide variants, and would appear to have reduced peptide specificity. By analogy with antibody : antigen interactions, we refer to the reactions with structurally related antigenic peptides as the ‘fine specificity’ (Fig. 3c).
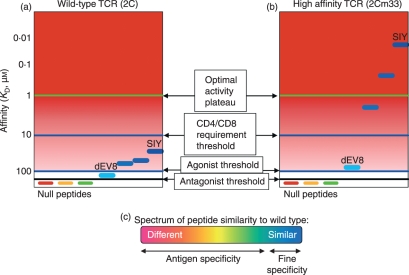
Figure 3.
Relationships among T-cell receptor (TCR) equilibrium binding constants, peptide specificity, and T-cell activity thresholds. For a given TCR, a variety of peptide ligands will have different stimulatory effects, depending on their binding affinity. The concept is highlighted using the 2C TCR system as a model. (a) Wild-type 2C is activated by a group of structurally similar peptides (of which SIY is the strong agonist) bound to Kb within a narrow affinity range, but only in the presence of the CD8 coreceptor. Those peptide–major histocompatibility complex (pepMHC) complexes that bind more weakly, such as dEV8/Kb, elicit antagonist signals. Complexes with even lower affinity are not stimulatory; they may give weak survival signals or be completely inactive (null). (b) By contrast, the high-affinity TCR 2Cm33 can be activated by agonist pepMHCs such as SIY/Kb without the CD8 coreceptor, and can be fully activated by the peptide dEV8 in the presence of CD8. This increase in affinity allows 2Cm33 to be stimulated by peptides with greater degrees of dissimilarity from wild-type, resulting in an apparent reduced ‘fine specificity’ by 2Cm33. (c) We refer to the ability of the TCR to distinguish between structurally unrelated peptides as ‘antigen specificity’ and the ability of the TCR to distinguish between structurally similar peptides (such as altered peptide ligands, in this case, of SIY) as ‘fine specificity’.
Observations about the role of TCR affinity in the fine specificity of T cells have now been made with in vitro-engineered TCRs that have affinities in the nanomolar range.16,27,29,96 The basis of this affect is illustrated most simply by a diagram that compares the effects of the wild-type 2C TCR affinity (Fig. 3a) and a high-affinity TCR mutant called 2C-m33 (Fig 3b). Change of a single amino acid residue on the peptide could reduce the binding affinity of either the low or high receptor by 10-fold, if the same atomic interactions operate at that region of the TCR : peptide interface. However, in the case of the TCR with low affinity, the peptide variant now has an affinity that is below the activity threshold (e.g. 500 µm). In contrast, a reduction in affinity of 10-fold for the high-affinity TCR still yields a TCR : pepMHC interaction that is above the threshold and the variant is active.
One advantage of evolving a T-cell immune system with TCRs that have a relatively low affinity threshold is to maintain a high degree of peptide specificity. This feature of the system may however have sacrificed the ability of T cells to recognize and eliminate peptide variants, as might arise from virus mutation. The apparent increase in the overall avidity of T cells after immunization may provide the system with an enhanced ability to recognize such variants.47,97 However, the equilibrium binding affinities of TCRs that are associated with these ‘avidity matured’ T cells have not been directly compared to the affinities associated with T cells from primary responses. In this respect, it is interesting that measurements of the best-studied T-cell clones may actually be such avidity-matured clones because generally these were derived after multiple immunizations with immunogen. Accordingly, the TCR affinities of T cells from primary responses may actually be quite low (e.g. in the 100 µm range).
Another aspect of peptide specificity that directly impacts T-cell function is the role of the multiple self (endogenous) peptides that are expressed by an individual. If the endogenous peptides have structures that are related to the foreign agonist peptide, then the peptide(s) may act not only in the process of positive selection, but also in the process of homeostatic proliferation and in enhancing the signalling that leads to full T-cell activation. Self peptides that are structurally homologous to a microbial foreign peptide may also be involved in autoimmune reactions, as has been suggested in various systems.98 There is in general very little known about the binding affinities of TCRs for this collection of self-peptide/MHC ligands, including what might be the minimal affinity necessary to achieve T-cell signalling that drives homeostatic proliferation.99
An intriguing feature of TCR-mediated binding involves the complexity of outcomes that can result from interactions that do not lead to full activity. For example, TCR binding to self peptide/MHC ligands could either enhance or antagonize signalling mediated by binding of the same TCR to agonist pepMHC ligands. Accordingly, self pepMHC : TCR interactions could amplify T-cell activity through coreceptor involvement, or could inhibit activity if partial ‘antagonist’ signals are initiated through such interactions. This has the important consequence that peptide variants, for example from viruses or potential tumour antigens, may not only escape recognition that leads to a productive T-cell reaction, but may also antagonize T-cell reactions. That is, binding of the mutated pepMHC through the TCR may actually lead to an inhibitory signal that operates through an antagonist reaction.
Finally, it is now clear that some TCRs cross-react with peptides and/or MHC molecules that are not structurally similar.100–102 The 2C TCR is such an example, as it has specificity for both the SIY/Kb ligand and an alloantigen called QL9/Ld. These cross-reactions may be the result of the structural plasticity of TCRs; it has now been shown that the 2C TCR docks onto Ld in a quite different mode from that with which it docks onto the SIY/Kb ligand, and it uses largely different chemistries to achieve binding.103 A conformer model, analogous to antibody reactions with diverse antigens,104 suggests that distinct conformations of the same TCR could bind to structurally distinct ligands.105,106 Nevertheless, the affinity and specificity rules hold for each distinct TCR : pepMHC interaction, whether the pepMHC are structurally related or not.
Conclusion
The binding properties of TCRs for their pepMHC ligands are critically important in the function of T cells, leading to outcomes that can involve T-cell selection in the thymus or full peripheral T-cell responsiveness or homeostatic T-cell proliferation in the periphery. The processes are even more complicated because the same TCR could interact with multiple pepMHC ligands on the same antigen-presenting cell, each with heterogeneous binding properties. These reactions would result in a complex integration of signals that ultimately determine the nature of the T-cell response. While there have been numerous studies to elucidate the precise binding parameters that correlate with different T-cell activities, various questions remain unanswered (in part because of the technical difficulties associated with performing binding experiments on low-affinity reactions). Further understanding of the TCR binding properties that generate defined signals is important, not only from a basic science perspective but also toward developing optimal strategies that improve T-cell responses to foreign antigens and tumour antigens.
Acknowledgments
We thank members of the laboratory for their contributions and discussions. This work was supported by NIH grant GM55767 (to D.M.K.), a grant from the James S. McDonnell Foundation (to D.M.K.), and the Samuel and Ruth Engelberg/Irvington Institute Fellowship of the Cancer Research Institute (to J.D.S.).
Footnotes
Half-life (t1/2) = ln 2/koff
It is worth noting that for many low-affinity interactions, it can be difficult to measure kinetic parameters with confidence because the high protein concentrations required [e.g. for surface plasmon resonance (SPR)] can lead to a higher background signal. Therefore, in KD ranges of > 10 µm it becomes increasingly difficult to determine reliable association and dissociation rates.
In these experiments, it is assumed that the total TCR concentration is approximately equal to the free, unbound TCR, a valid assumption given the typically high concentrations of TCR relative to the amount of immobilized pepMHC.
The model assumes a maximum bound valency of three due to geometrical constraints, despite a total reagent valency of four. The version of the model applied here does not include the possible effects of MHC coreceptor, nor does it assume any binding/cross-linking co-operation beyond an increase in local concentration.
References
- 1.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–66. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 2.Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–44. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 3.Slansky JE, Rattis FM, Boyd LF, Fahmy T, Jaffee EM, Schneck JP, Margulies DH, Pardoll DM. Enhanced antigen-specific antitumor immunity with altered peptide ligands that stabilize the MHC-peptide-TCR complex. Immunity. 2000;13:529–38. doi: 10.1016/s1074-7613(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 4.Boulter JM, Schmitz N, Sewell AK, Godkin AJ, Bachmann MF, Gallimore AM. Potent T cell agonism mediated by a very rapid TCR/pMHC interaction. Eur J Immunol. 2007;37:798–806. doi: 10.1002/eji.200636743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia KC, Tallquist MD, Pease LR, et al. Alphabeta T cell receptor interactions with syngeneic and allogeneic ligands: affinity measurements and crystallization. Proc Natl Acad Sci USA. 1997;94:13838–43. doi: 10.1073/pnas.94.25.13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alam SM, Davies GM, Lin CM, et al. Qualitative and quantitative differences in T cell receptor binding of agonist and antagonist ligands. Immunity. 1999;10:227–37. doi: 10.1016/s1074-7613(00)80023-0. [DOI] [PubMed] [Google Scholar]
- 7.Rosette C, Werlen G, Daniels MA, et al. The impact of duration versus extent of TCR occupancy on T cell activation: a revision of the kinetic proofreading model. Immunity. 2001;15:59–70. doi: 10.1016/s1074-7613(01)00173-x. [DOI] [PubMed] [Google Scholar]
- 8.Jones LL, Colf LA, Stone JD, Garcia KC, Kranz DM. Distinct CDR3 conformations in T cell receptors determine the level of cross-reactivity for diverse antigens, but not the docking orientation. J Immunol. 2008;181:6255–64. doi: 10.4049/jimmunol.181.9.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buslepp J, Kerry SE, Loftus D, Frelinger JA, Appella E, Collins EJ. High affinity xenoreactive TCR:MHC interaction recruits CD8 in absence of binding to MHC. J Immunol. 2003;170:373–83. doi: 10.4049/jimmunol.170.1.373. [DOI] [PubMed] [Google Scholar]
- 10.Davis-Harrison RL, Armstrong KM, Baker BM. Two different T cell receptors use different thermodynamic strategies to recognize the same peptide/MHC ligand. J Mol Biol. 2005;346:533–50. doi: 10.1016/j.jmb.2004.11.063. [DOI] [PubMed] [Google Scholar]
- 11.Lee JK, Stewart-Jones G, Dong T, et al. T cell cross-reactivity and conformational changes during TCR engagement. J Exp Med. 2004;200:1455–66. doi: 10.1084/jem.20041251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole DK, Pumphrey NJ, Boulter JM, et al. Human TCR-binding affinity is governed by MHC class restriction. J Immunol. 2007;178:5727–34. doi: 10.4049/jimmunol.178.9.5727. [DOI] [PubMed] [Google Scholar]
- 13.Ishizuka J, Stewart-Jones GB, van der Merwe A, Bell JI, McMichael AJ, Jones EY. The structural dynamics and energetics of an immunodominant T cell receptor are programmed by its Vbeta domain. Immunity. 2008;28:171–82. doi: 10.1016/j.immuni.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Gakamsky DM, Lewitzki E, Grell E, Saulquin X, Malissen B, Montero-Julian F, Bonneville M, Pecht I. Kinetic evidence for a ligand-binding-induced conformational transition in the T cell receptor. Proc Natl Acad Sci USA. 2007;104:16639–44. doi: 10.1073/pnas.0707061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ely LK, Green KJ, Beddoe T, et al. Antagonism of antiviral and allogeneic activity of a human public CTL clonotype by a single altered peptide ligand: implications for allograft rejection. J Immunol. 2005;174:5593–601. doi: 10.4049/jimmunol.174.9.5593. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Bennett AD, Zheng Z, et al. High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J Immunol. 2007;179:5845–54. doi: 10.4049/jimmunol.179.9.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia KC, Radu CG, Ho J, Ober RJ, Ward ES. Kinetics and thermodynamics of T cell receptor–autoantigen interactions in murine experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2001;98:6818–23. doi: 10.1073/pnas.111161198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kersh GJ, Kersh EN, Fremont DH, Allen PM. High- and low-potency ligands with similar affinities for the TCR: the importance of kinetics in TCR signaling. Immunity. 1998;9:817–26. doi: 10.1016/s1074-7613(00)80647-0. [DOI] [PubMed] [Google Scholar]
- 19.McKeithan TW. Kinetic proofreading in T-cell receptor signal transduction. Proc Natl Acad Sci USA. 1995;92:5042–6. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabinowitz JD, Beeson C, Wulfing C, Tate K, Allen PM, Davis MM, McConnell HM. Altered T cell receptor ligands trigger a subset of early T cell signals. Immunity. 1996;5:125–35. doi: 10.1016/s1074-7613(00)80489-6. [DOI] [PubMed] [Google Scholar]
- 21.Kersh EN, Shaw AS, Allen PM. Fidelity of T cell activation through multistep T cell receptor zeta phosphorylation. Science. 1998;281:572–5. doi: 10.1126/science.281.5376.572. [DOI] [PubMed] [Google Scholar]
- 22.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide–MHC complexes. Nature. 1995;375:148–51. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 23.Kalergis AM, Boucheron N, Doucey MA, Palmieri E, Goyarts EC, Vegh Z, Luescher IF, Nathenson SG. Efficient T cell activation requires an optimal dwell-time of interaction between the TCR and the pMHC complex. Nat Immunol. 2001;2:229–34. doi: 10.1038/85286. [DOI] [PubMed] [Google Scholar]
- 24.Carreno LJ, Bueno SM, Bull P, Nathenson SG, Kalergis AM. The half-life of the T-cell receptor/peptide-major histocompatibility complex interaction can modulate T-cell activation in response to bacterial challenge. Immunology. 2007;121:227–37. doi: 10.1111/j.1365-2567.2007.02561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 2003;18:255–64. doi: 10.1016/s1074-7613(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Moysey R, Molloy PE, et al. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol. 2005;23:349–54. doi: 10.1038/nbt1070. [DOI] [PubMed] [Google Scholar]
- 27.Weber KS, Donermeyer DL, Allen PM, Kranz DM. Class II-restricted T cell receptor engineered in vitro for higher affinity retains peptide specificity and function. Proc Natl Acad Sci USA. 2005;102:19033–8. doi: 10.1073/pnas.0507554102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holler PD, Lim AR, Cho BK, Rund LA, Kranz DM. CD8(−) T cell transfectants that express a high affinity T cell receptor exhibit enhanced peptide-dependent activation. J Exp Med. 2001;194:1043–52. doi: 10.1084/jem.194.8.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holler PD, Chlewicki LK, Kranz DM. TCRs with high affinity for foreign pMHC show self-reactivity. Nat Immunol. 2003;4:55–62. doi: 10.1038/ni863. [DOI] [PubMed] [Google Scholar]
- 30.Tian S, Maile R, Collins EJ, Frelinger JA. CD8+ T cell activation is governed by TCR-peptide/MHC affinity, not dissociation rate. J Immunol. 2007;179:2952–60. doi: 10.4049/jimmunol.179.5.2952. [DOI] [PubMed] [Google Scholar]
- 31.Krogsgaard M, Prado N, Adams EJ, He XL, Chow DC, Wilson DB, Garcia KC, Davis MM. Evidence that structural rearrangements and/or flexibility during TCR binding can contribute to T cell activation. Mol Cell. 2003;12:1367–78. doi: 10.1016/s1097-2765(03)00474-x. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong KM, Insaidoo FK, Baker BM. Thermodynamics of T-cell receptor-peptide/MHC interactions: progress and opportunities. J Mol Recognit. 2008;21:275–87. doi: 10.1002/jmr.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–6. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Huang Y, Lue J, Quandt JA, Martin R, Mariuzza RA. Structure of a human autoimmune TCR bound to a myelin basic protein self-peptide and a multiple sclerosis-associated MHC class II molecule. Embo J. 2005;24:2968–79. doi: 10.1038/sj.emboj.7600771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tissot AC, Pecorari F, Pluckthun A. Characterizing the functionality of recombinant T-cell receptors in vitro: a pMHC tetramer based approach. J Immunol Methods. 2000;2:147–65. doi: 10.1016/s0022-1759(99)00226-4. [DOI] [PubMed] [Google Scholar]
- 36.Ogg GS, McMichael AJ. HLA-peptide tetrameric complexes. Curr Opin Immunol. 1998;10:393–6. doi: 10.1016/s0952-7915(98)80110-6. [DOI] [PubMed] [Google Scholar]
- 37.Vollers SS, Stern LJ. Class II major histocompatibility complex tetramer staining: progress, problems, and prospects. Immunology. 2008;123:305–13. doi: 10.1111/j.1365-2567.2007.02801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daniels MA, Teixeiro E, Gill J, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–9. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 39.Holmberg K, Mariathasan S, Ohteki T, Ohashi PS, Gascoigne NR. TCR binding kinetics measured with MHC class I tetramers reveal a positive selecting peptide with relatively high affinity for TCR. J Immunol. 2003;171:2427–34. doi: 10.4049/jimmunol.171.5.2427. [DOI] [PubMed] [Google Scholar]
- 40.Perelson AS. Receptor clustering on a cell surface III. Theory of receptor cross-linking by multivalent ligands: description by ligand states. Math Biosci. 1981;53:1–39. [Google Scholar]
- 41.Stone JD, Cochran JR, Stern LJ. T-cell activation by soluble MHC oligomers can be described by a two-parameter binding model. Biophys J. 2001;81:2547–57. doi: 10.1016/S0006-3495(01)75899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fahmy TM, Bieler JG, Edidin M, Schneck JP. Increased TCR avidity after T cell activation: a mechanism for sensing low-density antigen. Immunity. 2001;14:135–43. [PubMed] [Google Scholar]
- 43.Schamel WW, Risueno RM, Minguet S, Ortiz AR, Alarcon B. A conformation- and avidity-based proofreading mechanism for the TCR-CD3 complex. Trends Immunol. 2006;27:176–82. doi: 10.1016/j.it.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Wooldridge L, van den Berg HA, Glick M, et al. Interaction between the CD8 coreceptor and major histocompatibility complex class I stabilizes T cell receptor-antigen complexes at the cell surface. J Biol Chem. 2005;280:27491–501. doi: 10.1074/jbc.M500555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cameron TO, Cochran JR, Yassine-Diab B, Sekaly RP, Stern LJ. Cutting edge: detection of antigen-specific CD4+ T cells by HLA-DR1 oligomers is dependent on the T cell activation state. J Immunol. 2001;166:741–5. doi: 10.4049/jimmunol.166.2.741. [DOI] [PubMed] [Google Scholar]
- 46.Naeher D, Daniels MA, Hausmann B, Guillaume P, Luescher I, Palmer E. A constant affinity threshold for T cell tolerance. J Exp Med. 2007;204:2553–9. doi: 10.1084/jem.20070254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10:485–92. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 48.Boniface JJ, Rabinowitz JD, Wulfing C, et al. Initiation of signal transduction through the T cell receptor requires the multivalent engagement of peptide/MHC ligands [corrected] Immunity. 1998;9:459–66. doi: 10.1016/s1074-7613(00)80629-9. [DOI] [PubMed] [Google Scholar]
- 49.Cochran JR, Cameron TO, Stern LJ. The relationship of MHC-peptide binding and T cell activation probed using chemically defined MHC class II oligomers. Immunity. 2000;12:241–50. doi: 10.1016/s1074-7613(00)80177-6. [DOI] [PubMed] [Google Scholar]
- 50.Minguet S, Swamy M, Alarcon B, Luescher IF, Schamel WW. Full activation of the T cell receptor requires both clustering and conformational changes at CD3. Immunity. 2007;26:43–54. doi: 10.1016/j.immuni.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 51.Stone JD, Stern LJ. CD8 T cells, like CD4 T cells, are triggered by multivalent engagement of TCRs by MHC-peptide ligands but not by monovalent engagement. J Immunol. 2006;176:1498–505. doi: 10.4049/jimmunol.176.3.1498. [DOI] [PubMed] [Google Scholar]
- 52.Kao C, Daniels MA, Jameson SC. Loss of CD8 and TCR binding to class I MHC ligands following T cell activation. Int Immunol. 2005;17:607–17. doi: 10.1093/intimm/dxh340. [DOI] [PubMed] [Google Scholar]
- 53.Call ME, Pyrdol J, Wiedmann M, Wucherpfennig KW. The organizing principle in the formation of the T cell receptor-CD3 complex. Cell. 2002;111:967–79. doi: 10.1016/s0092-8674(02)01194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Exley M, Wileman T, Mueller B, Terhorst C. Evidence for multivalent structure of T-cell antigen receptor complex. Mol Immunol. 1995;32:829–39. doi: 10.1016/0161-5890(95)00046-h. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez-Miguel G, Alarcon B, Iglesias A, Bluethmann H, Alvarez-Mon M, Sanz E, de la Hera A. Multivalent structure of an alphabetaT cell receptor. Proc Natl Acad Sci USA. 1999;96:1547–52. doi: 10.1073/pnas.96.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schamel WW, Arechaga I, Risueno RM, van Santen HM, Cabezas P, Risco C, Valpuesta JM, Alarcon B. Coexistence of multivalent and monovalent TCRs explains high sensitivity and wide range of response. J Exp Med. 2005;202:493–503. doi: 10.1084/jem.20042155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–6. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, Dustin ML, Saito T. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–62. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 59.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–27. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krummel MF, Sjaastad MD, Wulfing C, Davis MM. Differential clustering of CD4 and CD3zeta during T cell recognition. Science. 2000;289:1349–52. doi: 10.1126/science.289.5483.1349. [DOI] [PubMed] [Google Scholar]
- 61.Daniels MA, Devine L, Miller JD, et al. CD8 binding to MHC class I molecules is influenced by T cell maturation and glycosylation. Immunity. 2001;15:1051–61. doi: 10.1016/s1074-7613(01)00252-7. [DOI] [PubMed] [Google Scholar]
- 62.Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–9. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 63.Morgan R, Gao G, Pawling J, Dennis JW, Demetriou M, Li B. N-acetylglucosaminyltransferase V (Mgat5)-mediated N-glycosylation negatively regulates Th1 cytokine production by T cells. J Immunol. 2004;173:7200–8. doi: 10.4049/jimmunol.173.12.7200. [DOI] [PubMed] [Google Scholar]
- 64.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–7. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 65.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–6. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 66.Dustin ML. T-cell activation through immunological synapses and kinapses. Immunol Rev. 2008;221:77–89. doi: 10.1111/j.1600-065X.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- 67.Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–42. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 68.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5:524–30. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 69.Cemerski S, Das J, Giurisato E, Markiewicz MA, Allen PM, Chakraborty AK, Shaw AS. The balance between T cell receptor signaling and degradation at the center of the immunological synapse is determined by antigen quality. Immunity. 2008;29:414–22. doi: 10.1016/j.immuni.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cemerski S, Das J, Locasale J, et al. The stimulatory potency of T cell antigens is influenced by the formation of the immunological synapse. Immunity. 2007;26:345–55. doi: 10.1016/j.immuni.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee KH, Dinner AR, Tu C, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–22. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 72.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–9. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 73.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–71. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 74.Wulfing C, Sumen C, Sjaastad MD, Wu LC, Dustin ML, Davis MM. Costimulation and endogenous MHC ligands contribute to T cell recognition. Nat Immunol. 2002;3:42–7. doi: 10.1038/ni741. [DOI] [PubMed] [Google Scholar]
- 75.Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–43. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 76.Wang JH, Meijers R, Xiong Y, Liu JH, Sakihama T, Zhang R, Joachimiak A, Reinherz EL. Crystal structure of the human CD4 N-terminal two-domain fragment complexed to a class II MHC molecule. Proc Natl Acad Sci USA. 2001;98:10799–804. doi: 10.1073/pnas.191124098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Germain RN. T-cell activation: the power of one. Curr Biol. 2003;13:R137–9. doi: 10.1016/s0960-9822(03)00075-7. [DOI] [PubMed] [Google Scholar]
- 78.Krogsgaard M, Davis MM. How T cells ‘see’ antigen. Nat Immunol. 2005;6:239–45. doi: 10.1038/ni1173. [DOI] [PubMed] [Google Scholar]
- 79.Kern P, Hussey RE, Spoerl R, Reinherz EL, Chang HC. Expression, purification, and functional analysis of murine ectodomain fragments of CD8alphaalpha and CD8alphabeta dimers. J Biol Chem. 1999;274:27237–43. doi: 10.1074/jbc.274.38.27237. [DOI] [PubMed] [Google Scholar]
- 80.Garcia KC, Scott CA, Brunmark A, Carbone FR, Peterson PA, Wilson IA, Teyton L. CD8 enhances formation of stable T-cell receptor/MHC class I molecule complexes. Nature. 1996;384:577–81. doi: 10.1038/384577a0. [DOI] [PubMed] [Google Scholar]
- 81.Gao GF, Willcox BE, Wyer JR, et al. Classical and nonclassical class I major histocompatibility complex molecules exhibit subtle conformational differences that affect binding to CD8alphaalpha. J Biol Chem. 2000;275:15232–8. doi: 10.1074/jbc.275.20.15232. [DOI] [PubMed] [Google Scholar]
- 82.Gao GF, Jakobsen BK. Molecular interactions of coreceptor CD8 and MHC class I: the molecular basis for functional coordination with the T-cell receptor. Immunol Today. 2000;21:630–6. doi: 10.1016/s0167-5699(00)01750-3. [DOI] [PubMed] [Google Scholar]
- 83.Konig R. Interactions between MHC molecules and co-receptors of the TCR. Curr Opin Immunol. 2002;14:75–83. doi: 10.1016/s0952-7915(01)00300-4. [DOI] [PubMed] [Google Scholar]
- 84.Wyer JR, Willcox BE, Gao GF, Gerth UC, Davis SJ, Bell JI, van der Merwe PA, Jakobsen BK. T cell receptor and coreceptor CD8 alphaalpha bind peptide-MHC independently and with distinct kinetics. Immunity. 1999;10:219–25. doi: 10.1016/s1074-7613(00)80022-9. [DOI] [PubMed] [Google Scholar]
- 85.Laugel B, van den Berg HA, Gostick E, et al. Different T cell receptor affinity thresholds and CD8 coreceptor dependence govern cytotoxic T lymphocyte activation and tetramer binding properties. J Biol Chem. 2007;282:23799–810. doi: 10.1074/jbc.M700976200. [DOI] [PubMed] [Google Scholar]
- 86.Potter TA, Bluestone JA, Rajan TV. A single amino acid substitution in the alpha 3 domain of an H-2 class I molecule abrogates reactivity with CTL. J Exp Med. 1987;166:956–66. doi: 10.1084/jem.166.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Potter TA, Rajan TV, Dick RF, II, Bluestone JA. Substitution at residue 227 of H-2 class I molecules abrogates recognition by CD8-dependent, but not CD8-independent, cytotoxic T lymphocytes. Nature. 1989;337:73–5. doi: 10.1038/337073a0. [DOI] [PubMed] [Google Scholar]
- 88.Salter RD, Benjamin RJ, Wesley PK, et al. A binding site for the T-cell co-receptor CD8 on the alpha 3 domain of HLA-A2. Nature. 1990;345:41–6. doi: 10.1038/345041a0. [DOI] [PubMed] [Google Scholar]
- 89.Daniels MA, Jameson SC. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J Exp Med. 2000;191:335–46. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cho BK, Lian KC, Lee P, Brunmark A, McKinley C, Chen J, Kranz DM, Eisen HN. Differences in antigen recognition and cytolytic activity of CD8(+) and CD8(−) T cells that express the same antigen-specific receptor. Proc Natl Acad Sci USA. 2001;98:1723–7. doi: 10.1073/pnas.98.4.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuball J, Schmitz FW, Voss RH, et al. Cooperation of human tumor-reactive CD4+ and CD8+ T cells after redirection of their specificity by a high-affinity p53A2.1-specific TCR. Immunity. 2005;22:117–29. doi: 10.1016/j.immuni.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 92.Mareeva T, Lebedeva T, Anikeeva N, Manser T, Sykulev Y. Antibody specific for the peptide major histocompatibility complex. Is it T cell receptor-like? J Biol Chem. 2004;279:44243–9. doi: 10.1074/jbc.M407021200. [DOI] [PubMed] [Google Scholar]
- 93.Yachi PP, Ampudia J, Gascoigne NR, Zal T. Nonstimulatory peptides contribute to antigen-induced CD8-T cell receptor interaction at the immunological synapse. Nat Immunol. 2005;6:785–92. doi: 10.1038/ni1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clements CS, Dunstone MA, Macdonald WA, McCluskey J, Rossjohn J. Specificity on a knife-edge: the alphabeta T cell receptor. Curr Opin Struct Biol. 2006;16:787–95. doi: 10.1016/j.sbi.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 95.Manning TC, Kranz DM. Binding energetics of T-cell receptors: correlation with immunological consequences. Immunol Today. 1999;20:417–22. doi: 10.1016/s0167-5699(99)01508-x. [DOI] [PubMed] [Google Scholar]
- 96.Donermeyer DL, Weber KS, Kranz DM, Allen PM. The study of high-affinity TCRs reveals duality in T cell recognition of antigen: specificity and degeneracy. J Immunol. 2006;177:6911–9. doi: 10.4049/jimmunol.177.10.6911. [DOI] [PubMed] [Google Scholar]
- 97.Malherbe L, Mark L, Fazilleau N, McHeyzer-Williams LJ, McHeyzer-Williams MG. Vaccine adjuvants alter TCR-based selection thresholds. Immunity. 2008;28:698–709. doi: 10.1016/j.immuni.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Benoist C, Mathis D. Autoimmunity provoked by infection: how good is the case for T cell epitope mimicry? Nat Immunol. 2001;2:797–801. doi: 10.1038/ni0901-797. [DOI] [PubMed] [Google Scholar]
- 99.Kieper WC, Burghardt JT, Surh CD. A role for TCR affinity in regulating naive T cell homeostasis. J Immunol. 2004;172:40–4. doi: 10.4049/jimmunol.172.1.40. [DOI] [PubMed] [Google Scholar]
- 100.Sercarz EE, Maverakis E. Recognition and function in a degenerate immune system. Mol Immunol. 2004;15:1003–8. doi: 10.1016/j.molimm.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 101.Wucherpfennig KW. T cell receptor crossreactivity as a general property of T cell recognition. Mol Immunol. 2004;15:1009–17. doi: 10.1016/j.molimm.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 102.Wilson DB, Wilson DH, Schroder K, Pinilla C, Blondelle S, Houghten RA, Garcia KC. Specificity and degeneracy of T cells. Mol Immunol. 2004;15:1047–55. doi: 10.1016/j.molimm.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 103.Colf LA, Bankovich AJ, Hanick NA, Bowerman NA, Jones LL, Kranz DM, Garcia KC. How a single T cell receptor recognizes both self and foreign MHC. Cell. 2007;129:135–46. doi: 10.1016/j.cell.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 104.James LC, Roversi P, Tawfik DS. Antibody multispecificity mediated by conformational diversity. Science. 2003;299:1362–7. doi: 10.1126/science.1079731. [DOI] [PubMed] [Google Scholar]
- 105.Gakamsky DM, Luescher IF, Pecht I. T cell receptor–ligand interactions: a conformational preequilibrium or an induced fit. Proc Natl Acad Sci USA. 2004;101:9063–6. doi: 10.1073/pnas.0402840101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Holler PD, Kranz DM. T cell receptors: affinities, cross-reactivities, and a conformer model. Mol Immunol. 2004;15:1027–31. doi: 10.1016/j.molimm.2003.11.013. [DOI] [PubMed] [Google Scholar]