Abstract
Interleukin-15 (IL-15) is a proinflammatory cytokine that is overexpressed in rheumatoid arthritis (RA), a disease characterized by activation of monocytes/macrophages (MΦ), and by expansion of autoreactive CD4+ T cells. We hypothesized that IL-15 plays a major role for this expansion of CD4+ T cells and modulates the phenotype of monocytes/MΦ and their interaction with CD4+ T cells. Here, we show that IL-15 enhances the proliferation of CD4+ T cells from patients with RA in peripheral blood mononuclear cell cocultures. To further dissect the underlying mechanisms, we employed MΦ from IL-15−/− or IL-15 transgenic mice. These were induced to differentiate or were stimulated with IL-15. Here we show that addition of IL-15 during differentiation of MΦ (into ‘IL-15MΦ’) and overexpression of IL-15 by MΦ from IL-15tg mice leads to increased levels of major histocompatibility complex class II expression. This resulted in enhanced stimulation of antigen-specific CD4+ T cells in vitro and was accompanied by reduced messenger RNA expression in MΦ for immunosuppressive SOCS3. The proliferation rates of IL-15MΦ and IL-15tgMΦ were high, which was reflected by increased p27Kip1 and reduced p21Waf1 levels. In view of high serum and synovial levels of IL-15 in patients with RA, our data suggest the possibility that this excess IL-15 in RA may stimulate monocytes/MΦ to activate the characteristic autoreactive CD4+ T cells in RA.
Keywords: cytokines, macrophages, rheumatoid arthritis, T cells
Introduction
Interleukin-15 (IL-15) is expressed in a wide variety of cell types, including fibroblasts, keratinocytes, epithelial cells, osteoclasts and activated monocytes. It is also produced by haematopoietic progenitors and bone marrow stromal cells1 and by differentiated antigen-presenting cells and phagocytes, such as dendritic cells (DCs) and macrophages (MΦ).2 We have reported that interaction between IL-15 and the IL-15 receptor α (IL-15Rα) in DCs is critical for maturation and antigen presentation to T cells.3,4 Studies with IL-15Rα−/− and IL-15−/− mice show that IL-15 is an essential cytokine for the development and survival of natural killer (NK) cells, NK T cells, and memory and activated CD8+ T cells. Interleukin-15 is a potent proinflammatory cytokine that induces, for example, tumour necrosis factor-α (TNF-α), IL-1β and inflammatory chemokines. It inhibits self-tolerance and facilitates the maintenance of memory T-cell survival, including that of self-directed cells. Deregulated IL-15 expression has been reported in patients with an array of inflammatory autoimmune diseases.5
However, little is known about the role played by IL-15 in the development, functional maturation and terminal differentiation of MΦ. Previous studies have shown that IL-15 is up-regulated in MΦ in response to different innate stimuli like lipopolysaccharide (LPS) and TNF-α2,6 and acts as a potent autocrine regulator of proinflammatory cytokine production (e.g. of TNF-α and IL-6).2,7 This positive cytokine feedback loop may be a key event in diseases where IL-15 overexpression and release are prominent.
The best-characterized disease in which IL-15 is of substantial clinical importance is rheumatoid arthritis (RA). Here, local IL-15 expression is enhanced in both the joint tissue and the synovial fluid. In addition, IL-15 serum levels are elevated in patients with RA, and this increased concentration correlates with disease severity.8,9 Blocking IL-15 activity using soluble high-affinity IL-15Rα protein prevents collagen-induced arthritis in a mouse model.10 Nevertheless, the mechanisms by which IL-15 influences disease progression and/or severity in RA are currently ill-understood.
The progression of RA is speculated to be accompanied by recruitment and expansion of oligoclonal autoreactive CD4+ CD28− T cells. These cells would promote not only joint destruction, but also enhanced atherosclerosis in patients with RA.11,12 However, this is still a controversially discussed subject. On this background, we hypothesized, that IL-15 may be responsible for the differentiation and activation of monocytes/MΦ into efficient antigen-presenting cells and for the proliferation maintenance of autoreactive CD4+ T cells in the local joint tissue and/or in peripheral blood.
To verify this hypothesis, we stimulated human peripheral blood mononuclear cell (PBMC) cultures with IL-15 and analysed its effect on CD4+ T-cell proliferation. We investigated how long- and short-term exposure to IL-15 influences the differentiation, function and activation of bone-marrow-derived murine MΦin vitro. To this end we generated MΦ in the presence of macrophage colony-stimulating factor (M-CSF) alone or with IL-15 plus M-CSF. In parallel, MΦ were also generated from IL-15-deficient and IL-15-overexpressing mice and their phenotype, cytokine expression and antigen-presenting activity were analysed in vitro. Furthermore, the phenotype of MΦ isolated directly from wild-type, IL-15 transgenic and IL-15−/− mice was studied.
Here, we present new evidence that IL-15 is a major modulator of MΦ maturation and function and promotes MΦ activation of autoreactive CD4+ T cells in RA.
Materials and methods
Study population and PBMC analysis
Peripheral blood mononuclear cells from patients fulfilling the American College of Rheumatology criteria for Rheumatoid Arthritis13 and five healthy donors were analysed. This study was carried out according to the 1997 Declaration of Helsinki of the World Medical Association. The design of the work has been approved by the ethics committee of the University of Schleswig-Holstein, Campus Lübeck, and each patient gave informed consent before participation in the study.
Patient characteristics
Three male and three female patients were included in the study. Their mean age was 62 ± 8 years and they had suffered from erosive RA for a mean time of 12 years. Five of them were positive for rheumatoid factor. At the time of analysis five of them showed clinically active disease represented by a mean DAS 28 (disease activity score14) of 4·8 ± 2·2, and as serological markers of inflammation, C-reactive protein was elevated to 34 ± 16 mg/l and erythrocyte sedimentation rate was 26 ± 25 mm/hr. The PBMCs were separated from peripheral blood by Ficoll–Hypaque density gradient centrifugation.
Carboxy-fluorescein diacetate succinimidyl ester (CFSE) staining of PBMCs was performed using a Vybrant™ CFDA SE Cell Tracer Kit (Invitrogen, Karlsruhe, Germany). Briefly 10 × 106 PBMCs were resuspended with 0·5 ml RPMI-1640 (37°). In parallel, 10 μm CFSE–RPMI solution was prepared (37°); 0·5 ml CFSE–RPMI solution was added to 0·5 ml PBMC suspension, resuspended and incubated for 5 min at 37°. Then an equal volume of fetal calf serum (1 ml, 37°) was added and the cells were washed. Afterwards, two washing steps using RPMI-1640 containing fetal calf serum were performed. Stained PBMCs were stimulated for 7 days with 1 ng/ml anti-CD3 monoclonal antibody (clone X35; Beckman-Coulter, Krefeld, Germany) alone or combined with 10 ng/ml IL-15 (Strathmann, Hamburg, Germany) in culture medium (RPMI-1640 supplemented with 2 mmol/l l-glutamine, 100 U/ml penicillin G, 100 μg/ml streptomycin and 10% heat-inactivated fetal calf serum) at 37° and 5% CO2. At day 7, cells were washed and counterstained with phycoerythrin-conjugated anti-CD4 monoclonal antibody (clone MT310; Dako, Hamburg, Germany). Cell division status was then determined by flow cytometry using fluorescence-acitvated cell sorting (FACS) Calibur (BD Biosciences, Heidelberg, Germany).
Mice, culture medium and antibodies
Bone marrow (BM) was taken from C57BL/6 wild-type and IL-15-deficient or transgenic mice on a C57BL/6 background. In addition, we used (also on a BL/6 background) OTIItg mice, transgenic for a CD4+ T-cell restricted T-cell receptor, recognizing the ovalbumin 323–339 (OVA323–339) peptide. All mice were bred at the Research Center Borstel, maintained in specific pathogen-free conditions and were used between 8 and 10 weeks of age.
Murine cells were cultured in RPMI-1640 medium supplemented with 10% (v/v) heat-inactivated fetal calf serum (Biochrom, Berlin, Germany), 2 mm l-glutamine (Invitrogen), 50 μmβ-mercaptoethanol, 100 U/ml penicillin and 100 μg/ml streptomycin (PAA, Pasching, Austria), at 37° in 5% CO2.
MΦ preparation
Murine BM cells from femurs were freshly isolated and red blood cells were lysed by osmotic shock. Washed cells were seeded (1 × 106/ml) in bacterial grade Petri dishes (BD Falcon, Heidelberg, Germany) and induced to differentiate for 8 days in 10 ml medium plus 20 ng/ml M-CSF (Tebu, Offenbach, Germany) either alone or plus 5 ng/ml IL-15 (Tebu). After 3 days, 10 ml fresh medium with M-CSF or M-CSF plus IL-15 was added. At day 8, cells were harvested using Accutase (PAA) and macrophages represented > 98% of the total cells according to cell surface expression of F4/80, CD11c, CD3, CD45B220 and NK1.1.
Reverse transcription–polymerase chain reaction (RT-PCR)
Total RNA was extracted after an additional 24 hr stimulation with medium, IL-15 (100 ng/ml; Tebu) or LPS (100 ng/ml) using TriZol (Gibco, Karlsruhe, Germany) following the manufacturer's instructions. Complementary DNA (cDNA) was synthesized using random hexanucleotide primers and the Superscript preamplification system II (Invitrogen) and it was amplified using 1U AmpliTaq DNA polymerase (Roche, Mannheim, Germany). The final concentration of each primer was 0·5 μm. Cycling conditions were 5 min at 94°, 30 seconds at 60°, 30 seconds at 72° for 30 cycles and ended with 10 min at 72°. Primers were from Metabion (Planegg-Martinsried, Germany). The primer sequences were as follows: IL-15Rα sense: 5′-AACATCCACCCTGATTGAGTGT-3′, antisense: 5′-GTTTCCATGGTTTCCACCTCAA-3′; IL-6 sense: 5′-CAGAAAGCCAGAGTCCTTCAGAGAG-3′, antisense: 5′-CTAGGTTTGCCGACTAGATCTC-3′; IL-12 p40 sense: 5′-ATCGTTTTGCTGGTGTCTCC-3′, antisense: 5′-CTTTGTGGCAGGTGTACTGG-3′; SOCS 3 sense: 5′-TGCAAGGGGAATCTTCAAAC-3′, antisense: 5′-AGCTCACCAGCCTCATCTGT-3′; p27Kip1 sense: 5′-AATCTCATCACCCCACTTGC-3′, antisense: 5′-GGCCATTTTCCATCTCTGAA-3′; p21Waf1 sense: 5′-GGGATGGCAGTTAGGACTCA-3′; antisense: 5′-ACCCTAGACCCACAATGCAG-3′; β-actin sense: 5′-GTGGGGCGCCCCAGGCACCA-3′, antisense: 5′-CTCCTTAATGTCACGCACGATTTC-3′. To exclude contamination, all experiments were run with a mock PCR. β-actin was used to normalize the cDNA amount.
FACS analysis
MΦ were characterized using a panel of monoclonal antibodies including anti-F4/80 [fluorescein isothiocyanate (FITC)-labelled; Serotec, Martinsried, Germany) and major histocompatibility complex (MHC) class II (IA/IE,), CD80 and CD86 (Pharmingen, Heidelberg, Germany). Propidium iodide (Sigma, Taufkirchen, Germany) was added to exclude dead cells. For cell cycle analysis, cells were fixed in 70% ethanol for 24 hr, resuspended in 1 ml propidium iodide (50 μg/ml) and RNAse A (100 U/ml), stained for 1 hr and analysed by FACS staining. The diploid, dividing cells in G2/M phase are given. The FACS was performed using a FACSCalibur and cellquest software (BD Biosciences).
Analysis of endocytosis by flow cytometry
To quantify the endocytic activity, FITC–Dextran uptake (Molecular Probes, Karlsruhe, Germany) was monitored by FACS as described by Stumbles et al.15 In brief, 5 × 105 MΦ were resuspended in 100 μl RPMI-1640 containing 0·5 mg/ml FITC–Dextran (molecular weight 70 000) for 30 min at 37° or on ice. Cells were washed twice with ice-cold phosphate-buffered saline with bovine serum albumin and fluorescence intensity was determined by FACS.
Analysis of protease activity
This assay was performed using an EnzCheck Protease assay kit (Molecular Probes). Results are presented as fluorescence units.
LPS/IL-15 stimulation
The MΦ were generated as described above and were cultured for 24 hr with LPS or IL-15 (100 ng/ml) to induce MΦ activation. Cells were harvested for PCR analysis and supernatants were harvested for cytokine detection using a Bioplex kit (Bio-Rad, München, Germany).
Proliferation assays
T cells were isolated from lymph nodes of OTII T-cell receptor transgenic mice; aliquots of 1 × 105 MΦ were placed in 96-well flat-bottom culture plates (Costar Corning, Shiphol-Rijk, the Netherlands) and allowed to adhere for 2 hr. CD4+ T cells were added at 2 × 105 cells per well and OVA323–339 peptide was supplemented to a final volume of 200 μl. In some experiments, IL-15 was added to the MΦ during the initial 2 hr and then washed away. The cultures were incubated for 72 hr and labelled for an additional 18 hr with 0·2 μCi/well [3H]thymidine (Amersham, Midland, ON, Canada). Thymidine uptake was analysed using liquid scintillation counting (Perkin Elmer, Waltham, MA). The experiments were repeated three times with six replicates each. To analyse the proliferative response of MΦ alone, they were harvested at day 8, washed, seeded in fresh medium containing 20 ng/ml M-CSF alone or plus 20 ng/ml IL-15 in 96-well flat-bottom plates (50 000 cells/well; Nunc, Roskilde, Denmark) and incubated for 4 days. During the last 18 hr, plates were pulsed with 0·2 μCi/well [3H] thymidine and thymidine uptake was analysed by liquid scintillation counting.
Statistical analysis
Results are presented as mean ± SD from pooled data of two to five identical experiments. The FACS and PCR data from one representative experiment are shown. Student's t-test for unpaired samples was used for the determination of statistical differences (*P ≤ 0·05; **P ≤ 0·01).
Results
IL-15 supports CD4+ T-cell proliferation in PBMC cocultures from patients with RA
Although the identity of the (auto-) antigen remains to be revealed, it is hypothesized that RA is characterized by the expansion of autoreactive CD4+ T cells in an antigen-specific manner. To analyse whether IL-15 can promote the proliferation of T-cell receptor-activated human CD4+ T cells, we labelled these cells isolated from patients with RA with CFSE and stimulated them with anti-CD3 antibodies (α-CD3) to mimic T-cell receptor activation alone or together with IL-15. The T cells were cultured in the presence of monocytes for 7 days, and CD4+ proliferation was monitored.
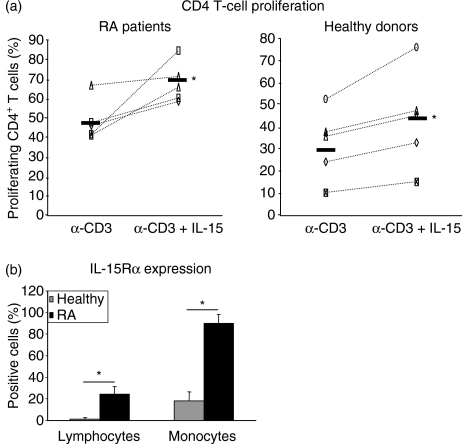
Our data show that in general CD4+ T cells from patients with RA display a higher proliferative response to α-CD3 than T cells from healthy donors. This is further significantly enhanced by the addition of IL-15, mimicking the situation in patients with RA, who are characterized by both high IL-15 expression and high proliferating CD4+ T cells.16 In addition, we analysed the expression of the high-affinity IL-15Rα on monocytes and lymphocytes ex vivo by FACS and, compared to healthy donors, found a highly significant overexpression of this receptor chain on both cell types in patients with RA (Fig. 1b).
Figure 1.
Interleukin-15 (IL-15) promotes CD4+ T-cell proliferation from patients with rheumatoid arthritis (RA) and healthy donors. (a) Peripheral blood mononuclear cell (PBMC) cocultures were stimulated for 7 days with α-CD3 monoclonal antibody alone or in combination with IL-15 and proliferation of CD4+ T cells was analysed by distribution of carboxy-fluorescein diacetate succinimidyl ester. Percentage of proliferating CD4+ T cells is given. Differences between healthy donors and patients with RA are statistically significant (P ≤ 0·05). (b) Expression of IL-15 receptor α (IL-15Rα) on lymphocytes and monocytes from healthy donors and patients with RA was measured by fluorescence-activated cell sorting. Statistical significance was calculated using Student's t-test, *P ≤ 0·05).
F4/80+ macrophages differentiate in the presence of IL-15 in vitro
The results presented in Fig. 1 indicate an important role for IL-15 in CD4+ T-cell expansion, induced in vivo by antigen-presenting cells expressing MHC class II, such as monocytes. To better dissect the relevant steps of MΦ biology we next turned to the murine system, where monocytes/MΦ can be generated from myeloid progenitors under standard conditions in vitro and genetically modified MΦ from IL-15-deficient or transgenic mice can be studied. Interleukin-15 has been shown to promote joint destruction in murine models of RA.10 The trimeric IL-15 receptor is expressed in haematopoietic stem cells. Since IL-15Rβ-chain stimulation leads to enhanced differentiation of the monocytic lineage,17 we were interested to determine whether IL-15, in combination with M-CSF, modulates MΦ generation from BM myeloid progenitor cells in vitro. Therefore, murine BM cells were cultured in the presence of M-CSF plus 5 ng/ml IL-15 or with M-CSF alone.
After 8 days, both the M-CSF-differentiated MΦ and those MΦ exposed to IL-15 for 8 days plus M-CSF (designated in the following text as ‘IL-15MΦ’) showed comparable size as well as granularity, and high purity, assessed by a similar percentage of F4/80+ cells. Addition of IL-15 to BM cells in combination with M-CSF did not lead to differentiation of other cell types like B, NK, Tcells, DCs or granulocytes (as assessed by FACS analysis of CD45B220, NK1.1, CD3, CD11c or GR1, which were all negative on the generated cells; data not shown). Interleukin-15 alone (without M-CSF) induced no differentiation of myeloid MΦ (not shown). Similar effects of IL-15 on the modulation of myeloid cell differentiation were observed when granulocyte–macrophage CSF – instead of M-CSF – was used to differentiate DCs, having been used as a control in one of our previous publications.18 Therefore, IL-15 neither altered the purity of the generated macrophages nor their total yield and did not shift the M-CSF-induced haematopoietic stem-cell differentiation towards the NK/lymphoid lineage.
IL-15 enhances MHC class II expression on murine MΦ
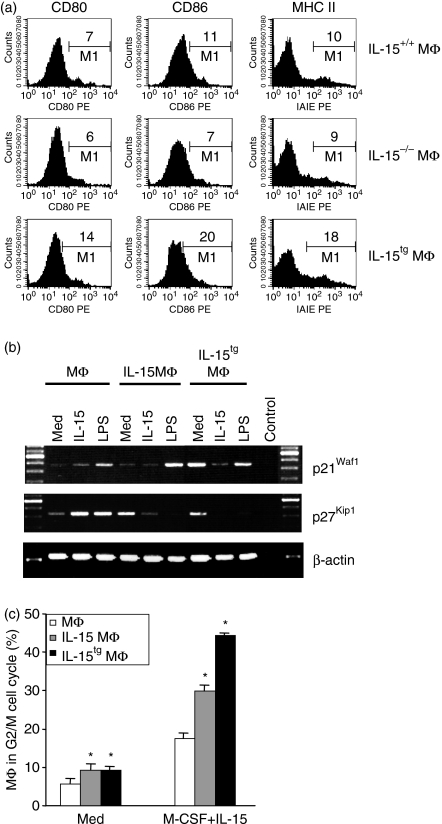
A unique feature of RA is that high concentrations of IL-15 are detected in the serum and synovial fluid of affected patients. Monocytes in the circulation and tissue MΦ in synovial cavities of patients with RA are therefore continuously exposed to IL-15 and, on the other hand, come into close contact with (autoreactive) CD4+ T cells, a characteristic hallmark of RA.12 It is currently not clear how these autoreactive CD4+ T cells are continuously activated and expand. Antigen presentation to CD4+ T cells is mediated by MHC class II molecules and is modulated by a variety of costimulatory molecules on the MΦ surface. To assess the long-term effects of IL-15 on these molecules, we examined their expression in murine MΦ and IL-15MΦ after 8 days culture by FACS. Interestingly, IL-15MΦ showed a similar morphology to MΦ, but expressed significantly higher levels of CD86 and MHC class II (Fig. 2b). In contrast, CD80, CD40 and CD11b showed a similar expression in MΦ and IL-15MΦ (Fig. 2b and data not shown). As expected, short-term treatment of MΦ for just 2 hr with IL-15 did not significantly enhance the expression of MHC class II (not shown). These data suggest that IL-15 significantly changes the MΦ phenotype only if present for a long-time during the MΦ differentiation.
Figure 2.
Murine interleukin-15-stimulated macrophages (IL-15MΦ) display an increased major histocompatibility complex (MHC) class II expression and CD4+ T-cell stimulatory activity. (a) MΦ were differentiated from murine bone marrow cells with macrophage colony-stimulating factor (M-SCF) alone or M-CSF plus IL-15 (IL-15MΦ) and analysed by fluorescence-activated cell sorting (FACS) at day 8. They show comparable size (forward scatter) and granularity (side scatter) as well as F4/80 expression. (b) CD86 and MHC class II expression was significantly enhanced in IL-15MΦ (mean fluorescence intensity is given on the graphs). (c) MΦ express the high-affinity IL-15 receptor α after 24 hr of IL-15 and lipopolysaccharide stimulation. (d) Antigen-specific proliferation assays were performed using MΦ and IL-15MΦ as antigen-presenting cells and T-cell receptor transgenic CD4+ cells from OTII mice. (e) Short-term incubation of MΦ (for 2 hr) with IL-15 did not significantly enhance their ability to stimulate CD4+ T cells. (f) In-vitro-generated IL-15−/− MΦ were equally potent in inducing CD4+ T-cell expansion as IL-15+/+ MΦ and short-term MΦ IL-15 stimulation did not increase their proliferation. Representative experiments are shown (mean ± SD) of three to six replicates; c.p.m., counts per minute; NS, not significant; **P ≤ 0·01 compared to IL-15+/+ MΦ or MΦ without addition of IL-15.
After activation, murine MΦ express the high-affinity IL-15 receptor
As a prerequisite for IL-15 stimulation and high-affinity cytokine binding, MΦ need to express the IL-15Rα. Therefore, MΦ and IL-15MΦ were stimulated with IL-15 and LPS. This induced message expression for different isoforms of the IL-15Rα in contrast to vehicle-stimulated MΦ (Fig. 2c), suggesting that IL-15-preactivated MΦ may respond to subsequent IL-15 stimulation even at very low ligand concentrations.
IL-15MΦ are highly potent stimulators of antigen-specific CD4+ T cells
The MΦ contribute to immunosurveillance as they continuously capture and process antigens in the periphery and present them to T cells. To analyse the effect of IL-15 on MΦ–T-cell interactions, and to specifically dissect the antigen-presenting and immunostimulatory capacities of IL-15MΦ, with enhanced MHC class II expression (see Fig. 2b), we used a coculture assay with T-cell receptor transgenic CD4+ T cells as responder18 to obtain data comparable to those obtained for human CD4+ T cells shown in Fig. 1. MΦ were incubated with a specific peptide derived from OVA and were cultured with T cells from OTII mice in vitro. These T cells express a CD4+ T-cell restricted, transgenic T-cell receptor, which recognizes the OVA323–339 peptide bound to MHC class II. The stimulatory capacity of the MΦ peaks at 0·1 μm by inducing T-cell proliferation, corresponding to thymidine incorporation of about 20 000 counts/min. As shown in Fig. 2(d), IL-15MΦ induced a highly significant (i.e. almost 10-fold) enhanced proliferation of antigen-specific CD4+ T cells compared to the proliferation induced by MΦ that had been generated in the presence of M-CSF alone. To analyse whether short-term stimulation with IL-15 affects MΦ antigen presentation to CD4+ T cells, we used M-CSF-generated MΦ and incubated them for only 2 hr with IL-15. The IL-15 was taken up within 30 min by APCs. After washing the IL-15 away, OVA323–339 peptide was added and the MΦ were cocultured with the transgenic CD4+ T cells. Unlike long-term exposure to IL-15, this short-term IL-15 stimulation had no significant effect on MΦ-induced CD4+ T-cell proliferation (Fig. 2e). This correlated with the observation that costimulatory and MHC class II molecules were not up-regulated after 2 hr of stimulation either, as analysed by FACS (data not shown).
These findings suggest that IL-15, if present during MΦ differentiation, facilitates ‘acquired’ immune responses such as MΦ-induced CD4+ T-cell expansion.
To confirm that only the long-term (over-) exposure to IL-15, and not normal, physiological IL-15 levels, is responsible for the observed effects on CD4+ T-cell proliferation, we next used MΦ generated from IL-15-deficient mice, which (unlike normal MΦ) do not produce IL-15. To assess the possible functional consequences of IL-15 deficiency on the induction of T-cell proliferation, the in-vitro-generated MΦ and IL-15-deficient murine MΦ were studied in the CD4+ T-cell proliferation assays. These showed that CD4+ T cells are induced to proliferate independently from the constitutive IL-15 expression by MΦ (Fig. 2f), underscoring that the long-term effect of IL-15 on MΦ, but not MΦ-derived IL-15, is important for T-cell activation. Instead, it is the chronic exposure to IL-15 that turns MΦ into highly efficient antigen-presenting and T-cell-activating cells, so serving as a potential positive feedback loop for CD4+ T-cell activation.
IL-15 is not essential for MΦ differentiation in vitro
Interleukin-15 is an essential factor in lymphopoiesis; while its role in the differentiation of myeloid MΦ has not yet been clarified. To address this, BM cells from wild-type or IL-15-deficient mice were employed and differentiated in the presence of M-CSF for 8 days. As shown in Fig. 3(a), normal, F4/80+ MΦ can be generated in vitro from IL-15-deficient BM cells with similar expression of CD80, CD86 and MHC class II as wild-type controls, demonstrating that IL-15 is dispensable for MΦ differentiation.
Figure 3.
Interleukin-15 (IL-15) is not essential for macrophage (MΦ) differentiation in vitro but modulates cell-cycle regulators and MΦ proliferation. (a) Wild-type (IL-15+/+) and IL-15−/− bone marrow cells equally differentiate by macrophage-colony-stimulating factor (M-CSF) stimulation into F4/80+ MΦ with comparable expression of costimulatory molecules in vitro. The IL-15tgMΦ express about twofold higher costimulatory and MHC class II molecules. Cells were analysed after 8 days of in vitro differentiation. (b) Reverse transcription–polymerase chain reaction of unstimulated or stimulated MΦ, IL-15MΦ or IL-15tgMΦ for detection of the cyclin-dependent kinase inhibitor p21Waf1 and p27Kip1 after 24 hr stimulation. (c) MΦ were stimulated for another 4 days with medium or with M-CSF in combination with IL-15, and the cell cycle analysis was performed using propidium iodide staining. The dividing cells in the G2/M phase are shown. All experiments were repeated two to five times.
Constitutive IL-15 overexpression mimics the effects of long-term IL-15 stimulation on MΦ
To corroborate the data shown above, namely that IL-15 (if added during the entire period of MΦ differentiation) affects the phenotype of the IL-15MΦ generated in this manner, BM from IL-15 transgenic mice, which overexpress and secrete large amounts of IL-15, were employed. After stimulating these BM cells for 8 days with M-CSF, FACS analysis revealed the differentiation of normal numbers of F4/80+ MΦ. However, they showed an approximately twofold higher expression of costimulatory molecules (CD80, CD86) as well as MHC class II molecules (Fig. 3a). This correlates well with the effects seen after the addition of IL-15 to IL-15MΦ for the entire period of MΦ generation (Fig. 2b). The data support the concept that high levels of IL-15 during differentiation transform MΦ into highly efficient antigen-presenting, T-cell-activating cells.
IL-15 regulates expression of the cell-cycle regulators p21Waf1 and p27Kip1
Cellular development, proliferation and differentiation are tightly controlled by cell-cycle promoting and inhibiting proteins, of which the cyclin-dependent kinases and their inhibitors are the best studied. Reasoning that the enhanced MΦ activity leading to improved CD4+ T-cell stimulation could be the result of an altered expression of cell-cycle regulators, we next analysed the message expression (which correlates with the protein expression19) of two critical cyclin-dependent kinase inhibitors. This revealed that p21Waf1, which protects MΦ from apoptosis,20 is expressed in MΦ and IL-15MΦ at comparable levels (Fig. 3b), and is induced by both IL-15 and LPS. This could in part explain why IL-15 and LPS could serve as strong anti-apoptotic stimuli for MΦ. Interestingly, IL-15tgMΦ already showed a very high baseline expression of p21Waf1, suggesting that overexpression of IL-15 directly up-regulates this anti-apoptotic factor.
Another very important regulator of the MΦ life cycle is p27Kip1, which is tightly regulated during MΦ terminal differentiation and proliferation.20 We found a high expression of p27Kip1 in unstimulated IL-15tgMΦ or long-term IL-15-exposed MΦ, while p27Kip1 was up-regulated in normal MΦ by IL-15 (and also by LPS) stimulation (Fig. 3b).
Suppressed terminal differentiation as the result of p27Kip1 expression should result in enhanced MΦ proliferation. To analyse this, we performed a proliferation assay, assessing the response of the MΦ, IL-15MΦ and IL-15tgMΦ to M-CSF. After harvesting the MΦ at day 8, they were stimulated for an additional 4 days with M-CSF + IL-15. As shown in Fig. 3(c), the IL-15MΦ and IL-15tgMΦ indeed proliferated significantly more than the MΦ if stimulated further with M-CSF + IL-15.
It might be speculated that the high potency of IL-15MΦ to induce CD4+ T-cell activation is directly correlated with the up-regulation of cell-cycle regulators, which leads to the suggestion that continuous exposure to IL-15 induces MΦ to enter an over-activated state, which results in chronic maintenance of CD4+ T-cell stimulation in patients with RA.
Long-term IL-15 exposure suppresses negative regulators of MΦ activation
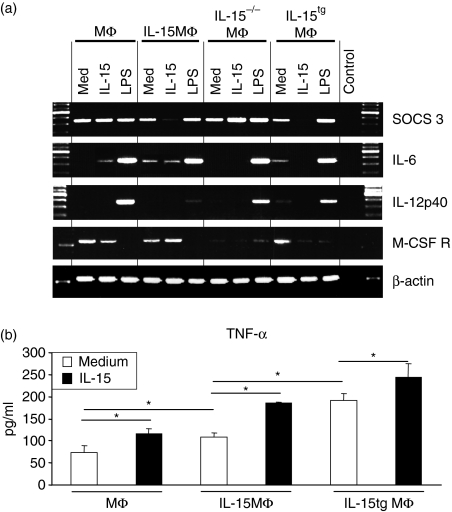
As a first step to further characterize the different activity of MΦ and the role played by IL-15, we analysed messenger RNA expression for molecules that are known to inhibit leucocyte activation or to modulate the induction of T-cell proliferation. One molecule that is important for the induction of tolerance and CD4+ regulatory T cells is the suppressor of cytokine signalling 3 (SOCS3),21 an antagonist of MΦ activation, which can be down-regulated by IL-15 in IL-15MΦ and in IL-15tgMΦ (Fig. 4a). These data show that the high activation state of IL-15MΦ and the potency of IL-15MΦ to induce CD4+ T-cell activation correlates with inhibited expression of the immunoinhibitory molecule SOCS3.
Figure 4.
Macrophage (MΦ) cytokine expression profile and secretion. (a) MΦ were stimulated for 24 hr with the indicated stimuli and reverse transcription–polymerase chain reaction was performed for the indicated suppressive/tolerogenic molecules and cytokines. (b) MΦ were stimulated for 24 hr, supernatants were collected and tumour necrosis factor-α was analysed by enzyme-linked immunosorbent assay.
MΦ and IL-15MΦ do not differ substantially in their cytokine expression profile after LPS stimulation
One key function of MΦ is to serve as key cellular protagonists of innate responses to infectious agents, such as bacterial products, which bind to pattern recognition receptors. Bacterial membrane LPS binds to Toll-like receptor (TLR) 2 and TLR4, which in turn activate cytokine production by MΦ.22 Therefore, we investigated whether the expression of MΦ cytokines is also regulated by IL-15. TLR2 and TLR4 messenger RNA expression was comparable in all the different MΦ populations examined (data not shown). The IL-6 transcription was induced by IL-15 and LPS, whereas IL-12 transcripts were only detectable after LPS stimulation (Fig. 4a). Interestingly, IL-15 stimulated MΦ to release TNF-α into the supernatants. This effect was much stronger when IL-15MΦ and IL-15tgMΦ were stimulated. Also, according to their activated state, these IL-15MΦ and IL-15tgMΦ produced significantly more TNF-α even at baseline medium stimulation compared to wild-type cells (Fig. 4b).
IL-15 does not modulate phagocytosis by and protease activity of MΦ
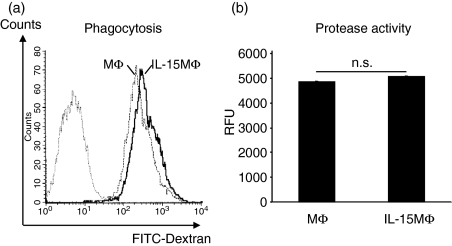
One additional key function of MΦ is the phagocytosis of antigens, pathogens and dying cells. To assess whether MΦ and IL-15MΦ differ in their phagocytic activity of antigens, FITC–Dextran was used as a model antigen and its uptake was measured by FACS. As shown in Fig. 5(a), FITC–Dextran is efficiently taken up by both MΦ and IL-15MΦ without apparent differences. Prolonged incubation did not increase the FITC–Dextran uptake. To control passive FITC-diffusion, all experiments were also performed on ice, which resulted in a very low, but still comparable, FITC–Dextran uptake. The internalization of FITC–Dextran was further confirmed by fluorescence microscopy (data not shown). In addition, phagocytosis of fluorescence-labelled (i.e. cycloheximide- or dexamethasone-treated) apoptotic murine thymocytes was efficiently carried out by the generated MΦ but again no differences were revealed between MΦ and IL-15MΦ (data not shown).
Figure 5.
Interleukin-15 (IL-15) did not alter macrophage (MΦ) phagocytosis and protease activity. (a) Both MΦ and IL-15MΦ display a similar phagocytosis of fluorescein isothiocyanate (FITC) –Dextran after 30 min incubation at 37°. (b) Both MΦ and IL-15MΦ show comparable unspecific protease activity after lipopolysaccharide (LPS) activation; RFU, relative fluorescence units.
The expression of proteases is essential for the migration of these cells and for the cleavage of phagocytosed material. When comparing the total protease activity (endo- and exonucleases as well as metallo-, serine-, acid and sulfhydryl proteases) within MΦ and IL-15MΦ, we found it at comparable levels (Fig. 5b). Summarizing the data presented above we conclude that because the cytokine expressions in response to LPS, phagocytosis and protease activity were widely comparable, IL-15-treatment during MΦ differentiation did not significantly affect ‘innate’ immune functions of the IL-15MΦ.
IL-15 overexpression in vivo results in highly activated MΦ in different mouse tissues
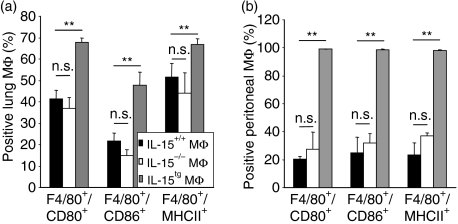
Our data demonstrate that overexpression of IL-15 leads to over-activated MΦ. To finally verify our in vitro findings on the in vivo level as well during murine MΦ differentiation, pulmonary MΦ were isolated from the lungs of wild-type, IL-15-deficient and IL-15tg mice. The absolute MΦ numbers were comparable (7·6 ± 2·7, 8·4 ± 1·6, and 8·1 ± 2·1 × 106 cells/lung, respectively n = 4) as were the expressions of the costimulatory molecules CD80/86 and of MHC class II on IL-15+/+ wild-type and IL-15−/− mice. However, MΦ isolated from IL-15tg mice showed a significant enhanced expression of costimulatory and antigen-presenting molecules (Fig. 6a). The same was seen with MΦ isolated from the peritoneal cavity, where IL-15tgMΦ also displayed a significantly elevated expression of surface markers of MΦ activation (Fig. 6b). Therefore, while IL-15 is not a prerequisite for the development of murine MΦin vitro, IL-15 serves as a potent MΦ activator, both in vitro and in vivo.
Figure 6.
Interleukin-15 (IL-15) is not essential for macrophage (MΦ) differentiation but strongly induces MΦ activation in vivo. MΦ were freshly isolated from wild-type IL-15+/+, IL-15−/− and IL-15tg lung (a) and peritoneal cavity (b). The expression of costimulatory molecules and major histocompatibility complex (MHC) class II was analysed by fluorescence-activated cell sorter staining on F4/80+ gated MΦ; (n = 4 mice/group). Significance was calculated using Student's t-test.
Discussion
Our findings help to clarify the mechanisms by which IL-15 stimulates MΦ and their interaction with CD4+ T cells. This helps us to understand in more detail how IL-15 may contribute to the pathogenesis of RA: IL-15 stimulates CD4+ T-cell proliferation induced by monocytes and so can contribute to the activation or expansion of autoreactive T cells. Our available data from human patients with RA are corroborated by a variety of experiments in the murine system, where more elaborate tools, such as IL-15-deficient and transgenic mice and T-cell receptor transgenic, antigen-specific T cells, can be used to investigate IL-15 activities. The effects of IL-15 on murine MΦ have been studied in the past, but these investigations were mainly focused on short-term effects after cytokine stimulation. In contrast, here we have compared long-term effects of IL-15, from the initiation of MΦ differentiation from hematopoietic stem cells until the final MΦ maturation (by adding IL-15 to M-CSF during in vitro MΦ generation). The IL-15MΦ were characterized by high MHC class II expression in vitro. This was confirmed in vivo by analysing MΦ from IL-15tg mice. Functionally, this resulted in an almost 10-fold enhanced stimulation of antigen-specific CD4+ T cells in vitro. This might be, at least in part, attributed to the much higher expression of costimulatory molecules, and in particular of MHC class II molecules, on IL-15MΦ. In our view, these data underline the magnitude of the activating, ‘proimmunogenic’ effects of IL-15 if present during macrophage differentiation.
We have demonstrated a mitogenic effect of IL-15 on both human and murine monocyte/MΦ-induced CD4+ T-cell proliferation. Analysing the IL-15Rα protein expression on human monocytes and T cells revealed that both cell types showed a significantly higher IL-15Rα protein expression in patients with RA compared to healthy controls. Evidently, these differences in the level of IL-15Rα expression do not necessarily prove differences in the level of response. However, there are numerous examples in the IL-15 literature that clearly document a close positive correlation of the level of the high-affinity IL-15 receptor (i.e. IL-15Rα) with the level of response-to-stimulation by its cognate ligand. If this mechanism really reflects how IL-15 contributes to the T-cell-mediated pathology in RA, it could also explain why a therapy aimed to block IL-15 might ameliorate the disease activity in patients with RA. The importance of IL-15 for CD4+ T cells in RA is supported by findings where blocking IL-15 inhibits the interaction of CD4+ T cells with B cells and therefore the anti-collagen antibody production in a mouse model.10 Furthermore, we have demonstrated IL-15-mediated CD4+ T-cell–B-cell interaction in a model of allergic sensitization; injection of IL-15 in vivo massively accelerated and enhanced antibody production in vivo up to 50-fold.23 The MΦ itself are a source as well as a target of IL-157 and MΦ express receptors for IL-15. Many findings from cell lines and murine MΦ have been confirmed in human MΦ,24 suggesting comparable regulatory mechanisms in humans and mice; this would encourage the extrapolation of the findings in murine MΦ presented here to the human system. The enhanced induction of CD4+ proliferation by IL-15 (Fig. 1) supports our findings in the murine system and underlines their clinical relevance.
A recent report has identified an IL-15-mediated cross-talk between synovial fibroblasts and T cells in patients with RA, and has demonstrated a high expression of IL-15 by these fibroblasts as well as contact-dependent activation of T cells, without specificity for CD4+ or CD8+ T cells.25 This might be explained by the missing expression of costimulatory and MHC class II molecules, hence T cells are not activated in an antigen-specific manner but unspecifically by surface-bound IL-15 on fibroblasts. Surface-bound IL-15 on monocytes can indeed mediate juxtacrine T-cell activation.26 As we could show for DCs, this probably depends on the IL-15–IL-15Rα interaction.4 The assumed specificity of IL-15 for CD8+ T cells (as seen in many models and in the initial reports about IL-15 and IL-15Rα knockout mice27,28) has recently been demonstrated to be a dose-dependent effect, because high IL-15 doses also potently activate CD4+ T cells.29 However, besides a direct effect of IL-15 on CD4+ T cells, we propose a new aspect of this cytokine by activating monocytes to induce and/or support CD4+ T-cell proliferation. Since IL-15 trans-presentation has already been demonstrated to be effective for CD8+ T cells, NK cells and germinal B cells, but to our knowledge not for CD4+ T cells, it deserves careful future exploration, especially in the context of RA. However, mice deficient for the IL-15 cytokine and the IL-15Rα chain do not display any defect in the CD4+ T-cell compartment, suggesting that IL-15 trans-presentation is not required for the homeostatic control of CD4 survival/proliferation. Therefore, the effects reported here are very likely to be secondary to the IL-15 exposure of MΦ. The high IL-15 concentration in the synovial tissue of patients with RA could shift MΦ towards highly efficient antigen-presenting cells.
Here for the first time we report that SOCS3 is down-regulated by IL-15 exposure in IL-15MΦ and IL-15tgMΦ. The increased expression of IL-6 (which up-regulates SOCS3), along with SOCS3 suppression, is not necessarily mutually contradictory because SOCS3 is down-regulated by different cytokines (including IL-10, IL-21 and IL-15 itself), which might easily have overcome the effect of IL-6. However, whether this SOCS suppression is a direct effect or secondary to the production of other cytokines known to regulate SOCS expression still needs to be defined.
The finding that the cyclin-dependent kinase inhibitor p27Kip1 is down-regulated in IL-15MΦ and IL-15tgMΦ but up-regulated in normal MΦ suggests a block in the end-stage differentiation of these MΦ, where p27Kip1 has been shown to be important by inducing a cell-cycle arrest.19,30 Rapid down-regulation of p27Kip1 is observed after stimulation with growth factors, when cells re-enter the cell cycle.31 This suggests that IL-15MΦ and IL-15tgMΦ are highly proliferating cells, which in addition are protected from apoptosis by IL-15.32 The expression of p21Waf1, (which is induced by IL-15 in our hands, or IL-6 or interferon-γ20,33) also promotes the inhibition of apoptosis.20 Since the regulation of the cell cycle, proliferation, terminal differentiation and apoptosis is highly complex, this should be analysed in future studies, based on the alterations observed here. As a first hint, we analysed M-CSF-induced proliferation with or without IL-15 and indeed found highly proliferative responses in the IL-15-exposed MΦ.
Long-term exposure to IL-15 during MΦ differentiation does not significantly affect functions of ‘innate immunity’ such as cytokine release, LPS-induced MΦ activation and phagocytosis. In addition to phagocytosis, another antigen uptake pathway, notably more relevant in RA, is the Fcγ receptor-mediated pathway. It will be very interesting for further studies to investigate whether IL-15 leads to alterations in this system, especially as the importance of this system has now been realized. Furthermore, normal MΦ differentiation, the lack of functional differences in MΦ functions in IL-15-deficient murine MΦ and normal MΦ development in IL-15R (common-) γ-chain-deficient mice34 argue against a significant role of IL-15 in MΦ development, differentiation and function under physiological conditions. The role of IL-2, which also – like IL-15 – signals through the common γ and IL-2/15Rβ chains, differs significantly from that of IL-15. Myelopoiesis in IL-2-deficient mice is severely disturbed, resulting in an abnormal generation of MΦ.35 Interestingly, addition of IL-2 during M-CSF-induced MΦ generation results in a NK-cell-like phenotype with lytic activity.36 These findings – in spite of the two shared receptor components argue for an important role of the IL-15Rα chain in mediating the effects during MΦ differentiation that are described here.
Taken together, excess of IL-15 in inflammatory and autoimmune diseases is likely to contribute to the respective T-cell-mediated immunopathology. The overall importance of IL-15 in RA was demonstrated some years ago.37,38 This has been verified by successful phase 1 studies in patients with RA, where blocking IL-15 ameliorated the clinical parameters of affected patients. Our data might help to explain this effect and foster selective and specific therapeutic strategies using IL-15 inhibitors.
Acknowledgments
We thank Katrin Seeger, Stephanie Belz and André Jenckel for excellent technical assistance and Christoph Hölscher for critically reading the manuscript.
Abbreviations
- BM
bone marrow
- CFSE
carboxy-fluorescein diacetate succinimidyl ester
- DC
dendritic cell
- FACS
fluorescence-activated cell sorting
- IL-15MΦ
bone-marrow derived macrophages generated for 8 days by M-CSF in the presence of interleukin-15
- LPS
lipopolysaccharide
- MΦ
macrophages
- M-CSF
macrophage colony-stimulating factor
- MHC
major histocompatibility complex
- NK
natural killer
- OVA
ovalbumin
- PBMC
peripheral blood mononuclear cell
- RA
rheumatoid arthritis
- RT-PCR
reverse transcription–polymerase chain reaction
- SOCS3
suppressor of cytokine signalling 3
- TLR
Toll-like receptor
- TNF
tumour necrosis factor
References
- 1.Giron-Michel J, Caignard A, Fogli M, et al. Differential STAT3, STAT5, and NF-kappaB activation in human hematopoietic progenitors by endogenous interleukin-15: implications in the expression of functional molecules. Blood. 2003;102:109–17. doi: 10.1182/blood-2002-09-2760. [DOI] [PubMed] [Google Scholar]
- 2.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 3.Bulfone-Paus S, Bulanova E, Budagian V, Paus R. The interleukin-15/interleukin-15 receptor system as a model for juxtacrine and reverse signaling. Bioessays. 2006;28:362–77. doi: 10.1002/bies.20380. [DOI] [PubMed] [Google Scholar]
- 4.Rückert R, Brandt K, Bulanova E, Mirghomizadeh F, Paus R, Bulfone-Paus S. Dendritic cell-derived IL-15 controls the induction of CD8 T cell immune responses. Eur J Immunol. 2003;33:3493–03. doi: 10.1002/eji.200324545. [DOI] [PubMed] [Google Scholar]
- 5.Waldmann T. The contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for the immunotherapy of rheumatological diseases. Arthritis Res. 2002;4(Suppl. 3):S161–7. doi: 10.1186/ar584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doherty TM, Seder RA, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J Immunol. 1996;156:735–41. [PubMed] [Google Scholar]
- 7.Alleva DG, Kaser SB, Monroy MA, Fenton MJ, Beller DI. IL-15 functions as a potent autocrine regulator of macrophage proinflammatory cytokine production: evidence for differential receptor subunit utilization associated with stimulation or inhibition. J Immunol. 1997;159:2941–51. [PubMed] [Google Scholar]
- 8.McInnes IB, Leung BP, Sturrock RD, Field M, Liew FY. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat Med. 1997;3:189–95. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- 9.McInnes IB, Gracie JA, Harnett M, Harnett W, Liew FY. New strategies to control inflammatory synovitis: interleukin 15 and beyond. Ann Rheum Dis. 2003;62(Suppl. 2):ii51–4. doi: 10.1136/ard.62.suppl_2.ii51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruchatz H, Leung BP, Wei XQ, McInnes IB, Liew FY. Soluble IL-15 receptor alpha-chain administration prevents murine collagen-induced arthritis: a role for IL-15 in development of antigen-induced immunopathology. J Immunol. 1998;160:5654–60. [PubMed] [Google Scholar]
- 11.Gerli R, Schillaci G, Giordano A, et al. CD4+CD28− T lymphocytes contribute to early atherosclerotic damage in rheumatoid arthritis patients. Circulation. 2004;109:2744–8. doi: 10.1161/01.CIR.0000131450.66017.B3. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7− CD28− T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996;97:2027–37. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 14.van Gestel AM, Haagsma CJ, van Riel PL. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998;41:1845–50. doi: 10.1002/1529-0131(199810)41:10<1845::AID-ART17>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 15.Stumbles PA, Thomas JA, Pimm CL, Lee PT, Venaille TJ, Proksch S, Holt PG. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J Exp Med. 1998;188:2019–31. doi: 10.1084/jem.188.11.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gravallese EM. Bone destruction in arthritis. Ann Rheum Dis. 2002;61(Suppl. 2):ii84–6. doi: 10.1136/ard.61.suppl_2.ii84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondo M, Scherer DC, Miyamoto T, King AG, Akashi K, Sugamura K, Weissman IL. Cell-fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature. 2000;407:383–6. doi: 10.1038/35030112. [DOI] [PubMed] [Google Scholar]
- 18.Brandt K, Bulfone-Paus S, Foster DC, Rückert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood. 2003;102:4090–8. doi: 10.1182/blood-2003-03-0669. [DOI] [PubMed] [Google Scholar]
- 19.Klausen P, Bjerregaard MD, Borregaard N, Cowland JB. End-stage differentiation of neutrophil granulocytes in vivo is accompanied by up-regulation of p27kip1 and down-regulation of CDK2, CDK4, and CDK6. J Leukoc Biol. 2004;75:569–78. doi: 10.1189/jlb.1003474. [DOI] [PubMed] [Google Scholar]
- 20.Xaus J, Cardo M, Valledor AF, Soler C, Lloberas J, Celada A. Interferon gamma induces the expression of p21waf-1 and arrests macrophage cell cycle, preventing induction of apoptosis. Immunity. 1999;11:103–13. doi: 10.1016/s1074-7613(00)80085-0. [DOI] [PubMed] [Google Scholar]
- 21.Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003;4:1169–76. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- 22.Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol. 2003;33:597–605. doi: 10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- 23.Rückert R, Herz U, Paus R, Ungureanu D, Pohl T, Renz H, Bulfone-Paus S. IL-15-IgG2b fusion protein accelerates and enhances a Th2 but not a Th1 immune response in vivo, while IL-2-IgG2b fusion protein inhibits both. Eur J Immunol. 1998;28:3312–20. doi: 10.1002/(SICI)1521-4141(199810)28:10<3312::AID-IMMU3312>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 24.Agostini C, Zambello R, Facco M, et al. CD8 T-cell infiltration in extravascular tissues of patients with human immunodeficiency virus infection. Interleukin-15 upmodulates costimulatory pathways involved in the antigen-presenting cells–T-cell interaction. Blood. 1999;93:1277–86. [PubMed] [Google Scholar]
- 25.Miranda-Carus ME, Balsa A, Benito-Miguel M, Perez dA, Martin-Mola E. IL-15 and the initiation of cell contact-dependent synovial fibroblast–T lymphocyte cross-talk in rheumatoid arthritis: effect of methotrexate. J Immunol. 2004;173:1463–76. doi: 10.4049/jimmunol.173.2.1463. [DOI] [PubMed] [Google Scholar]
- 26.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 in trans to neighboring cells. Immunity. 2002;17:537–47. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–80. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–76. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 29.Niedbala W, Wei X, Liew FY. IL-15 induces type 1 and type 2 CD4+ and CD8+ T cells proliferation but is unable to drive cytokine production in the absence of TCR activation or IL-12/IL-4 stimulation in vitro. Eur J Immunol. 2002;32:341–7. doi: 10.1002/1521-4141(200202)32:2<341::AID-IMMU341>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama K, Ishida N, Shirane M, et al. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–20. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 31.Nourse J, Firpo E, Flanagan WM, et al. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–3. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 32.Bulfone-Paus S, Ungureanu D, Pohl T, Lindner G, Paus R, Rückert R, Krause H, Kunzendorf U. Interleukin-15 protects from lethal apoptosis in vivo. Nat Med. 1997;3:1124–8. doi: 10.1038/nm1097-1124. [DOI] [PubMed] [Google Scholar]
- 33.Steinman RA, Hoffman B, Iro A, Guillouf C, Liebermann DA, el Houseini ME. Induction of p21 (WAF-1/CIP1) during differentiation. Oncogene. 1994;9:3389–96. [PubMed] [Google Scholar]
- 34.Andersson A, Grunewald SM, Duschl A, Fischer A, DiSanto JP. Mouse macrophage development in the absence of the common gamma chain: defining receptor complexes responsible for IL-4 and IL-13 signaling. Eur J Immunol. 1997;27:1762–8. doi: 10.1002/eji.1830270725. [DOI] [PubMed] [Google Scholar]
- 35.Reya T, Contractor NV, Couzens MS, Wasik MA, Emerson SG, Carding SR. Abnormal myelocytic cell development in interleukin-2 (IL-2) -deficient mice: evidence for the involvement of IL-2 in myelopoiesis. Blood. 1998;91:2935–47. [PubMed] [Google Scholar]
- 36.Lohmann-Matthes ML, Emmendoerffer A, Hao L. Influence of interleukin-2 on the differentiation of macrophages. Pathobiology. 1991;59:117–21. doi: 10.1159/000163627. [DOI] [PubMed] [Google Scholar]
- 37.Liew FY, McInnes IB. Role of interleukin 15 and interleukin 18 in inflammatory response. Ann Rheum Dis. 2002;61(Suppl. 2):ii 100–2. doi: 10.1136/ard.61.suppl_2.ii100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miranda-Carus ME, Benito-Miguel M, Balsa A, Cobo-Ibanez T, Perez de Ayala C, Pascual-Salcedo D, Martin-Mola E. Peripheral blood T lymphocytes from patients with early rheumatoid arthritis express RANKL and interleukin-15 on the cell surface and promote osteoclastogenesis in autologous monocytes. Arthritis Rheum. 2006;54:1151–64. doi: 10.1002/art.21731. [DOI] [PubMed] [Google Scholar]