Abstract
Glutamate transporters remove the excitatory neurotransmitter glutamate from the extracellular space after neurotransmission is complete, by taking glutamate up into neurons and glia cells. As thermodynamic machines, these transporters can also run in reverse, releasing glutamate into the extracellular space. Because glutamate is excitotoxic, this transporter-mediated release is detrimental to the health of neurons and axons, and it, thus, contributes to the brain damage that typically follows a stroke. This review highlights current ideas about the molecular mechanisms underlying glutamate uptake and glutamate reverse transport. It also discusses the implications of transporter-mediated glutamate release for cellular function under physiological and patho-physiological conditions.
Introduction
The plasma membrane transporters for the excitatory neurotransmitter glutamate play important roles in many physiological processes (reviewed in (1–5)). Most importantly, glutamate transporters control signal transmission at glutamatergic synapses, initially by buffering, and subsequently by removing pre-synaptically-released glutamate from the synaptic cleft into adjacent neurons and glia cells. Thus, these transporters perform their part in an elaborate interplay of membrane and soluble proteins that recycle and repackage glutamate for subsequent pre-synaptic release events. These proteins all contribute to the glutamate-glutamine cycle (6, 7), in which astrocytes convert glutamate into glutamine, export glutamine to neurons, where glutamine finally is recycled to glutamate. The cycle is completed by repackaging of glutamate into pre-synaptic vesicles, a task which is performed by the vesicular glutamate transporters (8, 9).
Many of the players of the glutamate-glutamine cycle have now been identified and characterized at the molecular level, including the plasma membrane glutamate transporters, which are the subject of this review. Recent findings have significantly improved our understanding of how glutamate transporters operate and how their function is regulated. This review will summarize current ideas on the structural basis of glutamate transport in light of the large body of available mechanistic information. It will also put special emphasis on the reversibility of the glutamate transport process, discussing recent information on the mechanism of glutamate reverse transport and its importance in pathophysiological conditions.
Function and Structure
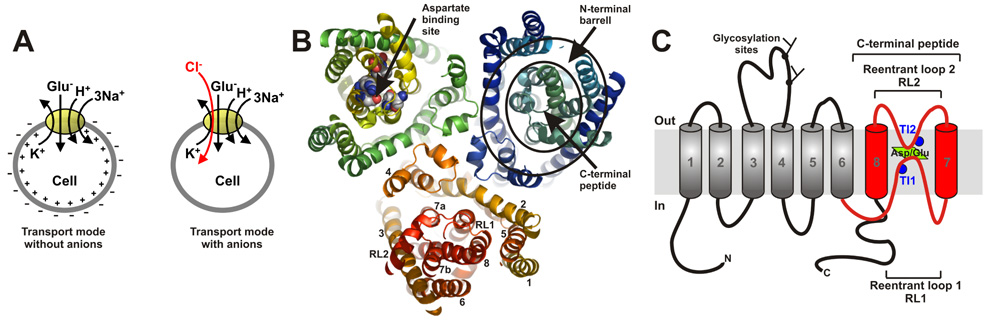
Glutamate transporters belong to the solute carrier 1 (SLC1) family of transport proteins (10). They are secondary-active transporters, using the free energy stored in the transmembrane concentration gradient of Na+, which in turn is established by the sodium pump through hydrolysis of the primary energy source ATP, to take up glutamate into the cell against its own concentration gradient (11). Inward transport of one glutamate molecule is coupled to the inward movement of three Na+ and one H+, and the outward movement of one K+ ion (12–14) for the mammalian transporters (Fig. 1A). This outward movement of K+ occurs in a step independent from the Na+/glutamate translocation step (15). According to this stoichiometry, glutamate transport is electrogenic, meaning that it is associated with net charge transport across the membrane. Coupling of glutamate uptake to cotransport of three Na+ ions together with the utilization of negative membrane potential as a driving force allows glutamate transporter-expressing neurons to maintain an up to 106-fold [glutamate] gradient across their membranes (14).
Figure 1.
(A) Illustration of the stoichiometry of glutamate transporters (left panel). The plus sign (+) inside the cell indicates depolarization of the cell induced by inward glutamate transport. The right panel illustrates the glutamate-induced anion conductance. (B) Top view of the trimeric assembly of GltPh adapted from (32). (C) Transmembrane topology of glutamate transporters. The two Tl+ (thallium+) binding sites found in GltPh are highlighted in blue, the bound substrate is shown in green. The functionally important C-terminal peptide is highlighted in red.
Glutamate transport is associated with an anion conductance (Fig. 1A). This anion conductance is activated by glutamate binding to the transporter (16–18). The anion conductance is not stoichiometrically coupled to the transport of glutamate and can be activated in the presence of external sodium alone. However, it is activated by glutamate only under ionic conditions that also favor glutamate translocation (e.g. presence of external sodium) (16). Hence, glutamate uptake can take place already in the absence of chloride ions, but no, or only little kinetically-coupled anion flux occurs without the activation of the glutamate transporter by substrate and cation binding (16). Thus, glutamate transporters have mixed characteristics that are, in part, carrier-like and, in part, similar to ligand-gated chloride channels. In analogy to ligand-gated ion channels it was initially proposed that anion permeation may take place through a pore formed in the center of the multimeric subunit complex (19), although more detailed recent studies suggest that the anion-conducting pathway is more likely formed by each individual subunit of the homotrimer (20–22). The anion conductance is selective for hydrophobic anions. The anion permeability follows the sequence PSCN- > PNO3- > PI- > PBr- > PCl- > PF- >> PGluconate- (16, 23, 24). Interestingly, the anion conductance appears to be conserved in the glutamate transporter protein family, since it is also present in the related neutral amino acid transporters (25, 26) and in bacterial glutamate/aspartate transporters (27).
Glutamate transporter structure and location of the substrate binding site
Our understanding of the glutamate transport process has been greatly facilitated by the cloning of the transporters (28–31), as well as by the recent determination of a crystal structure of a bacterial glutamate transporter analogue from Pyrococcus horikoshii (Fig. 1B), GltPh (32), which confirmed previous determinations of the transmembrane topology through biochemical methods (33, 34). GltPh shares about 35% amino acid identity with the human EAATs and, thus, is believed to serve as a valid model for the structure of the human EAATs. A schematic of the transmembrane topology, including the binding site for the amino acid substrate (green), and potential binding sites for cations (blue), is shown in Fig. 1C. The functionally important part of the transporter is a C-terminal peptide (red in Fig. 1C see also top view in Fig. 1B), which is inserted into a N-terminal barrel formed by transmembrane domains (TMDs) 1–6 (32). This C-terminal peptide is composed of two helical transmembrane domains (TMDs 7 and 8) and two reentrant loop structures (RL 1 and 2), which dip into the transmembrane segment of the transporter from opposite sides of the membrane (31). As shown in Error! Reference source not found. 1B, GltPh is assembled as a homotrimer (32), confirming previous evidence from biochemical experiments (35) that suggested trimeric composition of the mammalian glutamate transporters. Each monomer contains a separate amino acid binding site, which appears to be buried within the protein and is closed off from the extracellular side by RL2 (32). The localization of this substrate binding site is consistent with mutagenesis studies, which implicated a conserved arginine residue (R446 in EAAC1) in the coordination of the γ-carboxylate of the acidic amino acid (36). Furthermore, the aspartate residue D443 was found to be important for interaction with zwitterionic acidic amino acids, but not dicarboxylic acids, consistent with the location of side-chain of the analogous GltPh residue in contact with the bound substrate (37).
A significant body of functional data suggests that the subunits of the trimer transport glutamate independent from each other (20–22, 38). Furthermore, the anion conductance may also be catalyzed independently by each subunit, at least for the glutamate transporter subtype EAAT3 (20–22). However, cooperativity has been observed for the activation of the EAAT4 anion conductance by glutamate (Hill coefficient > 1) (39), suggesting a fundamentally different mechanism by which EAAT4 conducts anions. Consistent with the idea of crosstalk between the subunits of the trimer, inter-subunit cooperativity has also been proposed for Na+ binding events to the glutamate-free, empty EAAT3 transporter (40), in order to explain the remarkably large Hill coefficient associated with apparent Na+ association.
Cation binding sites
In 2007 a second crystal structure of the bacterial glutamate transporter homologue GltPh was published, in which the transporter is in complex with two thallium (Tl+) ions (41). Tl+ was used as a cation instead of Na+ in the crystallographic study due to its anomalous scattering properties, facilitating the detection of the bound cations. Functional studies indicate that, like the mammalian counterparts, substrate transport by GltPh is driven by co-transport of at least two Na+ ions (41). Although substrate transport is not supported by Tl+, it was proposed that the two Tl+ ions bind to sites (termed Na1 and Na2 sites) that also bind Na+, because Tl+ and Li+ competed with Na+ in a binding assay, whereas K+ did not compete (41). This proposal was further supported by the fact that mutation of one of the amino acids contributing its side chain to the Na1 site, D405, to asparagine altered the 2:1 stoichiometry of Na+:aspartate binding to a stoichiometry of 1.5:1. If the D405 mutation would fully eliminate binding of a second Na+ ion, a coupling ratio of 1:1 should be observed. Thus, the less-than-expected reduction of the coupling ratio by this mutation points to the existence of a third GltPh Na+ binding site that may be resistant to Tl+ substitution (41).
In addition, our laboratory (42, 43) and others (44–47) have identified amino acids that potentially contribute to other cation binding sites by using site-directed mutagenesis. A highly-conserved aspartate residue in TMD7, D367 in EAAC1, was found, which upon mutagenesis to asparagine led to a >16 fold reduction in sodium affinity (42). In contrast to this dramatic effect of the D367N mutation, the D454N exchange did not reduce the Na+ affinity of EAAC1 (42). Based on these data, it was hypothesized that D454 does not contribute to Na+ coordination in mammalian glutamate transporters, and an additional Na+ binding site exists that is buried deeply in the membrane, which directly or indirectly involves the side chain of D367. This finding is consistent with the effects of mutations to D367, and the nearby N365, reported by the Kanner laboratory (47). The existence of additional cation binding site(s) in the mammalian transporters that is/are not present in the GltPh crystal structure is consistent with the amino acid:Na+ coupling stoichiometry of 1:3 of the mammalian transporters, in contrast to the hypothesized GltPh 1:2 coupling ratio. Thus, it is expected that at least one cation binding site of the mammalian transporters is not conserved in GltPh, or that the Na+ bound to this site cannot be replaced with Tl+.
Mechanism of Glutamate Transport
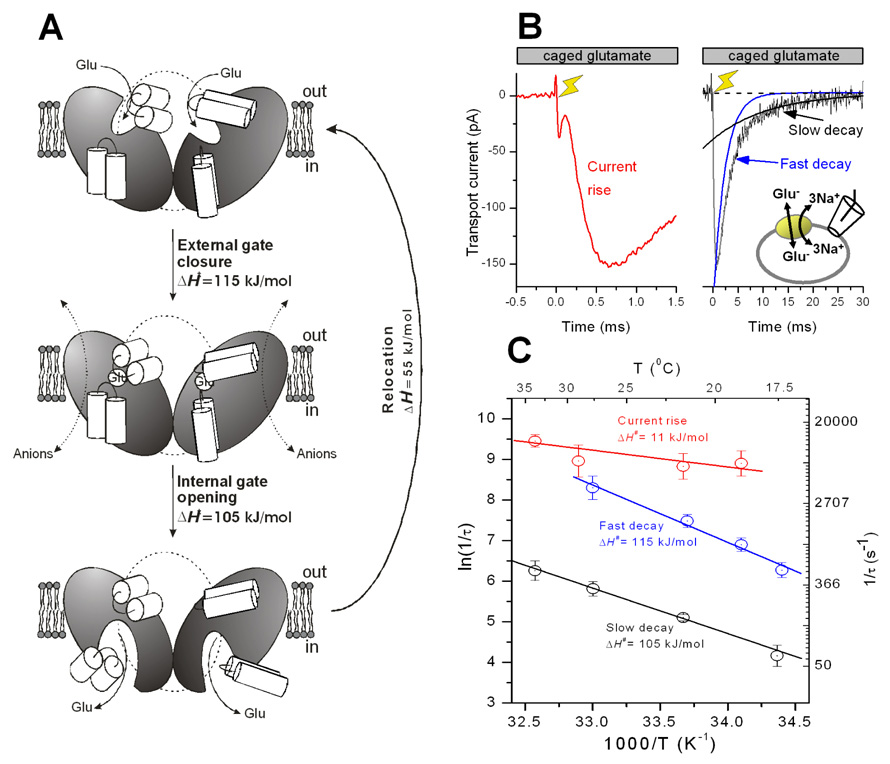
Glutamate transport by the members of the SLC1 family, as well as secondary-active transport by other solute carriers, is thought to occur through an alternating access mechanism. Such a mechanism assumes that the transporter cycles through two discrete conformational states, one of them allowing access of substrate to its binding site from the extracellular side, the other one allowing access from the cytoplasm (48). In addition, a third state may exist, in which the substrate binding site is closed to both sides of the membrane. This state is called “occluded” and may be the one observed in the aspartate-bound GltPh structure (32). Alternating access is hypothesized to be brought about by the coupled opening and closing of two gates, one gating extracellular access, and the other gating intracellular access to the substrate binding site (32, 41, 49). Only one of the two gates can be open at a time, preventing leak flux of substrate across the membrane. Experimental evidence for the alternating access model comes from the crystal structure of GltPh in complex with a competitive inhibitor, DL-threo-benzyloxyaspartate (TBOA), in which RL2 does not fully close off the inhibitor binding site to the extracellular side of the membrane (41). Thus, this structure may be analogous to the outward-facing conformation that has an outwardly-accessible substrate binding site. The three major states are illustrated in Fig. 2A.
Figure 2.
(A) Proposed mechanism for the transmembrane movement of glutamate catalyzed by the glutamate transporter, based on functional and structural data. (B) Pre-steady-state currents measured in response to a rapid glutamate concentration jump point to at least two independent processes associated with glutamate translocation. (C) The temperature dependence of pre-steady-state kinetics indicates the existence of two conformational changes.
Due to the complex stoichiometry of the glutamate transport process, it is thought that, in addition to the three major states shown in Fig. 2A, several sub-states exist with populations of these sub-states depending on the presence/absence of substrate and cations. A number of kinetic mechanisms have been proposed based on this alternating access, multi-step hypothesis, which vary in complexity from very simplified schemes including only three or four states (23, 50), to complex mechanisms with up to 16 states (51, 52). Simulations using some of these kinetic mechanisms fit the available data remarkably well (23, 52, 53). Therefore, these mechanisms can be used as working hypotheses for future mechanistic studies.
In addition to structural evidence, there is also functional data available supporting the alternating access, multi-step hypothesis for the transport mechanism. For example, pre-steady-state transport currents recorded in response to glutamate concentration jumps decay with two exponential components (53–55), both of which are also present when the transporter is restricted to access states associated with translocation, by employing the Na+/glutamate-exchange mode (Fig. 2B). In this exchange mode, ionic conditions are used that prevent Na+/glutamate dissociation to both the intra- and extracellular solutions, but allow relaxation of the translocation equilibrium. The existence of two exponential components of this relaxation suggests that the translocation process consists of at least two separable steps (55), consistent with the structural evidence for an intermediate on the translocation reaction coordinate (possible occluded state). It can be hypothesized that the two molecular processes underlying the two exponential components are related to consecutive closing of an extracellular gate and opening of an intracellular gate, allowing alternating access of the substrate binding site from the extracellular or intracellular medium (Figs. 2A and C). While the extracellular gate is most likely formed by the two α-helices of RL2 (32, 41), the structural basis of the intracellular gate remains less clear, However, RL1 was suggested to play the role of the intracellular gate, based on the striking symmetry of the RL1-TMD7-RL2 structure (32) and a recent accessibility study of cysteine residues that were introduced into RL1 (56). Consistent with the assignment of the two exponential components of the overall translocation reaction to gate closing and opening, the relaxation rate constants associated with both components show strong temperature dependence (Fig. 2C), which is indicative of conformational changes (55). In contrast, a third phase of the exchange current (rising phase, Fig. 2B) is only weakly accelerated at elevated temperatures, as expected for a diffusion-controlled process. This result is consistent with the assignment of this phase to glutamate binding, which most likely occurs by diffusion-controlled access of glutamate to its binding site through an aqueous pathway (55), as expected for the outward-facing conformation with open extracellular gate (Fig. 2A).
Additional mechanistic insight into structural changes accompanying glutamate transport was obtained from biochemical cross-linking experiments. If RL1 plays the role of the intracellular gate, which has to move (open) to allow glutamate dissociation to the cytoplasm, it is expected that covalently attaching RL1 to other, neighboring transporter elements should eliminate glutamate transport. This expectation is met (transport is inhibited) when the tip of RL1 is crosslinked with introduced cysteines in RL2 (57), as well as when it is covalently attached to the central part of TMD7 (58). In contrast, crosslinking of the tip of RL2 with TMD7 did not result in inhibition of the maximum rate of glutamate transport, but in a reduction of the apparent affinity of the transporter for glutamate (58). These results were interpreted such that only small conformational changes of RL2 relative to the rest of the protein are required for transport, as also suggested by fluorescence resonance energy transfer (FRET) experiments (38), or that RL2 and TMD7 move during transport, but not relative to each other. To summarize, although the structural, functional and biochemical data available at present is consistent with the dual-gate alternating access model, alternative mechanisms cannot be categorically ruled out. Furthermore, direct experimental evidence for RL1 playing the role of the intracellular gate has yet to be obtained.
Recent Insights into the Reverse Transport Process
Glutamate transport by the plasma membrane transporters is reversible. While the conventional transport direction is inward under physiological conditions, glutamate can also be transported in the outward direction when extracellular [Na+] / intracellular [K+] decrease and/or intracellular [Na+] / extracellular [K+] increase (59–61). Due to the electrogenicity of glutamate transport, membrane depolarization will also result in a reversal of the transport direction because the driving force for uptake decreases under depolarized conditions. Glutamate transport in the outward direction was also termed reverse(d) transport, to indicate the reversal from the conventional transport direction.
Methods for studying reverse glutamate transport
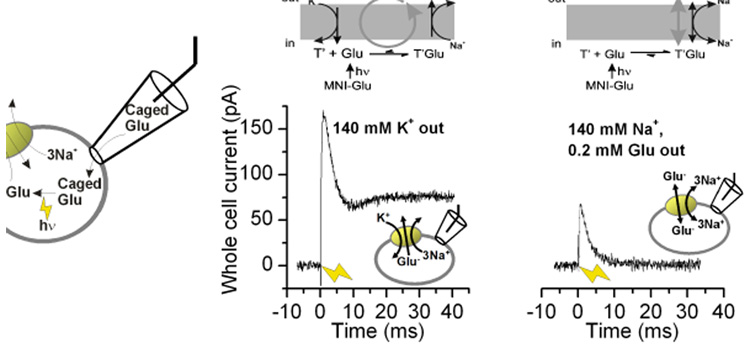
Traditionally, reverse glutamate transport has been studied by applying K+ to the extracellular side of the transporter (14, 59), followed by measurement of glutamate efflux or reverse transport current. This method has proven powerful for determining the properties of the reverse transport process at steady state. For example, it has allowed the characterization of glutamate release from isolated retinal Mueller cells through reverse transport, as well as its modulation by protons (59, 60). More recently, reverse transport current has been studied after directly applying glutamate to the intracellular face of the transporter. This was achieved by using inside-out patches (62–64), in which the inside of the transporter faces the bath solution, thus making it possible to change intracellular [glutamate] through bath perfusion. This method allows one to also control and alter intracellular [Na+] for the determination of Na+ association kinetics with the transporters. Finally, reverse glutamate transport was studied after rapid, sub-millisecond intracellular application of glutamate through photolysis of an inactive, caged precursor ((64), illustrated in Fig. 3A). The power of this method lies in the fast time resolution, allowing the determination of the pre-steady-state kinetics of reverse transport.
Figure 3.
Reverse translocation of glutamate is associated with transmembrane charge movement. (A) Illustration of the rapid delivery of glutamate to the cytosolic face of the membrane by laser photolysis of intracellular caged glutamate. (B) and (C) show reverse transport currents induced by photolysis of 6 mM MNI-Glu at t = 0 ms (yellow arrow) in the reverse transport mode (B, illustrated in top panel) and reverse exchange mode (C, illustrated in top panel).
The kinetic properties of glutamate transporters depend strongly on the transport direction
The crystal structure of GltPh shows strong asymmetry with respect to the plane of the membrane, exhibiting a solvent-filled basin protruding half-way into the membrane from the extracellular side, but not from the intracellular side (41). Therefore, it should not be surprising that the kinetic properties of glutamate transport (but not the general alternating access transport mechanism) depend on the transport direction. First, the amino acid binding site is optimized for tight binding of extracellular, but not intracellular substrate (the apparent affinity of the transporter for glutamate is about 50-times higher when the substrate binding site faces the extracellular side as compared to intracellular glutamate binding (62)). This functional feature is important because it promotes dissociation of glutamate into the cytoplasm against a high intracellular glutamate concentration. At a physiological [Na+]i of about 5 mM, the intracellular Km for glutamate is > 50 mM. Cytosolic glutamate concentration in neurons, [Glu−]i, is assumed to be 1–10 mM, consistent with the Kd of vesicular glutamate transport measured between 0.3 and 2 mM (65). Thus the intracellular glutamate binding site of the plasma membrane transporters is far from being saturated ([Glu−]i < 1/5 Km), preventing the transporters from being trans-inhibited by intracellular glutamate under normal uptake conditions (64).
Second, steady-state glutamate transport is faster in the reverse direction (55 s−1) than in the forward transport mode (24 s−1), given equal but opposite electrochemical driving forces (64). This difference in the transport rates in the two transport directions is caused by a difference in the rate limitation. While forward transport is strongly rate limited by one single reaction step, the K+ induced relocation (52, 66) taking place with a rate constant of about 30 s−1 at 0 mV, the reverse transport rate is limited by a number of reactions, including the K+-induced relocation, glutamate reverse translocation, and, possibly, glutamate dissociation to the extracellular solution (64). Thus, glutamate transporters are optimized for efficient capture, translocation, and release of glutamate to the cytosol, but not for rapid steady-state turnover. Consistently, in their physiological environment at the synapse rapid steady-state turnover is not required because each transporter most likely completes only one transport cycle per pre-synaptic release event. GABA transporters also operate more rapidly in the reverse than in the forward transport mode (67, 68). As new functional information is gathered on other secondary-active transporters it will be interesting to determine if rapid steady-state reverse transport is a general property also shared by other transporters.
Third, the voltage dependence of glutamate transport depends on the transport direction. While the forward transport current increases exponentially with increasing electrical driving force, the reverse transport current saturates at increasingly positive potentials (V > 0 mV) (59, 64), indicating that at these voltages reverse transport becomes rate-limited by an electroneutral or weakly electrogenic process.
Fourth, the glutamate transporter anion conductance, which is thought to be activated by substrate and/or Na+ binding to their transporter binding sites, exhibits sidedness with respect to the plane of the membrane. Although this anion conductance is activated by glutamate application to either side of the membrane, anion currents induced by intracellular glutamate are much smaller than those induced by extracellular glutamate (62). This finding suggest that the anion conducting states are more strongly populated (or have higher unitary conductance) in the forward transport than in the reverse transport mode, supporting the idea of strongly asymmetric transporter behavior with respect to the plane of the membrane.
Electrogenicity of the reaction steps in the reverse direction
Kinetically, the overall transport cycle can be separated into two independent branches, the glutamate/Na+ translocation branch and the K+ relocation branch. In the forward transport mode, inwardly-directed glutamate translocation was proposed to be associated with transient inward charge movement (50, 53). Thus, outward charge movement should be observed when the transporter moves through the translocation steps in the reverse direction. Consistent with this expectation, rapid glutamate application to the intracellular side of the membrane resulted in transient outward currents in the reverse transport mode as well as the reverse exchange mode (Fig. 3B and C) (64). In the reverse exchange mode (Fig. 3C) no net dissociation of glutamate and Na+ takes place to the extracellular side, thus restricting the transporter to the translocation steps. Thus, part of the outward charge movement has to be caused by the actual translocation process(es) and not by electrogenic dissociation of Na+ to the extracellular side of the membrane, which has been shown to be also electrogenic (53).
In addition to glutamate translocation, charge movement is also associated with Na+ binding from the cytosol (64). This is not surprising, given that the proposed cation binding sites are located in the center of the transmembrane domain of the transporter. Thus, Na+ may have to cross part of the transmembrane electric field when accessing its binding site from the cytoplasm, as has been previously shown for Na+ accesses of its binding site(s) from the extracellular side of the membrane (69). In contrast, binding of the negatively-charged glutamate molecule from the cytoplasm is associated with little, if any charge movement (64), suggesting that an aqueous access pathway exists that connects the glutamate binding site to the intracellular medium in the inward-facing conformation. Such an access pathway is not visible in the presently available crystal structures (32, 41), raising the possibility that the inward-facing conformation differs dramatically from the outward-facing and occluded conformations, which are believed to be represented by these crystal structures.
A comprehensive kinetic model for glutamate forward and reverse transport
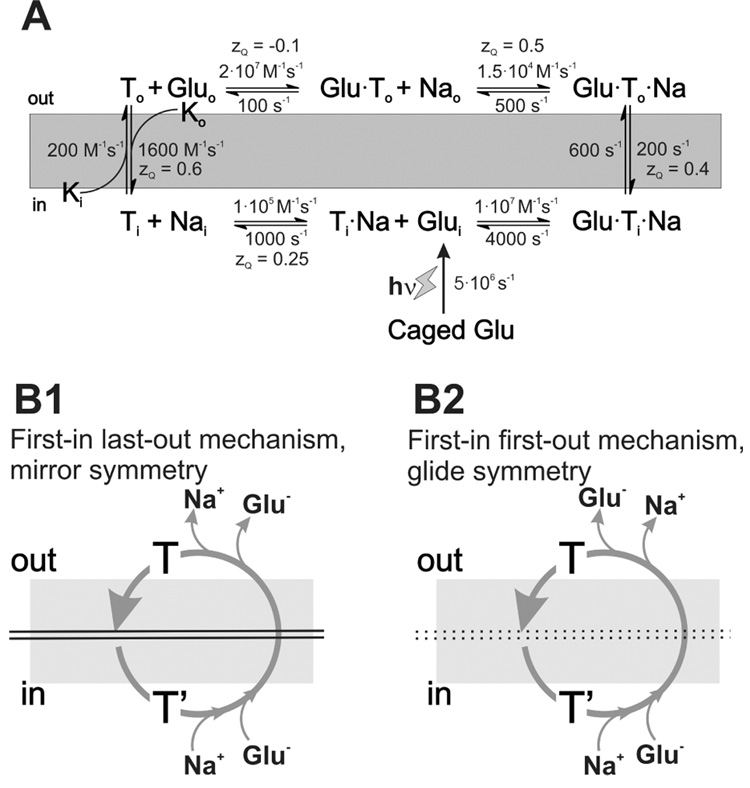
Many kinetic models have been proposed for glutamate transport, most of them based on functional data obtained in the forward transport mode (50–53, 66, 70). Although these models are useful for describing the kinetics of glutamate uptake, they do not account for actual experimental data on intracellular ion and substrate dissociation steps. With the recent availability of such experimental data (64), these models can now be extended to provide a more detailed view of how the properties of these dissociation steps affect the overall kinetic properties of forward and reverse glutamate transport. One possible kinetic model is shown in Fig. 4A. One of the most important features of this model is the symmetry of the sequence of the substrate/Na+ binding/dissociation events with respect to the plane of the membrane. When simplifying the model such that only one Na+ binding step is included, two possible symmetries have to be considered, namely mirror symmetry (Fig. 4B1) and glide symmetry (Fig. 4B2). These model symmetries are analogous to classical model symmetries for multi-substrate soluble enzyme reactions (71). In the mirror symmetry model, the species that associates first with its intracellular binding site dissociates last from its extracellular binding site (assuming reverse transport direction). For glide symmetry, the substrate that associates first intracellularly also dissociates first to the extracellular medium (also termed first-in-first-out model). Experimental evidence points to asymmetry of the substrate/Na+ binding sequence with respect to the plane of the membrane (64), supporting the glide symmetry, first-in-first-out model, at least for the binding sequence of one Na+ ion with respect to glutamate binding (Fig. 4B2), while the two remaining Na+ ions most likely bind in a first-in-last-out fashion (mirror symmetry) (64).
Figure 4.
A simple kinetic model based on a “first-in-first-out” mechanism can explain the experimental data (A). (B1) and (B2) illustrate possible substrate/Na+ association/dissociation sequences based on mirror-symmetry and glide symmetry models.
Glutamate Release from Neurons through Reverse Transport
Reverse transport in brain ischemia
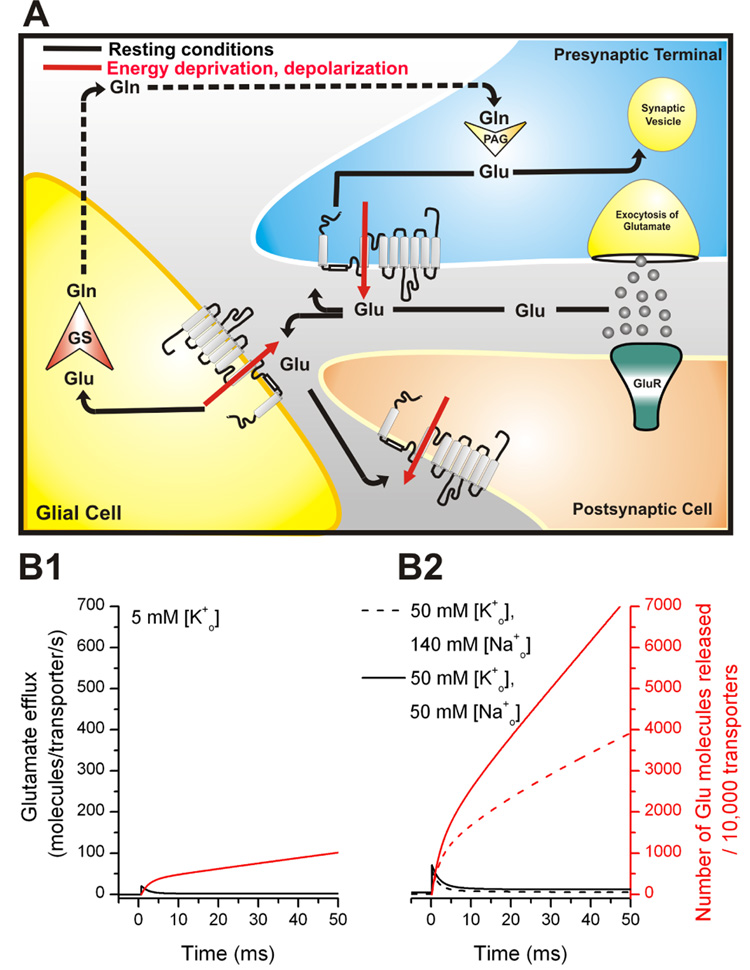
When neurons are deprived of their energy source (glucose and oxygen), lack of ATP synthesis will eventually lead to a run-down of the Na+ and K+ concentration gradients across the neuronal membrane, which are generated usually by the Na+/K+ ATPase, as well as a run-down of the membrane potential. Energy deprivation can occur after blockage of blood vessels, resulting in under-supply of the affected brain areas. The transmembrane potential and the Na+ and K+ concentration gradients power glutamate uptake. As a result, the run-down of both will cause the power-loss of the glutamate transporters with the consequence of transporter reversal (72). Although glutamate is released from neurons under ischemic conditions by exocytosis (73, 74), it has been well established that reversal of the transporters also strongly contributes to this release, as illustrated schematically in Fig. 5A. It should be noted that other pathways were also implicated in glutamate release from brain cells, such as a gap-junction hemichannel-mediated and P2X channel-dependent release from astrocytes (75). These mechanisms, as well as Ca2+-dependent exocytosis, will not be further discussed here. Kinetic data predict that glutamate release through reverse transport can be dramatic, with 1 µm2 of neuronal cell membrane releasing 140,000 molecules of glutamate per second (Fig. 5 B2, see next section for a detailed description of these estimates) at an elevated extracellular [K+] of 50 mM and reduced [Na+] of 50 mM (Vm = −40 mV). Such abnormal cation concentrations and mild membrane depolarization are reached even in partial ischemia (76).
Figure 5.
(A) Distribution and function of EAA transporters at the synapse during a synaptic event. Glutamate (circles) binding to glutamate receptors (GluR) mediates the signal to postsynaptic neurons. Rapid removal of glutamate from the synaptic cleft is achieved by glutamate transporters which are located in glial cells and neurons. Once glutamate is transported into the cell (indicated by arrows), it can be sequestered in synaptic vesicles, ready for a new cycle of neurotransmission (indicated by arrows in the presynapse) or is converted in glial cells by the glia-specific enzyme glutamine-synthetase (GS) to glutamine (Gln), which is released into the extracellular space, and subsequently taken up by glutamatergic neurons for a new cycle of transmitter synthesis (indicated by dotted lines) for a new transmitter synthesis. The red arrows indicate transporter-mediated glutamate release upon energy deprivation and/or membrane depolarization. (B1) Predicted rate of glutamate efflux (black lines) in response to a step depolarization from −80 mV to −40 mV at t = 0 ms in the presence of 5 mM extracellular [K+] (physiological case, left Y-axis) (B2) Predicted rate of glutamate efflux (black lines) in response to a step depolarization from −80 mV to −40 mV at t = 0 ms in the presence of 50 mM extracellular [K+] (normal [Na+]o, dashed lines) and 50 mM extracellular [K+] (reduced [Na+]o of 50 mM, solid lines, pathophysiological case). The red lines show the total number of glutamate molecules released by 10,000 transporters (right Y-axis).
Two independent reports concluded that the majority of glutamate is released by the reverse transport pathway upon oxygen deprivation (61, 77). This conclusion was reached because an inhibitor of glutamate transport, L-trans-pyrrolidine-2,4-dicarboxylic acid (PDC), strongly inhibited ischemia-induced glutamate release, whereas the absence of Ca2+ in the extracellular medium had no effect on release (77). This result suggests that Ca2+-dependent mechanisms, such as exocytosis, played only a minor role in the glutamate release induced by ischemia. PDC targets neuronal and astrocytic glutamate transporter subtypes alike. In contrast, dihydrokainate (DHK) is somewhat selective for the main astrocytic glutamate transporter GLT-1 (78). In contrast to PDC, DHK did not prevent glutamate release, suggesting that the ischemia-induced glutamate efflux arises predominantly from neurons, but not from astrocytes (77). This finding is consistent with the hypothesis that intracellular [glutamate] is lower in astrocytes than in neurons, which would prevent significant release through reverse transport (77). However, the hypothesis of low intracellular astrocytic [glutamate], which is affected by metabolic processes in this glia cell type, is not generally accepted, with other papers reporting cytoplasmic [glutamate] values as high as 8.5 mM (79), a value which corresponds well with that of intracellular [glutamate] found in neurons. An alternative explanation for the lack of astrocytic involvement in ischemic glutamate release would be their ability to maintain glutamate uptake in the absence of sufficient levels of oxygen, at least for a while. Thus, it is likely that astrocytes still take up glutamate in the early phase of the anoxic response (76, 80), when neurons already release glutamate. This delayed response of astrocytes to energy deprivation may be caused by their ability to step up glycolysis (81), as well as their higher resistance to anoxic depolarization (82, 83). In fact, astrocytes, in contrast to neurons, have the ability to metabolize glycogen (84), potentially allowing them to sustain glutamate transport after energy deprivation, at least for a limited amount of time. Neurons, on the other hand, cannot metabolize lactate supplied to them by astrocytes anaerobically (85). Thus, they cannot sustain glutamate transport after energy deprivation. The most likely sequence of events is, therefore, that in the initial phase of energy deprivation neurons already release glutamate through reverse transport, while astrocytes still take it up (76). In this initial phase extracellular [glutamate] may remain relatively constant. Only after astrocytic glycogen stores are depleted will the astrocytic glutamate transporters start running in reverse, resulting in a catastrophic surge of extracellular [glutamate]. The quest to directly identify the cellular sources of glutamate release would be aided by the development of subtype specific blockers of glutamate transport, a field which has been boosted by the recent development of an inhibitor that is partially selective for the neuronal EAAT3 subtype (86).
Does reverse transport occur under physiological conditions?
Although glutamate release through reverse transport is an accepted release mechanism under pathophysiological conditions, it is not clear whether such glutamate release also occurs in the absence of energy deprivation. While experimental evidence for or against transporter reversal under physiological conditions is lacking, the reverse transport model shown in Fig. 4A can be used to predict the kinetics of glutamate release from EAAC1-expressing cells upon a sudden decrease in the driving force for uptake (64). Such a decrease in the driving force could be, for example, a glutamate receptor-induced depolarization of the postsynaptic membrane, as encountered during normal excitatory transmission. Assuming such a sudden depolarization from −80 mV to −40 mV ([Na+]i = 5 mM, [Na+]o = 140 mM, [K+]i = 140 mM, [K+]o = 5 mM, [Glu−]i = 5 mM, [Glu−]o = 1 nM), the steady-state release rate of glutamate (1.2 molecules/transporter/s) is predicted to increase 20-fold at short times after the depolarization (Fig. 5B1). Glutamate transporters are expressed at densities of 15,000–20,000/µm2 in HEK293 cells (66), as well as in the vicinity of glutamatergic synapses (1). Thus, the large number of transporters present at the synapse may significantly and rapidly release hundreds of molecules of glutamate per ms and µm2 of membrane surface area upon depolarization. For example, in the simulation shown in Fig. 5B1, approximately 1000 molecules of glutamate are reverse transported by 20,000 transporters within 10 ms. This value is of the same magnitude as numbers of glutamate molecules exocytosed from one single vesicle (100 – 5000). Furthermore, this release is expected to be significantly stimulated as extracellular [K+] increases and extracellular [Na+] drops (Fig. 5B2). Therefore, neuronal transporters may release significant amounts of glutamate upon depolarization, possibly contributing to the regulation of glutamatergic neurotransmission.
Concluding remarks
This review has summarized data available in the literature on the kinetic, mechanistic, and structural properties of glutamate transporters, focusing specifically onto the kinetic characteristics of reverse transport of glutamate. A significant advance in our understanding of glutamate transporter function came with the availability of several structures of GltPh, with substrates and inhibitors bound. Although these structures answer many questions regarding transmembrane topology, assembly of subunits, and arrangement of TMDs, a transport pathway for glutamate and the co-transported ions, as well as the chloride permeation pathway are not immediately obvious. Thus, the challenge in the future will be to integrate the large body of functional work with the structure in order to obtain a clear picture of how glutamate is moved across the membrane. This review has made an attempt to begin with this integration, pointing out similarities between transport mechanisms developed on structural or functional bases.
Perhaps the most important feature of glutamate transporters with respect to their potential as a pharmaceutical target is their ability to reverse transport direction. While progress has been made with regard to our understanding of the biopysical mechanisms underlying the reverse transport process, as well as of its contribution to glutamate release under pathophysiological conditions, the cellular and molecular pathways of this glutamate release remain to be determined. Therefore, it is expected that reverse transport will remain a highly interesting topic for future studies.
Acknowledgements
This work was supported by the National Institutes of Health Grant R01-NS0493 to CG and by the Deutsche Forschungsgemeinschaft Grants GR 1393/2-2,3 to CG and RA 753/1-1,2 to TR.
References
- 1.Danbolt NC. Glutamate uptake. Prog. Neurobiol. 2001;65:101–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 2.Grewer C, Rauen T. Electrogenic Glutamate Transporters in the CNS: Molecular Mechanism, Pre-steady-state Kinetics, and their Impact on Synaptic Signaling. J. Membr. Biol. 2005;203:1–20. doi: 10.1007/s00232-004-0731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem. Int. 2007;51:333–355. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vandenberg RJ. Molecular pharmacology and physiology of glutamate transporters in the central nervous system. Clin. Exp. Pharmacol. Physiol. 1998;25:393–400. doi: 10.1111/j.1440-1681.1998.tb02221.x. [DOI] [PubMed] [Google Scholar]
- 5.Kanai Y, Hediger MA. The glutamate and neutral amino acid transporter family: physiological and pharmacological implications. Eur. J. Pharmacol. 2003;479:237–247. doi: 10.1016/j.ejphar.2003.08.073. [DOI] [PubMed] [Google Scholar]
- 6.Hertz L. Functional interactions between neurons and astrocytes I. Turnover and metabolism of putative amino acid transmitters. Prog. Neurobiol. 1979;13:277–323. doi: 10.1016/0301-0082(79)90018-2. [DOI] [PubMed] [Google Scholar]
- 7.Albrecht J, Sonnewald U, Waagepetersen HS, Schousboe A. Glutamine in the central nervous system: function and dysfunction. Front. Biosci. 2007;12:332–343. doi: 10.2741/2067. [DOI] [PubMed] [Google Scholar]
- 8.Bellocchio EE, Reimer RJ, Fremeau RT, Jr, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–960. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- 9.Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- 10.Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch. Eur. J. Phyiol. 2004;447:465–468. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- 11.Kanner BI, Sharon I. Active transport of L-glutamate by membrane vesicles isolated from rat brain. Biochemistry. 1978;17:3949–3953. doi: 10.1021/bi00612a011. [DOI] [PubMed] [Google Scholar]
- 12.Levy LM, Warr O, Attwell D. Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. J. Neurosci. 1998;18:9620–9628. doi: 10.1523/JNEUROSCI.18-23-09620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owe SG, Marcaggi P, Attwell D. The ionic stoichiometry of the GLAST glutamate transporter in salamander retinal glia. J. Physiol. 2006;577:591–599. doi: 10.1113/jphysiol.2006.116830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383:634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]
- 15.Kanner BI, Bendahan A. Binding order of substrates to the sodium and potassium ion coupled L-glutamic acid transporter from rat brain. Biochemistry. 1982;21:6327–6330. doi: 10.1021/bi00267a044. [DOI] [PubMed] [Google Scholar]
- 16.Wadiche JI, Amara SG, Kavanaugh MP. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995;15:721–728. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 17.Picaud SA, Larsson HP, Grant GB, Lecar H, Werblin FS. Glutamate-gated chloride channel with glutamate-transporter-like properties in cone photoreceptors of the tiger salamander. J. Neurophysiol. 1995;74:1760–1771. doi: 10.1152/jn.1995.74.4.1760. [DOI] [PubMed] [Google Scholar]
- 18.Eliasof S, Jahr CE. Retinal glial cell glutamate transporter is coupled to an anionic conductance. Proc. Natl. Acad. Sci. U S A. 1996;93:4153–4158. doi: 10.1073/pnas.93.9.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eskandari S, Kreman M, Kavanaugh MP, Wright EM, Zampighi GA. Pentameric assembly of a neuronal glutamate transporter. Proc. Natl. Acad. Sci. U S A. 2000;97:8641–8646. doi: 10.1073/pnas.97.15.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grewer C, Balani P, Weidenfeller C, Bartusel T, Tao Z, Rauen T. Individual subunits of the glutamate transporter EAAC1 homotrimer function independently of each other. Biochemistry. 2005;44:11913–11923. doi: 10.1021/bi050987n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leary GP, Stone EF, Holley DC, Kavanaugh MP. The Glutamate and Chloride Permeation Pathways Are Colocalized in Individual Neuronal Glutamate Transporter Subunits. J. Neurosci. 2007;27:2938–2942. doi: 10.1523/JNEUROSCI.4851-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch HP, Lane Brown R, Larsson HP. The Glutamate-Activated Anion Conductance in Excitatory Amino Acid Transporters Is Gated Independently by the Individual Subunits. J. Neurosci. 2007;27:2943–2947. doi: 10.1523/JNEUROSCI.0118-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wadiche JI, Kavanaugh MP. Macroscopic and microscopic properties of a cloned glutamate transporter/chloride channel. J. Neurosci. 1998;18:7650–7661. doi: 10.1523/JNEUROSCI.18-19-07650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melzer N, Biela A, Fahlke C. Glutamate Modifies Ion Conduction and Voltage-dependent Gating of Excitatory Amino Acid Transporter-associated Anion Channels. J. Biol. Chem. 2003;278:50112–50119. doi: 10.1074/jbc.M307990200. [DOI] [PubMed] [Google Scholar]
- 25.Broer A, Wagner C, Lang F, Broer S. Neutral amino acid transporter ASCT2 displays substrate-induced Na+ exchange and a substrate-gated anion conductance. Biochem. J. 2000;346:705–710. [PMC free article] [PubMed] [Google Scholar]
- 26.Grewer C, Grabsch E. New inhibitors for the neutral amino acid transporter ASCT2 reveal its Na+-dependent anion leak. J. Physiol. 2004;557:747–759. doi: 10.1113/jphysiol.2004.062521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan RM, Mindell JA. The uncoupled chloride conductance of a bacterial glutamate transporter homolog. Nat. Struct. Mol. Biol. 2007;14:365–371. doi: 10.1038/nsmb1230. [DOI] [PubMed] [Google Scholar]
- 28.Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- 29.Kanai Y, Hediger MA. Primary Structure and Functional Characterization of a High-Affinity Glutamate Transporter. Nature. 1992;360:467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- 30.Pines G, Danbolt NC, Bjoras M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm Mathisen J, Seeberg E, Kanner BI. Cloning and Expression of a Rat Brain L Glutamate Transporter. Nature. 1992;360:464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- 31.Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc. Natl. Acad. Sci. U S A. 1992;89:10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yernool D, Boudker O, Jin Y, Gouaux E. Structure of a glutamate transporter homologue from pyrococcus horikoshii. Nature. 2004;431:811–818. doi: 10.1038/nature03018. [DOI] [PubMed] [Google Scholar]
- 33.Grunewald M, Kanner BI. The accessibility of a novel reentrant loop of the glutamate transporter GLT-1 is restricted by its substrate. J. Biol. Chem. 2000;275:9684–9689. doi: 10.1074/jbc.275.13.9684. [DOI] [PubMed] [Google Scholar]
- 34.Slotboom DJ, Sobczak I, Konings WN, Lolkema JS. A conserved serine-rich stretch in the glutamate transporter family forms a substrate-sensitive reentrant loop. Proc. Natl. Acad. Sci. U S A. 1999;96:14282–14287. doi: 10.1073/pnas.96.25.14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gendreau S, Voswinkel S, Torres-Salazar D, Lang N, Heidtmann H, Detro-Dassen S, Schmalzing G, Hidalgo P, Fahlke C. A Trimeric Quaternary Structure Is Conserved in Bacterial and Human Glutamate Transporters. J. Biol. Chem. 2004;279:39505–39512. doi: 10.1074/jbc.M408038200. [DOI] [PubMed] [Google Scholar]
- 36.Bendahan A, Armon A, Madani N, Kavanaugh MP, BI, K Arginine 447 plays a pivotal role in substrate interactions in a neuronal glutamate transporter. J. Biol. Chem. 2000;275:37436–37442. doi: 10.1074/jbc.M006536200. [DOI] [PubMed] [Google Scholar]
- 37.Teichman S, Kanner BI. Aspartate-444 Is Essential for Productive Substrate Interactions in a Neuronal Glutamate Transporter. J. Gen. Physiol. 2007;129:527–539. doi: 10.1085/jgp.200609707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koch HP, Larsson HP. Small-Scale Molecular Motions Accomplish Glutamate Uptake in Human Glutamate Transporters. J. Neurosci. 2005;25:1730–1736. doi: 10.1523/JNEUROSCI.4138-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres-Salazar D, Fahlke C. Intersubunit interactions in EAAT4 glutamate transporters. J. Neurosci. 2006;26:7513–7522. doi: 10.1523/JNEUROSCI.4545-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koch HP, Hubbard JM, Larsson HP. Voltage-independent sodium-binding events reported by the 4B–4C loop in the human glutamate transporter EAAT3. J. Biol. Chem. 2007 doi: 10.1074/jbc.M704087200. M704087200. [DOI] [PubMed] [Google Scholar]
- 41.Boudker O, Ryan RM, Yernool D, Shimamoto K, Gouaux E. Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature. 2007;445:387–393. doi: 10.1038/nature05455. [DOI] [PubMed] [Google Scholar]
- 42.Tao Z, Zhang Z, Grewer C. Neutralization of the aspartic acid residue Asp-367, but not Asp-454, inhibits binding of Na+ to the glutamate-free form and cycling of the glutamate transporter EAAC1. J. Biol. Chem. 2006;281:10263–10272. doi: 10.1074/jbc.M510739200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tao Z, Grewer C. Cooperation of the conserved aspartate 439 and bound amino acid substrate is important for high-affinity Na+ binding to the glutamate transporter EAAC1. J. Gen. Physiol. 2007;129:331–344. doi: 10.1085/jgp.200609678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kavanaugh MP, Bendahan A, Zerangue N, Zhang Y, Kanner BI. Mutation of an amino acid residue influencing potassium coupling in the glutamate transporter GLT-1 induces obligate exchange. J.Biol. Chem. 1997;272:1703–1708. doi: 10.1074/jbc.272.3.1703. [DOI] [PubMed] [Google Scholar]
- 45.Zarbiv R, Grunewald M, Kavanaugh MP, Kanner BI. Cysteine scanning of the surroundings of an alkali-ion binding site of the glutamate transporter GLT-1 reveals a conformationally sensitive residue. J. Biol. Chem. 1998;273:14231–14237. doi: 10.1074/jbc.273.23.14231. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Kanner BI. Two serine residues of the glutamate transporter GLT-1 are crucial for coupling the fluxes of sodium and the neurotransmitter. Proc. Natl. Acad. Sci. U S A. 1999;96:1710–1715. doi: 10.1073/pnas.96.4.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosental N, Bendahan A, Kanner BI. Multiple consequences of mutating two conserved beta-bridge forming residues in the translocation cycle of a neuronal glutamate transporter. J. Biol. Chem. 2006;281:27905–27915. doi: 10.1074/jbc.M600331200. [DOI] [PubMed] [Google Scholar]
- 48.Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211:969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- 49.Kanner BI. Gate Movements in Glutamate Transporters. ACS Chem. Biol. 2007;2:163–166. doi: 10.1021/cb700040e. [DOI] [PubMed] [Google Scholar]
- 50.Otis TS, Kavanaugh MP. Isolation of current components and partial reaction cycles in the glial glutamate transporter EAAT2. J Neurosci. 2000;20:2749–2757. doi: 10.1523/JNEUROSCI.20-08-02749.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Auger C, Attwell D. Fast removal of synaptic glutamate by postsynaptic transporters. Neuron. 2000;28:547–558. doi: 10.1016/s0896-6273(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 52.Bergles DE, Tzingounis AV, Jahr CE. Comparison of Coupled and Uncoupled Currents during Glutamate Uptake by GLT-1 Transporters. J. Neurosci. 2002;22:10153–10162. doi: 10.1523/JNEUROSCI.22-23-10153.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watzke N, Bamberg E, Grewer C. Early intermediates in the transport cycle of the neuronal excitatory amino acid carrier EAAC1. J. Gen. Physiol. 2001;117:547–562. doi: 10.1085/jgp.117.6.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mim C, Balani P, Rauen T, Grewer C. The glutamate transporter subtypes EAAT4 and EAATs 1–3 transport glutamate with dramatically different kinetics and voltage dependence but share a common uptake mechanism. J. Gen. Physiol. 2005;126:571–589. doi: 10.1085/jgp.200509365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mim C, Tao Z, Grewer C. Two conformational changes are associated with glutamate translocation by the glutamate transporter EAAC1. Biochemistry. 2007;46:9007–9018. doi: 10.1021/bi7005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shlaifer I, Kanner BI. Conformationally Sensitive Reactivity to Permeant Sulfhydryl Reagents of Cysteine Residues Engineered into Helical Hairpin 1 of the Glutamate Transporter GLT-1. Mol. Pharmacol. 2007;71:1341–1348. doi: 10.1124/mol.106.032607. [DOI] [PubMed] [Google Scholar]
- 57.Brocke L, Bendahan A, Grunewald M, Kanner BI. Proximity of Two Oppositely Oriented Reentrant Loops in the Glutamate Transporter GLT-1 Identified by Paired Cysteine Mutagenesis. J. Biol. Chem. 2002;277:3985–3992. doi: 10.1074/jbc.M107735200. [DOI] [PubMed] [Google Scholar]
- 58.Leighton BH, Seal RP, Watts SD, Skyba MO, Amara SG. Structural Rearrangements at the Translocation Pore of the Human Glutamate Transporter, EAAT1. J. Biol. Chem. 2006;281:29788–29796. doi: 10.1074/jbc.M604991200. [DOI] [PubMed] [Google Scholar]
- 59.Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature. 1990;348:443–446. doi: 10.1038/348443a0. [DOI] [PubMed] [Google Scholar]
- 60.Billups B, Attwell D. Modulation of non-vesicular glutamate release by pH. Nature. 1996;379:171–174. doi: 10.1038/379171a0. [DOI] [PubMed] [Google Scholar]
- 61.Jabaudon D, Scanziani M, Gahwiler BH, Gerber U. Acute decrease in net glutamate uptake during energy deprivation. Proc. Natl. Acad. Sci. U S A. 2000;97:5610–5615. doi: 10.1073/pnas.97.10.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watzke N, Grewer C. The anion conductance of the glutamate transporter EAAC1 depends on the direction of glutamate transport. FEBS Lett. 2001;503:121–125. doi: 10.1016/s0014-5793(01)02715-6. [DOI] [PubMed] [Google Scholar]
- 63.Watzke N, Rauen T, Bamberg E, Grewer C. On the mechanism of proton transport by the neuronal excitatory amino acid carrier 1. J. Gen. Physiol. 2000;116:609–622. doi: 10.1085/jgp.116.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Z, Tao Z, Gameiro A, Barcelona S, Braams S, Rauen T, Grewer C. Transport direction determines the kinetics of substrate transport by the glutamate transporter EAAC1. Proc. Natl. Acad. Sci. U S A. 2007;104:18025–18030. doi: 10.1073/pnas.0704570104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okumoto S, Looger LL, Micheva KD, Reimer RJ, Smith SJ, Frommer WB. Detection of glutamate release from neurons by genetically encoded surface-displayed FRET nanosensors. Proc. Natl. Acad. Sci. U S A. 2005;102:8740–8745. doi: 10.1073/pnas.0503274102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grewer C, Watzke N, Wiessner M, Rauen T. Glutamate translocation of the neuronal glutamate transporter EAAC1 occurs within milliseconds. Proc. Natl. Acad. Sci. U S A. 2000;97:9706–9711. doi: 10.1073/pnas.160170397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hilgemann DW, Lu CC. GAT1 (GABA:Na+:Cl−) cotransport function. Database reconstruction with an alternating access model. J. Gen. Physiol. 1999;114:459–475. doi: 10.1085/jgp.114.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu CC, Hilgemann DW. GAT1 (GABA:Na+:Cl−) cotransport function. Steady state studies in giant Xenopus oocyte membrane patches. J. Gen. Physiol. 1999;114:429–444. doi: 10.1085/jgp.114.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wadiche JI, Arriza JL, Amara SG, Kavanaugh MP. Kinetics of a human glutamate transporter. Neuron. 1995;14:1019–1027. doi: 10.1016/0896-6273(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 70.Otis TS, Jahr CE. Anion currents and predicted glutamate flux through a neuronal glutamate transporter. J. Neurosci. 1998;18:7099–7110. doi: 10.1523/JNEUROSCI.18-18-07099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cleland WW. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim. Biophys. Acta. 1963;1000:213–220. [PubMed] [Google Scholar]
- 72.Nicholls D, Attwell D. The release and uptake of excitatory amino acids. Trends Pharmacol. Sci. 1990;11:462–468. doi: 10.1016/0165-6147(90)90129-v. [DOI] [PubMed] [Google Scholar]
- 73.Dawson LA, Djali S, Gonzales C, Vinegra MA, Zaleska MM. Characterization of transient focal ischemia-induced increases in extracellular glutamate and aspartate in spontaneously hypertensive rats. Brain Res. Bull. 2000;53:767–776. doi: 10.1016/s0361-9230(00)00363-4. [DOI] [PubMed] [Google Scholar]
- 74.Katayama Y, Kawamata T, Tamura T, Hovda DA, Becker DP, Tsubokawa T. Calcium-dependent glutamate release concomitant with massive potassium flux during cerebral ischemia in vivo. Brain Res. 1991;558:136–140. doi: 10.1016/0006-8993(91)90730-j. [DOI] [PubMed] [Google Scholar]
- 75.Parpura V, Scemes E, Spray DC. Mechanisms of glutamate release from astrocytes: gap junction "hemichannels", purinergic receptors and exocytotic release. Neurochem. Int. 2004;45:259–264. doi: 10.1016/j.neuint.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 76.Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat. Neurosci. 2007;10:1377–1386. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- 78.Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J. Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Longuemare MC, Rose CR, Farrell K, Ransom BR, Waxman SG, Swanson RA. K+-induced reversal of astrocyte glutamate uptake is limited by compensatory changes in intracellular Na+ Neuroscience. 1999;93:285–292. doi: 10.1016/s0306-4522(99)00152-9. [DOI] [PubMed] [Google Scholar]
- 80.Ottersen OP, Laake JH, Reichelt W, Haug FM, Torp R. Ischemic disruption of glutamate homeostasis in brain: quantitative immunocytochemical analyses. J. Chem. Neuroanat. 1996;12:1–14. doi: 10.1016/s0891-0618(96)00178-0. [DOI] [PubMed] [Google Scholar]
- 81.Swanson RA. Astrocyte glutamate uptake during chemical hypoxia in vitro. Neurosci. Lett. 1992;147:143–146. doi: 10.1016/0304-3940(92)90580-z. [DOI] [PubMed] [Google Scholar]
- 82.Silver IA, Deas J, Erecinska M. Ion homeostasis in brain cells: differences in intracellular ion responses to energy limitation between cultured neurons and glial cells. Neuroscience. 1997;78:589–601. doi: 10.1016/s0306-4522(96)00600-8. [DOI] [PubMed] [Google Scholar]
- 83.Duffy S, MacVicar BA. In vitro ischemia promotes calcium influx and intracellular calcium release in hippocampal astrocytes. J. Neurosci. 1996;16:71–81. doi: 10.1523/JNEUROSCI.16-01-00071.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown AM, Baltan Tekkok S, Ransom BR. Energy transfer from astrocytes to axons: the role of CNS glycogen. Neurochem. Int. 2004;45:529–536. doi: 10.1016/j.neuint.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 85.Schurr A, Payne RS, Miller JJ, Rigor BM. Brain Lactate Is an Obligatory Aerobic Energy Substrate for Functional Recovery After Hypoxia: Further In Vitro Validation. J. Neurochem. 1997;69:423–426. doi: 10.1046/j.1471-4159.1997.69010423.x. [DOI] [PubMed] [Google Scholar]
- 86.Esslinger CS, Agarwal S, Gerdes J, Wilson PA, Davis ES, Awes AN, O'Brien E, Mavencamp T, Koch HP, Poulsen DJ, Rhoderick JF, Chamberlin AR, Kavanaugh MP, Bridges RJ. The substituted aspartate analogue Lbeta-threo-benzyl-aspartate preferentially inhibits the neuronal excitatory amino acid transporter EAAT3. Neuropharmacology. 2005;49:850–861. doi: 10.1016/j.neuropharm.2005.08.009. [DOI] [PubMed] [Google Scholar]