Abstract
Rats with complete spinal transections are capable of acquiring a simple instrumentally trained response. If rats receive shock to one hindlimb when the limb is extended (controllable shock), the spinal cord will learn to hold the leg in a flexed position that minimizes shock exposure. If shock is delivered irrespective of leg position, subjects do not exhibit an increase in flexion duration and subsequently fail to learn when tested with controllable shock (learning deficit). Just 6 min of variable intermittent shock produces a learning deficit that lasts 24 hrs. Evidence suggests that the neural mechanisms underlying the learning deficit may be related to those involved in other instances of spinal plasticity (e.g., wind-up, long-term potentiation). The present paper begins to explore these relations by demonstrating that direct stimulation of the sciatic nerve also impairs instrumental learning. Six minutes of electrical stimulation (mono- or biphasic direct current [DC]) of the sciatic nerve in spinally transected rats produced a voltage-dependent learning deficit that persisted for 24 hr (Experiments 1–2) and was dependent on C-fiber activation (Experiment 7). Exposure to continuous stimulation did not produce a deficit, but intermittent burst or single pulse (as short as 0.1 ms) stimulation (delivered at a frequency of 0.5 Hz) did, irrespective of the pattern (fixed or variable) of stimulus delivery (Experiments 3–6, 8). When the duration of stimulation was extended from 6 to 30 min, a surprising result emerged; shocks applied in a random (variable) fashion impaired subsequent learning whereas shocks given in a regular pattern (fixed spacing) did not (Experiments 9–10). The results imply that spinal neurons are sensitive to temporal relations and that stimulation at regular intervals can have a restorative effect.
Keywords: plasticity, spinal cord, instrumental learning, operant, timing
The processing of afferent signals can result in behavioral modifications, even in the compromised nervous system. For instance, when the connections between the brain and spinal cord are severed, animals reacquire the motor skills necessary to step over a moving treadmill (Edgerton et al., 1997). Moreover, once a high degree of stepping proficiency is achieved, subjects can modify their stepping behavior to reduce the disruptive impact of perturbing forces on the step cycle (Heng and de Leon, 2007; Hodgson et al., 1994). Research has also shown that the spinal cord contains the circuitry needed to support a variety of learning phenomena, including habituation, sensitization, classical conditioning, and instrumental learning (e.g., Beggs et al., 1985; Durkovic, 1975; Joynes and Grau, 1996; Grau et al., 1998; Groves and Thompson, 1970; Segal and Wolf, 1994; Spencer et al., 1966; Wolpaw et al., 1991).
Over the past decade our laboratory has been examining the conditions under which spinally-mediated instrumental learning occurs (for a recent review, see Grau et al., 2006). To explore this type of plasticity, we utilize a simple learning procedure refined by Grau et al. (1998) in which rats with complete spinal transections are administered shock to one hindlimb whenever that leg is extended (controllable shock). Subjects in this condition (master) learn to maintain their leg in a flexed position that minimizes net shock exposure. A second group of subjects is experimentally coupled (yoked) to the master rats and receives stimulation at the same time and intensity, but independent of leg position (uncontrollable shock). Yoked rats do not exhibit an increase in flexion duration during training and later fail to learn when tested with controllable shock, a learning deficit that is reminiscent of learned helplessness (Maier and Seligman, 1976; Overmier and Seligman, 1967; Seligman and Maier, 1967). Uncontrollable legshock applied to one hind leg has a general, lasting, effect that inhibits learning for up to 48 hr when subjects are tested on the contralateral leg (Crown et al., 2002a).
To further explore the mechanisms that underlie the learning deficit, Crown et al. (2002a) developed a computer program that emulated the distribution of shocks produced by a typical master subject. Using this program, they found that 80 ms alternating current (AC) legshocks applied on a varying interstimulus interval (ISI; 0.2–3.8 s) produced a lasting deficit. Intermittent tailshock also produced a long-term impairment and has been shown to disrupt recovery after a contusion injury to the spinal cord (Grau et al., 2004). Because AC tailshock can be applied through cutaneous electrodes without inducing tissue damage, we have used it to investigate the neurobiological mechanisms that underlie the learning deficit (Ferguson et al., 2006; Ferguson et al., 2003; Joynes et al., 2003; Joynes and Grau, 2004; Patton et al., 2004).
Our current technique, however, has some limitations that stem from our use of constant current, AC, shocks. Although this type of shock has been widely used to examine the behavioral consequences of nociceptive stimulation in intact subjects (Fanselow, 1986; Grau et al., 1981; Maier, 1989; Meagher et al., 2001; Terman et al., 1984; Watkins and Mayer, 1986), physiological studies have routinely used direct current (DC) stimulation (Bliss and Lomo, 1973; Douglas and Goddard, 1975; Toth et al., 2000). DC stimulation has a number of advantages, including the fact that it can be produced with a rapid onset/offset (square wave) that allows quantification of key parameters (e.g., conduction velocity). Further, an extensive literature exists demonstrating that DC stimulation of selective nerves induces alterations in spinal processing (Azuke et al., 2003; Liu et al., 1998; Liu and Sandkühler, 1995, 1997; Mendell, 1966; Schouenborg, 1984; Schouenborg and Sjölund, 1983). For example, low frequency stimulation at an intensity that engages pain (C) fibers causes a progressive increase in the action potential output from dorsal horn neurons, a phenomenon known as wind-up (Mendell, 1966; Herrero et al., 2000; Schouenborg, 1984; Wall and Wolf, 1984, 1986; Woolf and King, 1987). More recently, researchers have shown that DC stimulation can induce both long-term potentiation (LTP) and depression (LTD) within the spinal cord (Liu et al., 1998; Liu and Sandkühler, 1995, 1997; Randić et al., 1993).
Work from other laboratories has explored the impact of environmental stimulation on spinal function using DC stimulation of the sciatic nerve (e.g., Liu and Sandkühler, 1995, 1997; Mendell, 1966; Schouenborg, 1984; Schouenborg and Sjölund, 1983; Wall and Woolf, 1986; Woolf and King, 1987). Here we employ similar methods and show that electrophysiological DC stimulation of the sciatic nerve in spinally transected rats also has a lasting effect on instrumental learning. Six min of DC stimulation (mono- or biphasic; see Figure 1) at a voltage that engages C-fibers induced a robust learning deficit. Parametric manipulations revealed that just 180, 0.5 ms, pulses produced the deficit and that this effect was most robust when the stimuli were presented at 0.5 Hz. Surprisingly, when pulse number was increased, a deficit was only observed when the interval between pulses varied; however, when the interval between shocks was kept constant (fixed), no deficit was observed.
Figure 1.

Experimental manipulations for Experiments 1, 3,4, and 5. A) Exp. 1a explored the impact of biphasic DC stimulation on instrumental learning. Independent groups were administered 6 min of intermittent DC simulation to the sciatic nerve at 0, 20, 30, or 40 V (100 Hz, 80 ms burst duration, 10 ms pulse width). Following sciatic stimulation subjects received 30 min of testing in which their capacity to acquire an instrumentally trained leg flexion response was assessed. B) Exp. 1b explored the effect of monophasic DC stimulation on instrumental learning. Subjects were administered 6 min of intermittent DC stimulation to the sciatic nerve at 0, 20, 30, or 40 V (100 Hz, 80 ms burst duration, 10 ms pulse width) prior to instrumental testing. Following stimulation subjects received 30 min of testing. C) Exp. 3 assessed the impact of continuous vs. intermittent sciatic stimulation on instrumental learning. Subjects received 360 s of intermittent or 3.6, 14.4 s or 360 s of continuous stimulation prior to instrumental testing. D) Exp. 4 was designed to determine the number of pulses per burst required to induce a learning deficit. Subjects received 6 min of intermittent sciatic stimulation (40V, 100 Hz, 10 ms pulse width) in which the bursts were 0, 20 (1 pulse), or 80 ms (4–5 pulses) in duration. Following stimulation subjects received 30 min of instrumental testing. E) Exp. 5 explored whether stimulation must occur on a variable ISI in order to induce the learning deficit. Subjects received 6 min of intermittent sciatic stimulation (40V, 100Hz, 20 ms burst duration, 10 ms pulse width) on a fixed (2 s) or variable (range = 0.2–3.8, M=2 s) ISI. Following stimulation subjects received 30 min of instrumental testing.
EXPERIMENTAL PROCECDURES
Animals
Subjects were male Sprague-Dawley rats obtained from Harlan (Houston, TX). Rats were 70–90 days old and weighed 350–400g at the time of spinal cord transection. They were housed in pairs with free access to food and water, and were maintained on a 12–12 hr light-dark cycle. All experiments were carried out in accordance with NIH standards for the care and use of laboratory animals (NIH publications No. 80-23), and were approved by the University Laboratory Animal Care Committee at Texas A&M University. Every effort was made to minimize suffering and limit the number of animals used.
Spinalization Surgery
Prior to surgery, the fur over the thoracic portion of the vertebral column was shaved and disinfected with betadine solution. Rats were anesthetized with isoflurane gas. The rat’s head was rendered immobile in a stereotaxic apparatus with a small (5 × 4 × 2.5 cm) gauze pillow under the subject’s chest. An anterior to posterior incision over the second thoracic vertebrae (T2) was made, the tissue just rostral to T2 was cleared using rongeurs, and the cord was exposed and cauterized. The remaining gap in the cord was filled with Gelfoam (Pharmacia Corp., Kalamazoo, MI) and the wound was closed with Michel clips (Fisher Scientific, Waltham, MA). Following closure of the wound, the surface of each leg was shaved for electrode placement. Intraperitoneal injections (3 mL) of 0.9% saline solution were administered postoperatively to prevent dehydration. Following surgery, rats were placed in a temperature-controlled environment (25.5 °C) and monitored until awake. All rats were checked every 6 to 8 hr during the 18–24 hr post-surgical period. During this time, hydration was maintained with supplemental injections of saline, and the rats’ bladders and colons were expressed as necessary.
Spinal transections were confirmed by inspecting the cord under a 10× dissection scope, and observing the behavior of the subjects after they recovered to ensure they exhibited paralysis below the level of the forepaws and did not exhibit any supraspinally-mediated pain responses to legshock.
Sciatic Nerve Exposure and Stimulation
Twenty-four hours following surgery, spinalized subjects were placed in a restraining tube with their rear legs exposed. Their legs were positioned so that they were lying flat and extended away from their body. An incision was made on the lateral surface of the leg (counterbalanced) to expose the biceps femoris and vastus lateralus muscles. These muscle groups were dissected away, exposing the sciatic nerve within the popliteal fossa. Bipolar hook electrodes were then placed around the sciatic nerve, with the electrodes 5 mm apart. A test pulse was delivered from the stimulator (model S9; Grass Medical Instruments, Quincy, MA.) to ensure contact between the nerve and electrodes. Once the electrodes were in place, the appropriate stimulation treatment was administered. Warm mineral oil was applied, as needed, to prevent dehydration of the exposed nerve. Immediately following sciatic nerve stimulation, the leg was closed with Michel clips and the subject was prepared for instrumental testing.
Instrumental Testing
The apparatus used was similar to that described by Grau et al. (1998). Briefly, during instrumental training, all subjects were loosely restrained in Plexiglas tubes, with their hindlimbs suspended above a saline solution contained in a rectangular plastic dish (11.5 cm [w] × 19 cm [l] × 5 cm [d]) positioned 7.5 cm below the restraining tube. Holes were drilled into the anterior portion of the tubes to allow for ventilation. Two slots were cut 4 cm apart and 1.5 cm from the posterior end of the tube to allow both hind legs to hang freely. To monitor leg position, a stainless steel rod (7 cm [l], 0.46 mm [w]) was attached to the pad of one foot (contact electrode) extending past the toes. The contact electrode was attached to the plantar surface of the rat’s foot with porous tape (Orthaletic, 1.3 cm [width]; Johnson and Johnson, New Brunswick, NJ), with one end positioned directly in front of the plantar protuberance. Heat-shrink tubing electrically insulated the rod from the paw. A fine wire (0.01 mm [36 AWG], magnet wire single beldsol) was attached to the end of the rod at a point under the insulation. This wire extended from the rear of the foot and was connected to a digital input board that was monitored by a Macintosh G4 computer. To minimize lateral leg movements, a piece of porous tape (Orthaletic, 1.3 cm [width]) was wrapped around the leg above the tarsus and attached under the front panel of the restraining tube.
Two electrodes were then inserted into one hindleg. The first electrode was constructed from stainless steel wire (0.05 mm [30 AWG]) and was inserted through the skin over the tibia 1.5 cm from the tarsus. The second was made of fine wire (0.01 mm [36 AWG], magnet wire single beldsol) and was inserted perpendicular to the leg, through the body of the tibialis anterior muscle 1.7 cm above the first electrode. Legshock was applied by attaching one lead from a constant current AC shock generator (Model SG-903; BRS/LVE, Laurel MD) to the electrode inserted into the tibialis anterior muscle. The second lead was attached to the wire implanted in the skin over the tibia. Shock (60 Hz, AC) intensity was adjusted for each subject to a level that produced a 0.4 Newton (N) flexion response. This value was determined prior to instrumental training by looping a monofilament plastic line (“6 lb.” test strength; Du Pont, Wilmington DE) around the rat’s ankle. The end of the line was attached to a strain gauge (Fort-1000; World Precision Instruments, New Haven, CT) and fastened to a ringstand. The strain gauge output was fed through a calibrated mutltimeter that allowed for a conversion from voltage to force in N. To determine the necessary flexion force a single 300 ms shock was applied to the leg and the shock intensity was adjusted to elicit the prescribed flexion force. After flexion force was set, the monofilament line was removed from the rat’s paw and the saline solution was adjusted so that the contact electrode sat 4 mm beneath the surface of the salt solution. Once each animal was prepared, the 30 min instrumental testing session began. Whenever the subject’s leg was in the down position, the end of the rod contacted the saline solution and completed an electrical circuit. When the circuit was closed, shock was delivered to the tibialis anterior muscle, which elicited a flexion response. The flexion response raised the contact electrode out of the saline solution, which broke the circuit and terminated the shock.
Measures of Instrumental Learning
Training and testing sessions were divided into 30, 1-min bins to examine learning across time. Response number and response duration were collected by the computer during these sessions, and were separately averaged across each 1-min bin. Every time the contact electrode left the solution, the number of responses was increased by one. The computer also recorded the amount of time the electrode remained out of the solution. Response duration served as the primary measure of learning and was calculated for each 1-min bin using the equation: response duration = (time out of solution) ÷ (response number + 1).
Statistics
Response duration was analyzed using a mixed-design analysis of variance (ANOVA) coupled with trend analyses to evaluate the impact of parametric variables over time (e.g., pulse number). Where appropriate, Tukey’s Honestly Significant Difference (HSD) was used to conduct post hoc analyses. In all cases P < .05 was used to determine statistical significance.
Measures of Baseline Behavioral Reactivity
Prior work has shown that the administration of shock, irrespective of controllability, causes a limb-specific decrease in response vigor (e.g., habituation) that grows as a function of shock duration/intensity (Grau et al., 1998). To insure that any learning deficit observed was not caused by the inability of shock to elicit the target flexion response, and to assure that our experimental procedures did not affect that shock-elicited flexion response on the test leg, we analyzed the amount of stimulation required to produce a 0.4 N flexion force and subjects’ initial flexion durations. ANOVAs performed on data obtained in Experiments 1–6 and 9 did not produce any significant effects of prior shock treatment on baseline behavioral reactivity, all Fs < 3.14, P > .05. However, analysis of initial flexion durations from Experiments 8 and 10 did reveal significant effects based on group assignment, all Fs > 3.56, P < .05. In Experiment 8 we found that subjects in the 0 Hz stimulation condition had significantly shorter initial response durations than subjects in the 0.05 Hz condition. In Experiment 10, subjects in the 180-pulse condition had significantly shorter initial response durations than subjects in the 900-pulse condition. To verify that these differences did not contribute to our results, we reanalyzed the response duration data (our primary dependent measure) using an analysis of covariance (ANCOVA), entering initial flexion duration as a cofactor. This covariate did not account for a significant proportion of the variance, all Fs > 1.00, p < .05. More importantly, including the covariate did not alter the profile of statistical significance obtained across our independent variables. For these reasons, we do not provide further analyses of the flexion force and initial response duration values, and use an ANOVA to analyze response duration over time.
Measures of Tactile Reactivity
To determine if sciatic stimulation produced any changes in mechanical reactivity, thresholds were assessed using von Frey filaments (Stoelting, Wood Dale, IL). Mechanical sensitivity was determined by stimulating the mid-plantar surface of each hindpaw in an ascending order until a flexion response was elicited. Stimuli were presented twice to each paw in an ABBA counterbalanced fashion (A = left, B = right), with testing on the same leg separated by a 2 min interval. Testing occurred in the instrumental testing apparatus described earlier, and occurred at three time points: prior to sciatic nerve exposure, following nerve exposure, and following nerve stimulation (or an equivalent period of restraint). Filament thickness/force was related to behavior using the transformation provided by the manufacturer: Intensity = log10 (10,000 × g). This transformation yields a scale that is approximately linear and amendable to parametric analyses. We found that nerve exposure, per se, produced no change in mechanical reactivity on either the ipsilateral (mean = 0.05, S.E. = 0.03) or contralateral (mean = 0.05, S.E. = 0.03) leg all Fs < 1.13, P > .05. Electrical stimulation generally reduced mechanical reactivity on the ipsilateral leg (Figure 2; all Fs > 8.56, P < .05). However, in no case, did sciatic stimulation impact behavioral reactivity on the contralateral leg (unshocked: mean = 0.06 ± 0.05; shocked: mean = 0.12 ± 0.08; all Fs < 1.95, P > .05). The reduction in behavioral reactivity observed on the stimulated leg is presumably related to the shock-induced habituation reported in earlier studies (Grau et al., 1998), and will be discussed further in the General Discussion. More importantly, there was no evidence that sciatic stimulation affected motor reactivity when tactile stimulation was applied to the test (contralateral) leg, and consequently, no further analyses of these data are reported.
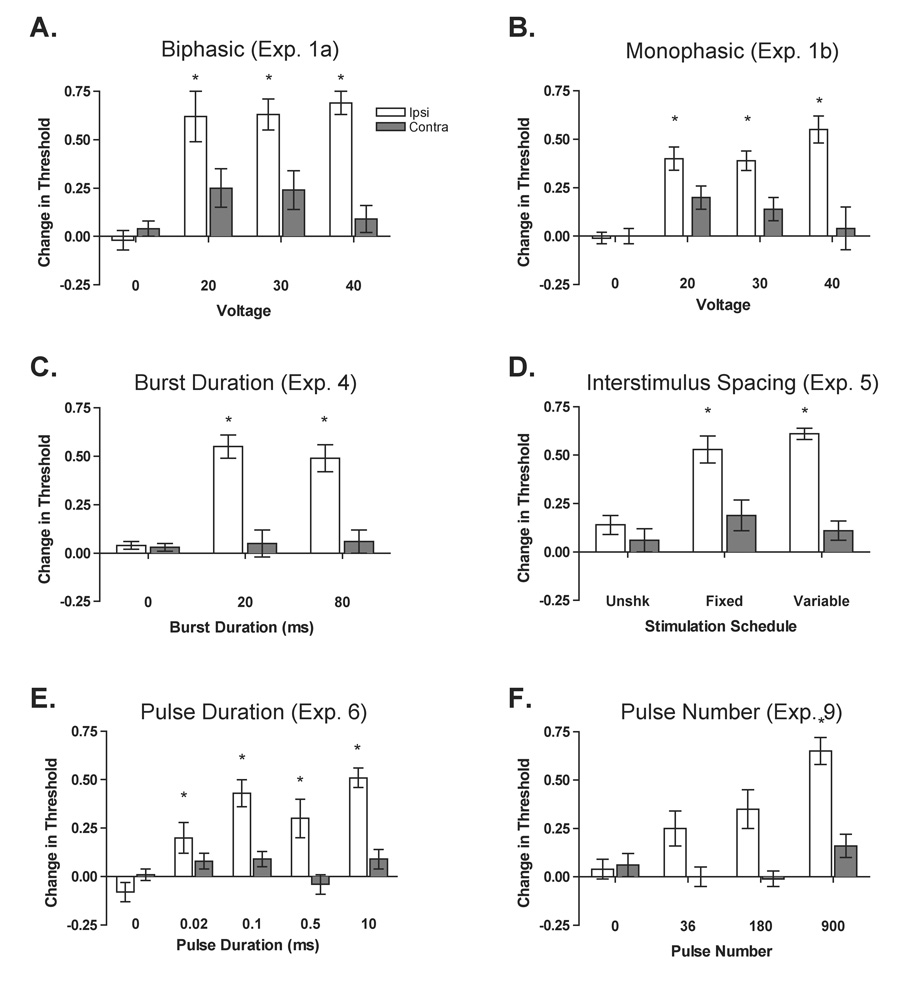
Figure 2.
The effect of sciatic stimulation on mechanical thresholds. Change from baseline scores were computed and used as our index of mechanical sensitivity. Representative data are presented from experiments examining biphasic stimulation (A), monophasic stimulation (B), burst duration (C), interstimulus spacing (D), pulse duration (E), and pulse number (F). Stimulation of the sciatic nerve increased response thresholds on the ipsilateral leg (all Fs > 8.56, P < .05) but had a minimal effect on response thresholds taken from the contralateral leg (all Fs < 1.95, P > .05). Asterisks indicate conditions with significant change from baseline scores on the same leg (P < .05), and error bars indicate ± S.E.
RESULTS
Experiment 1a: Biphasic sciatic stimulation impairs spinal learning
Previous work has shown that intermittent (80 ms bursts given on a variable ISI [range = 0.2–3.8 s; mean ISI = 2 s]) cutaneous or intramuscular AC (60Hz) stimulation is sufficient to produce a long-lasting learning deficit (Crown et al., 2002a). To determine if a learning deficit could be produced using biphasic, DC, stimulation, subjects had their sciatic nerves exposed, followed by placement of bipolar hook electrodes as described above. We chose biphasic stimulation to maintain congruency with our earlier work that has used biphasic AC shocks. To determine the minimum intensity required to induce a learning deficit, subjects received 0, 20, 30, or 40 V (50 Hz, 80 ms burst duration, 10 ms pulse width) of biphasic, DC stimulation on a variable ISI (0.2–3.8 s; mean = 2 s) for a total of 6 min (see Crown et al., 2002a). Subjects in the 0 V condition had electrodes placed around the sciatic nerve but received no stimulation (see Figure 1a). The highest voltage was chosen based on the observation that 40 V of sciatic nerve stimulation produces C-fiber evoked LTP (Liu and Sandkühler, 1995, 1997). After sciatic stimulation, rats were placed in the instrumental apparatus, and were tested with 30 min of controllable AC shock on the contralateral leg (n=6 per condition).
Sciatic stimulation had a voltage dependent effect on learning (Figure 3). Subjects that received 0 or 20V of sciatic stimulation exhibited a progressive increase in response duration. Subjects that received 30V of stimulation exhibited an intermediate amount of learning, while those that received 40V of stimulation failed. An ANOVA revealed significant main effects of Voltage, F(3, 20) = 6.59, P < .01, and Trials, F(29, 580) = 6.93, P < .001. No other effects approached significance, F(87, 580) = 1.22, P > .05. Subsequent post hoc comparisons of the group means revealed that subjects that received 40V of sciatic stimulation had significantly shorter flexion durations relative to subjects in the 0 and 20 V conditions (P < .05).
Figure 3.
The effect of biphasic DC sciatic stimulation on instrumental performance. Subjects had their sciatic nerves exposed and isolated in the popliteal fossa. Once electrodes were placed on the sciatic nerve, subjects received 6 min of sciatic biphasic DC stimulation at 0 (open squares), 20 (closed triangles), 30 (closed circles), or 40 V (closed diamonds). Immediately following stimulation, subjects’ legs were prepared for 30 min of instrumental testing. The upper panel depicts response durations across time (A) and collapsed across trials (B). The lower panel shows the number of responses made during the testing session over time (C) and collapsed across trials (D). Asterisks indicate groups that were significantly different from the 0 V condition (P < .05) and error bars indicate ± S.E. (n=6 subjects per group).
The number of responses made over the 30 min testing session is illustrated in Figure 3. As observed in prior studies (Baumbauer et al., 2007a, b; Crown et al., 2002a; Grau et al., 1998; Joynes et al., 2003), subjects that exhibited an increase in flexion duration made fewer responses. Thus, subjects that received 0 or 20 V of stimulation responded less than subjects that received 40 V of stimulation. An ANOVA yielded significant main effects of Voltage, F(3, 20) = 3.57, P < .05, and Trials F(29, 580) = 4.08, P < .001, as well as a significant Voltage × Trials interaction, F(87, 580) = 1.62, P < .01. Post hoc comparisons of the group means confirmed that subjects in the 0 and 20 V conditions made significantly fewer responses when compared to subjects in the 40 V condition. Subjects in the 30 V condition were not significantly different from any other group.
Here, as in prior studies, shocked rats failed to learn (did not exhibit an increase in response duration) and this was accompanied by an increase in response number. Thus, previously shocked subjects repeatedly experienced the response-outcome relation, but this did not produce an increase in response duration (our index of learning). This observation, in conjunction with our measures of baseline behavioral reactivity (tactile reactivity, the shock intensity needed to elicit a 0.4 N flexion response, and the duration of the first flexion response), all suggest that the impaired learning does not reflect an inability to produce the target response. Importantly, a similar pattern was observed in each of the subsequent experiments; subjects that failed to learn generally exhibited more responses. Because a similar pattern was observed across experiments, we focus on our primary measure of learning, response duration. Doing so allows us to side-step a number of interpretative problems that plagued earlier studies (Buerger et al., 1981; Church, 1964, 1989; Grau et al., 1998).
Experiment 1b: Monophasic sciatic stimulation impairs spinal learning
Although we have typically employed biphasic AC stimulation to examine spinally-mediated learning, electrophysiological analyses (e.g., LTP, LTD, wind-up) typically use monophasic DC stimulation. To determine if monophasic stimulation differentially affected learning, subjects received 0, 20, 30, or 40 V of monophasic sciatic stimulation as described in Experiment 1a (see Figure 1b). Following sciatic stimulation subjects were tested for instrumental learning (n=6 per condition).
As is shown in Figure 4, monophasic sciatic stimulation had a voltage-dependent effect on instrumental learning. Subjects that received 0 or 20 V of stimulation learned to maintain their legs in a flexed position, while subjects that received 30 V exhibited an intermediate amount of learning. Rats that received 40 V of stimulation exhibited a learning deficit. An ANOVA revealed significant main effects of Voltage, F(3, 20) = 7.70, P = .001, and Trials, F(29, 580) = 4.44, P < .001, as well as a significant Voltage × Trials interaction, F(87, 580) = 1.59, P = .001. Post hoc comparisons of the group means confirmed that subjects in the 0 V condition had significantly longer flexion durations than subjects that received 30 or 40 V of sciatic stimulation (P < .05). Subjects in the 20 V condition also had longer flexion durations than subjects that received 30 or 40 V of stimulation, and did not differ from subjects in the 0 V condition. Moreover, subjects that received 30 V of stimulation had longer flexion durations than subjects who received 40 V of stimulation (P < .05).
Figure 4.
The impact of monophasic DC sciatic stimulation on instrumental performance. Following sciatic nerve exposure and isolation, subjects received 6 min of sciatic monophasic DC stimulation at 0 (open squares), 20 (closed triangles), 30 (closed circles), or 40 V (closed diamonds). Immediately following stimulation, subjects’ legs were prepared for 30 min of instrumental testing. Panel A depicts response durations across time and panel B shows average response duration collapsed across trials. Asterisks indicate groups that were significantly different from the 0 V condition (P < .05) and error bars indicate ± S.E. (n=6 subjects per group).
Experiment 2: Sciatic nerve stimulation undermines spinal learning for 24 hr
Crown et al. (2002a) previously demonstrated that the learning deficit observed after 6 min of tail stimulation persists up to 24 hr. To determine if sciatic stimulation has a similar long-lasting effect, subjects received 6 min of 40 V DC pulse trains (50 Hz, 80 ms burst duration, 10 ms pulse width) on a variable ISI (or no shock) and were tested 24 hr later (n=6 per condition).
Twenty-four hours following stimulation, shocked rats exhibited a learning deficit while unshocked rats did not (Figure 5). An ANOVA yielded significant main effects of Shock Condition, F(1, 10) = 110.26, P < .001, and Trials, F(29, 290) = 2.98, P < .001, as well as a significant Shock Condition × Trials interaction, F(29, 290) = 3.04, P < .001, confirming that unshocked rats were capable of learning while shocked rats were not.
Figure 5.
The impact of sciatic stimulation on instrumental performance 24 hr following shock treatment. Subjects received 0 (Unshocked; open squares) or 6 min (Shocked; closed squares) of intermittent sciatic stimulation, followed by instrumental testing 24 hr later. Panel A depicts response durations across time and panel B shows the average response duration collapsed across trials. The asterisk indicates a significant difference when compared to the unshocked condition (P < .05) and error bars indicate ± S.E. (n=6 subjects per group).
Experiment 3: Intermittent, but not continuous, stimulation undermines learning
Crown et al. (2002a) previously demonstrated that intermittent, but not continuous, AC shock is capable of inducing the learning deficit. However, it is not known whether sciatic stimulation must occur in an intermittent fashion in order to produce a learning deficit. To examine this issue, one group of subjects received 6 min of intermittent stimulation in which 40 V, DC pulse trains (50 Hz, 80 ms burst duration, 10 ms pulse width) were delivered on a variable ISI. Separate groups of animals received continuous stimulation in which the temporal gaps between successive stimuli were removed. Subjects in the continuous stimulation conditions received 3.6 (180 pulses), 14.4 (total duration of shock delivered in the intermittent condition [180 pulses × 80 ms]), or 360 s (total duration of stimulation session) of 40 V (50 Hz), DC pulses (see Figure 1c). Following the stimulation period, subjects were tested as described earlier (n=8 per condition).
As usual, previously unshocked subjects exhibited a progressive increase in response duration (Figure 6). Prior exposure to intermittent, but not continuous, shock disrupted learning. An ANOVA yielded significant main effects of Stimulation Condition, F(4,35) = 7.59, P < .001, and Trials, F(29, 1015) = 17.81, P < .001, as well as a significant Stimulation Condition × Trials interaction, F(116, 1015) = 1.49, P < .01. Post hoc comparisons of the group means indicated that subjects in the intermittent shock condition had significantly shorter flexion durations than unshocked subjects, as well as subjects that received 14.4 or 360 s of continuous shock (P < .05). No other comparisons approached significance.
Figure 6.
The effect of continuous or intermittent sciatic stimulation on instrumental performance. Subjects received 6 min of intermittent sciatic stimulation (Int.; closed diamonds), no stimulation (0 s; open squares), or 3.6 (closed, downward triangles), 14.4 (closed circles) or 360 s (closed, upward triangles) of continuous sciatic stimulation. Following the stimulation period subjects received 30 min of instrumental testing. Panel A depicts response durations across time and panel B shows average response duration collapsed across trials. Asterisks indicate groups that were significantly different from the unshocked condition (P < .05) and error bars indicate ± S.E. (n=8 subjects per group).
Experiment 4: Pulse and burst stimulation undermine spinal learning
Our earlier experiments have used 80 ms bursts of stimulation to maintain consistency with prior work using AC shock (Crown et al., 2002a, b; Grau et al., 1998; Joynes and Grau, 2004). An 80 ms burst allows for approximately 5 AC (60 Hz) oscillations or 4–5 DC (50 Hz) pulses. While we have controlled our stimulus parameters to produce a similar number of “pulses” across our experiments, other work suggests that the consequences of stimulation may depend on the number of pulses delivered within each burst. For example, both burst stimulation and single pulses elicit the release of substance P from dorsal horn neurons. However, the release of brain-derived neurotrophic factor (BDNF) is only observed following burst stimulation (Lever et al., 2001). Both BDNF and substance P have been shown to play a role in learning and the learning deficit, respectively (Baumbauer et al., 2007b; Gómez-Pinilla et al., 2007). Given that single pulses versus burst stimulation can have distinct neurochemical consequences, we examined whether intermittent stimulation with single pulses would produce a learning deficit. Subjects were administered 0, 20, or 80 ms of intermittent DC bursts (see Figure 1d). Using a recording oscilloscope (TDS1001; Tektronics Inc., Beaverton, OR), we verified that 20 ms of stimulation produced a single pulse and that 80 ms of stimulation produced a minimum of 4 pulses (at 50 Hz). Subjects in the 20 and 80 ms burst duration conditions received 6 min of 40 V DC stimulation on a variable ISI. Following the stimulation period, subjects had their legs prepared and were tested as described earlier (n=6 per condition).
Subjects’ response durations are depicted in Figure 7. Subjects in the 0 ms condition were capable of maintaining a prolonged flexion response, while subjects that received intermittent stimulation in 20 or 80 ms bursts were not. An ANOVA yielded a significant main effect of Trials, F(29, 435) = 3.27, P < .001, as well as a significant Burst Duration × Trials interaction, F(58, 435) = 2.17, P < .001, confirming that subjects in the 0 ms condition had significantly longer flexion durations over the course of testing than subjects in the 20 ms and 80 ms conditions. No other effects approached significance, F(2, 15) = 2.21, P > .05.
Figure 7.
The impact of burst duration on instrumental performance. Subjects received 6 min of intermittent DC stimulation delivered in bursts of 0 (open squares), 20 (closed circles), or 80 ms (closed diamonds). The 20 ms burst duration was sufficient to allow one pulse to be delivered to the sciatic nerve, while the 80 ms pulse was sufficient to allow four to five. Following sciatic stimulation subjects had their legs prepared for 30 min of instrumental testing. Panel A depicts response durations across time and panel B shows average response duration collapsed across trials. Asterisks indicate groups that were significantly different from the 0 ms burst condition (P < .05) and error bars indicate ± S.E. (n=6 subjects per group).
Experiment 5: Six min of fixed or variable stimulation produces a learning deficit
Prior work examining the nature of the learning deficit has used variable ISIs that emulate the pattern of responding in the master-yoke paradigm. Because this pattern of stimulation yields a robust learning deficit, we have incorporated it into our usual experimental procedures. While we have tacitly assumed that variable stimulation is required, others investigating windup and spinal LTP typically employ fixed ISIs (Li et al., 1999; Liu and Sandkühler, 1995, 1997; Randić et al., 1993; Woolf and Thompson, 1991). The present experiment was designed to determine whether the pattern of stimulus delivery (variable versus fixed) influences how stimulation affects spinal learning. Subjects in the variable shock condition received 6 min of 40 V DC pulse trains (50 Hz, 20 ms burst duration, 10 ms pulse width) on a variable ISI (range = 0.2–3.8 s, mean = 2 s). Subjects in the fixed spacing condition received the same type of stimulation, but pulse trains were administered on a fixed ISI (2 s). Importantly, subjects in both conditions received the same number of pulses, only their distribution across time was manipulated (see Figure 1e). Following the stimulation period subjects were tested as described earlier (n=10 per condition).
Sciatic stimulation produced a learning deficit independent of whether the spacing between shocks was variable or fixed (Figure 8). An ANOVA revealed significant main effects of Stimulation Condition, F(2, 27) = 9.58, P < .05, and Trials, F(29, 783) = 7.66, P < .05, as well as a significant Stimulation Condition × Trials interaction, F(58, 783) = 1.93, P < .001. Post hoc comparisons of the group means showed that unshocked controls had significantly longer flexion durations than subjects in the variable and fixed spacing conditions (P < .05). No other comparisons approached significance.
Figure 8.
The impact of variable or fixed sciatic stimulation on instrumental performance. Subjects received 6 min of intermittent sciatic stimulation in which bursts were delivered on a fixed or variable ISI. Subjects in the fixed ISI condition received pulses every 2 s while subjects in the variable ISI condition received pulses every 2 s, on average. Once sciatic stimulation was completed, subjects were assessed for instrumental performance. Panel A depicts response durations across time and panel B shows average response duration collapsed across trials for unshocked subjects (Unshk; open squares), as well as subjects that received stimulation on a fixed (closed triangles) or variable (closed circles) ISI. Asterisks indicate groups that were significantly different from the unshocked condition (p < .05) and error bars indicate ± S.E. (n=10 subjects per group).
Experiment 6: Stimulation employing short pulse durations (> 0.02 ms) undermines subsequent performance
The previous experiments have demonstrated that just 6 min of single, 40 V, 10 ms pulses of intermittent sciatic stimulation impaired subsequent learning. Our next experiment determined the minimum pulse duration needed to produce a learning deficit. We have used 10 ms pulse durations because it mirrors the pulse width of stimuli used in our prior studies (Joynes et al., 2003; Joynes and Grau, 2004; Grau et al., 1998; Crown et al., 2002a). However, pulse durations of just 0.5 ms can induce spinal LTP, allodynia, and central sensitization (Li et al., 1999; Liu and Sandkühler 1995, 1997). To determine whether shorter pulse durations were effective in our paradigm, subjects received 6 min of 40 V DC pulses on a fixed ISI with pulse duration set to one of 5 values: 0.0, 0.02, 0.1, 0.5, or 10.0 ms. Following the stimulation period, subjects were tested as described earlier (n=10 per condition).
Subjects in the 0.0 and 0.02 ms conditions acquired the instrumental response while all other subjects failed to do so (Figure 9). An ANOVA revealed significant main effects of Pulse Duration, F(4, 45) = 5.04, P < .01, and Trials, F(29, 1305) = 15.10, P < .001, as well as a significant Pulse Duration × Trials interaction, F(116, 1305) = 1.34, P < .05. Post hoc comparisons of group means demonstrated that subjects in the 0.0 and 0.02 ms conditions had significantly longer flexion durations than all other subjects (P < .05). The 0.5 ms pulse duration was utilized in subsequent experiments because it yielded a robust effect and has been used in other studies (Li et al., 1999; Liu and Sandkühler, 1995, 1997; Wall and Woolf, 1986).
Figure 9.
The impact of pulse duration on instrumental performance. Subjects were administered 6 min of intermittent sciatic stimulation on a fixed ISI with pulse duration set to 0 (open squares), 0.02 (closed, upward triangles), 0.1 (closed circles), 0.5 (closed diamonds), or 10 ms (closed, downward triangles). Following sciatic stimulation instrumental performance was measured. Panel A depicts response durations across time and panel B shows average response duration collapsed across trials. Asterisks indicate groups that were significantly different from the 0 ms condition (P < .05) and error bars indicate ± S.E. (n=10 subjects per group).
Experiment 7: Sciatic stimulation results in voltage-dependent recruitment of Aδ and C-fibers
Prior work suggests that C-fiber activation is required to induce a learning deficit. Baumbauer et al. (2007b) demonstrated that blocking the NK1 receptor prior to uncontrollable stimulation prevented the induction of the learning deficit, while selectively activating the same receptor with a high-affinity analog of substance P resulted in a learning deficit. In addition, peripheral capsaicin, which selectively engages C fiber activity, induces a learning deficit (Hook et al., 2008). Taken together, these results suggest that C-fiber activation is required for the induction of the deficit.
To examine the fiber types engaged by sciatic stimulation, conduction velocities were measured from the sciatic nerves of 2 signalized rats using a platinum recording cuff electrode (Harvard Apparatus, Holliston, MA). Single, 0.5 ms pulses were administered to the exposed nerve at incrementing voltages (0.1 – 40 V), and signals were amplified at 1000×, stored to a PC computer using a Recorder Neural Data Acquisition system (Plexon, Inc., Dallas, TX), and analyzed using Offline Sorter (Plexon, Inc., Dallas, TX). The class of fiber activated was determined using the following conduction velocities: >24 m/s = Aβ, 2.5–24 m/s = Aδ, and < 2.5 m/s = C (Handwerker et al., 1991; Leem et al., 1993; Sanders and Zimmerman, 1986).
We found that Aβ mediated compound action potentials began to emerge at 0.5–0.6 V, Aδ fiber thresholds were reached at 1–2 V, and C-fiber activation was achieved at 3–4 V. These results demonstrate that C-fibers were activated at all voltages used for sciatic stimulation in Experiment 1.
To evaluate whether there were any electrophysiological changes that coincided with the differential performance observed in Experiment 1, compound action potentials were recorded and analyzed following stimulation at 10, 20, 30, and 40 V. As can be seen in Figure 10, 10 V of stimulation engaged a strong response in Aβ fibers, and a weak response in Aδ and C-fibers. A much larger Aδ and C-fiber response was observed when shock voltage was increased.
Figure 10.
The effect of voltage on primary afferent activation. The figure depicts compound action potentials recorded following 10, 20, 30, or 40 V of sciatic stimulation. Subjects received 5 individual 0.5 ms pulses separated by 1 s intervals. Each panel contains 5 representative waveforms. The lower-most panel illustrates time of stimulation relative to the detection of Aβ activity. The 10 V condition is labeled with Aβ, Aδ, and C as a reference for the activation of each type of primary afferent. The total time elapsed was 10 ms (N=2 subjects).
Experiment 8: Low-frequency sciatic stimulation produces a robust learning deficit
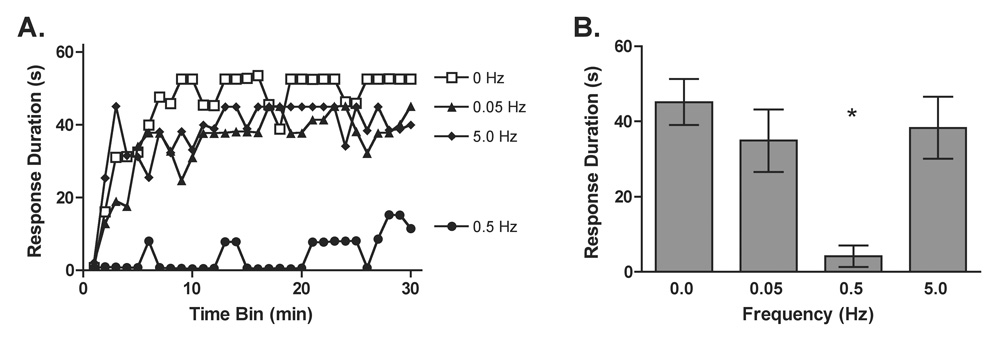
The frequency at which stimuli are administered over time has a significant impact on subsequent behavior. Crown et al. (2002a) demonstrated that tailshock delivered at a frequency of 0.5 Hz produced a robust learning deficit while an equal number of shocks presented at a lower (0.1 Hz) or higher (2.5 Hz) frequency produced a weaker effect. From a neural perspective, research has shown that high frequency stimulation results in LTP, while low frequency stimulation often yields LTD (Malenka, 1994; Sandkühler et al., 1997). To facilitate comparisons across paradigms, the present experiment explored the relationship between the frequency of sciatic stimulation and the induction of the learning deficit. Based on the results of Experiment 3 (demonstrating 3.6 s of continuous shock [180 pulses] at 50 Hz does not produce a learning deficit) and past studies (Crown et al., 2002a), we assessed the impact of shocks given between 0.05 and 5 Hz. Independent groups of subjects received 0, 0.05 (3,600 s), 0.5 (360 s), or 5 Hz (36 s) of sciatic nerve stimulation (0.5 ms pulse width) on a variable ISI. (A variable, rather than fixed, ISI was used because concurrent work [see Experiments 9–10] revealed that this shock schedule can sometimes produce a more robust effect). Following the stimulation period subjects were tested as described earlier (n=8 per condition).
Rats that received 0, 0.05, or 5 Hz of sciatic stimulation were capable of maintaining a prolonged flexion response while rats that received 0.5 Hz were not (Figure 11). An ANOVA yielded significant main effects of Frequency, F(3, 28) = 6.35, p < .01, and Trials, F(29, 812) = 10.46, P < .001, as well as a significant Frequency × Trials interaction, F(87, 812) = 1.74, P < .001. Post hoc comparisons of the group means demonstrated that subjects in the 0.5 Hz condition had shorter response durations relative to all other groups (P < .05). No other comparisons approached significance.
Figure 11.
The effect of stimulation frequency on instrumental performance. Subjects received 6 min of intermittent sciatic stimulation at 0 (open squares), 0.05 (closed, upward triangles), 0.5 (closed circles), or 5 Hz (closed diamonds) prior to instrumental testing. Panel A depicts response durations across time and the right panel B shows average response durations collapsed across trials. The asterisk indicates a significant difference when compared to the 0 Hz condition (P < .05) and error bars indicate ± S.E. (n=8 subjects per group).
Experiment 9: Increasing shock number can produce a less robust effect
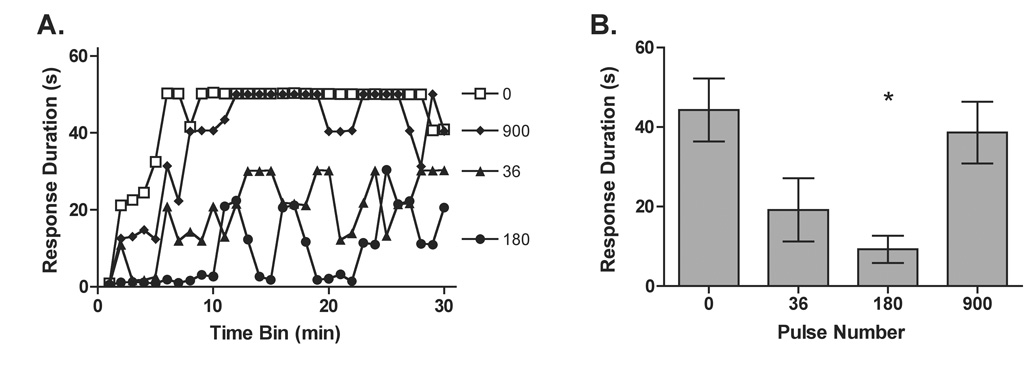
Previous research has shown that 360 or 1800 s, but not 72 s, of intermittent AC tailshock results in a robust learning deficit (Crown et al., 2002a). The present experiment examined whether a similar result would be observed using DC stimulation to the sciatic nerve. Subjects received 0, 72 (36 pulses), 360 (180 pulses), or 1800 s (900 pulses) of 40 V (0.5 ms pulse width) DC pulses on a fixed ISI (n=6 per condition). All subjects spent an equivalent amount of time in the restraining tubes. Half of the subjects in the 36 and 180 pulse conditions received stimulation at the beginning of the restraint period, while the remaining half received shock at the end of the session. To examine whether this difference affected the results, an ANOVA was performed comparing time of stimulation. The ANOVA confirmed that this variable did not have a significant effect on behavior, F(1, 8) < 1.0, P > .05. Consequently, the data were collapsed across this factor in subsequent analyses.
Untreated controls (0 pulse condition) and subjects in the 36 pulse condition exhibited increases in flexion duration when tested with controllable shock (Figure 12). Exposure to the shock schedule used earlier (180 pulses) disrupted learning whereas a more prolonged exposure (900 pulses) did not. An ANOVA yielded significant main effects of Pulse Number, F(3, 20) = 4.53, P < .05, and Trials, F(29, 580) = 8.57, P < .001, as well as a significant Trials × Pulse Number interaction,F(87, 580) = 1.32, P < .05. To further characterize the effect of sciatic stimulation over the course of time, trend analyses were conducted. These analyses yielded a significant quadratic (U-shaped) trend that accounted for 91.95% of the overall variance, F(1, 29) = 12.50, P < .01. This quadratic trend emerged because only the 180-pulse treatment produced a learning deficit.
Figure 12.
The effect of pulse number on the induction of the learning deficit. Subjects were administered 0 (open squares), 36 (closed triangles),180 (closed circles), or 900 (closed diamonds) intermittent DC pulses to their sciatic nerves on a fixed ISI. Once sciatic stimulation was completed subjects received 30 min of instrumental testing. Panel A depicts response durations across time and panel B shows average response duration collapsed across trials. Asterisks indicate groups that were significantly different from the 0 pulse condition (P < .05) and error bars indicate ± S.E. (n=6 subjects per group).
Experiment 10: Extended variable, but not fixed, stimulation produces a learning deficit
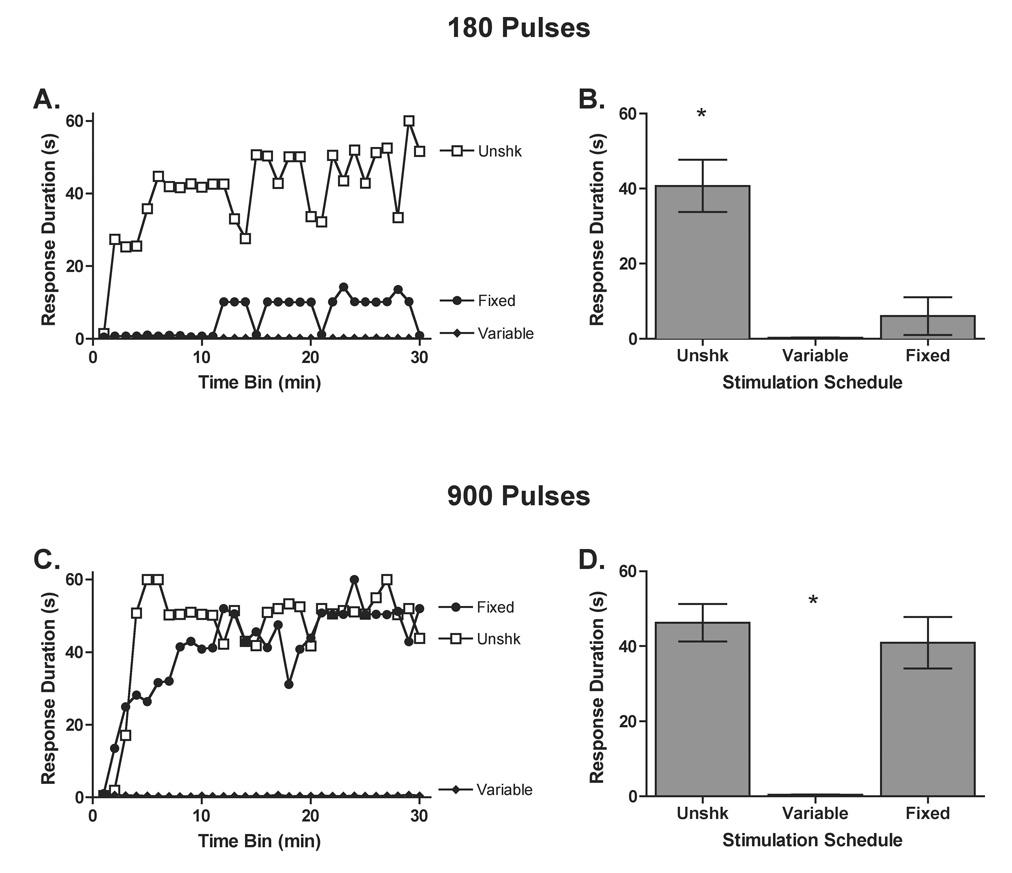
In Experiments 5, 6, and 9, we found that 6 min of intermittent shock (180 pulses) applied using a fixed spacing produced a robust learning deficit. Surprisingly, when subjects received more shock (900 pulses), no deficit was observed. It appears that, when shocks are presented at regular intervals and for an extended period of time, the adverse consequences of shock treatment lessen. Yet, this has not been observed when intermittent AC shock is used to induce a learning deficit (Baumbauer et al., 2007b; Crown et al., 2002a; Ferguson et al., 2006; Grau et al., 2004; Patton et al., 2004). Beyond the mode of presentation (AC shock through cutaneous electrodes versus DC stimulation of the sciatic nerve) a key is that the ISI was fixed in the present experiments, whereas earlier studies used a variable ISI. Experiment 10 evaluated whether this temporal variable matters by comparing the impact fixed versus variable shock given for 6 or 30 min. Subjects received 180, or 900, 40 V DC pulses (0.5 ms pulse width) on a fixed or variable ISI. Following the stimulation period subjects were tested as described earlier (n=6 per condition).
The pattern and duration of shock exposure had a significant effect on subjects’ test performance. Unshocked subjects were capable of maintaining a prolonged flexion response, while subjects that received 6 min of fixed or variable stimulation were not (Figure 13). Moreover, subjects that received 900 pulses on a variable ISI also exhibited a learning deficit, while those that received 900 pulses on a fixed ISI learned. An ANOVA yielded significant main effects of Pulse number, F(1, 30) = 9.43, P < .01, Schedule, F(2, 30) = 32.02, P < .001, and Trials, F(29, 870) = 8.24, P < .001. The ANOVA also revealed significant Pulse Number × Schedule, F(2, 30) = 6.01, P < .01, Pulse Number × Trials, F(29, 870) = 1.70, P < .05, and Schedule × Trials, F(58,870) = 3.50, P < .001, interactions. No other statistical effects approached significance, F(58, 870) = 1.28, P > .05. Post hoc comparisons of the group means showed that unshocked subjects maintained significantly longer flexion responses relative to subjects in both 180-pulse conditions and subjects that received 900 shocks on a variable ISI (P < .05). Subjects that received 900 pulses on a fixed ISI also differed from both 180-pulse conditions and the 900-pulse group that received shocks in a variable manner (P < .05). No other comparisons approached significance.
Figure 13.
The impact of increasing shock number using a fixed or variable ISI. Subjects received 0 (Unshk), 180, or 900 pulses of sciatic stimulation delivered on a fixed (closed circles) or variable (closed diamonds) ISI. Following sciatic stimulation subjects’ capacity to learn was assessed. The uppermost panels depict data for subjects administered 180 pulses, and the lowermost panels show data for subjects administered 900 pulses. Panels A and B depict response durations across time and panels C and D show average response durations collapsed across trials. Asterisks indicate groups that were significantly different from the unshocked conditions (P < .05) and error bars indicate ± S.E. (n=6 subjects per group).
DISCUSSION
Prior work has shown that intermittent AC shock applied through intramuscular or cutaneous electrodes inhibits learning when subjects are subsequently tested with controllable legshock. The present experiments demonstrate that DC stimulation of the sciatic nerve also induces a learning deficit. Intermittent shock pulses (0.1–10 ms), at an intensity (40V, monophasic or biphasic) that engages significant C-fiber activity, inhibited subsequent learning, an effect that lasted 24 hrs. The induction of the deficit required a temporal gap between stimuli and a similar deficit was observed independent of whether shocks were given as single DC pulses or bursts of 4–5 pulses. A robust learning deficit was observed when subjects received stimuli in a variable fashion, approximately 2 s (0.5 Hz) apart; variable shock given at a lower (0.05 Hz) or a higher (5 Hz) frequency had no adverse effect. An even higher frequency (50 Hz) of shock treatment had no effect across a range of stimulus durations (3.6–360 s).
Six min of intermittent shock (180 pulses at 0.5 Hz) produced a learning deficit independent of whether the spacing between shocks was fixed or variable. When stimulus exposure was increased 5 fold, to 30 min (900 pulses at 0.5 Hz), only variable shock produced a learning deficit. Because a briefer period (6 min) of fixed spaced shock induces a learning deficit, this finding suggests that continued training with fixed spaced shock engages a process that effectively reverses the learning deficit.
In the sections that follow we discuss the relation of these effects to work in the pain literature, the role of temporal spacing, controllability, and prediction, and the implications of this work for understanding the underlying neurobiological mechanisms.
Consequences of Pain Fiber Activation
Prior work suggests that prolonged C-fiber activity is both necessary and sufficient to induce the learning deficit (Baumbauer et al., 2007b; Hook et al., 2008; Ferguson et al., 2006). Here, we observed a direct relationship between the amount of stimulation administered and the level of pain fiber recruitment. Stimulation at 10 V did activate C-fibers, but as voltage increased, greater levels of Aδ and C-fiber recruitment were observed, with the highest levels of activation occurring at 40 V. This result suggests that it was not the activation of C-fibers, per se, that caused a learning deficit, but rather the degree of C-fiber recruitment that determines whether a learning deficit is observed. This is consistent with a number of other observations. Elsewhere, we have suggested that uncontrollable stimulation may impair selective response modifications (instrumental learning) by saturating NMDAR-mediated neural plasticity (Baumbauer 2007a, b; Moser et al., 1998; Ferguson et al., 2006). Such an effect should only be observed after prolonged stimulation. Similarly, prolonged C-fiber activity (e.g., from peripheral capsaicin treatment) is required to induce a lasting increase (central sensitization) in nociceptive reactivity (Coderre and Katz, 1997; Salter, 2002; Woolf, 1983).
Relation to Central Sensitization
A number of observations suggest that the induction of the learning deficit may be related to the phenomenon of central sensitization. Central sensitization is induced by peripheral nociceptive input (C-fiber activity resulting from injury or inflammation) and produces an increase in mechanical reactivity (allodynia) that has been linked to the development of neuropathic pain (Hohmann et al., 2005; Ma and Woolf, 1996; Yaksh et al., 1999; Young et al., 2007). Prior work has shown that central sensitization, and the induction of the learning deficit, are similarly affected by a host of pharmacological treatments (Baumbauer et al., 2007; Baumbauer et al., 2007b; Ferguson et al., 2004; Ferguson et al., 2006; Joynes and Grau, 2004; Joynes et al., 2004). Further, peripheral inflammation (from capsaicin or formalin) impairs subsequent learning (Ferguson et al., 2006; Hook et al., 2008). Just as controllable stimulation can prevent, and reverse, the adverse effects of uncontrollable shock (Crown et al., 2001), controllable stimulation can also prevent and reverse the adverse effects of capsaicin treatment (Hook et al., 2008). Finally, uncontrollable shock has been shown to induce a bilateral tactile allodynia and controllable shock attenuates capsaicin-induced allodynia (Ferguson et al., 2006; Hook et al., 2008).
Based on previous work demonstrating that intermittent shock produces allodynia (Ferguson et al., 2006), and that regular stimulation of the sciatic nerve can enhance neural activity within the dorsal horn (wind-up; Schouenborg and Sjölund 1983; Wall and Woolf, 1984, 1986), we expected to observe allodynia in response to sciatic stimulation. As shown in Figure 2, no evidence of allodynia was observed. Instead, shock treatment produced a unilateral reduction in mechanical reactivity. This effect was not observed on the contralateral (test) leg, and was not modulated by variables related to the induction of the learning deficit (e.g., whether shocks occurred in a fixed or variable fashion). Therefore, the reduction in behavioral reactivity appears to be unrelated to the learning deficit. Elsewhere (Grau et al., 1998), we reported that shock per se (independent of whether it was controllable or uncontrollable) produces a limb-specific reduction in shock reactivity. Like the reduction in tactile reactivity observed in the present experiment, this habituation-like effect grew as a function of shock intensity and duration (Grau et al., 1998). This effect may emerge, in part, because the minimum amount of stimulation that reliably produces a learning deficit (6 min) is far longer than that normally used to induce wind-up (Schouenborg and Sjölund, 1983). Supporting this, others have reported a non-monotonic effect between shock exposure and behavioral reactivity/neural responsiveness, with an enhancement (sensitization/wind-up) observed with briefer periods of stimulation and a decrement (habituation/wind-down) emerging with more prolonged stimulation (see Groves and Thompson, 1970; Herrero et al., 2000).
What is difficult to determine from the present results is whether the reduction in behavioral reactivity observed after sciatic stimulation reversed –or–masked spinally-mediated alterations related to the induction of central sensitization (and allodynia). We know from a concurrent experiment that behavioral evidence of allodynia resulting from shock fades far faster (within 3 hrs; K.M. Baumbauer unpublished results) than the learning deficit, which can last up to 48 hrs (Crown et al., 2002a). To the extent that the induction of the deficit and allodynia are tied, the linkage is limited to the initial induction (and possibly, consolidation) of the deficit. From this perspective, sciatic stimulation engaged some of the same processes as leg or cutaneous shock (because it produced a lasting deficit), but failed to yield evidence of allodynia because a masking (habituation-like) effect was also elicited. This effect may have occurred because stimulation was done in the presence of tissue damage (required to uncover the nerve) or perhaps because the stimulation also engaged robust A-β and A-δ fiber activity. Independent of why sciatic stimulation did not produce behavioral allodynia, a crucial prediction remains to be tested: If the induction of the learning deficit is related to central sensitization and behavioral allodynia, then procedures that help unveil the underlying allodynia should demonstrate that fixed and variable stimuli have divergent effects. Recent work has explored this issue using shock to the tibialis anterior muscle. As reported by Ferguson et al. (2006), intermittent shock given on a variable ISI produced a bilateral allodynia. Subjects that received the same amount of shock, but with fixed spacing, were less responsive to tactile stimulation on the ipsilateral leg immediately after shock treatment (the habituation-like effect) and exhibited only a weak allodynia 1 hr later (K.M. Baumbauer, unpublished results). Thus, both the induction of the learning deficit and the emergence of allodynia were attenuated when shocks were presented at a regular (predictable) interval.
Role of Shock Onset and Temporal Spacing
Prior work has shown that both the induction of the learning deficit, and instrumental learning, depend on repeated stimulation and that the onset of shock plays a greater role than its offset (Grau et al., 1998). We obtained evidence that shock onset is important to instrumental learning in a study examining the impact of manipulating response-outcome contiguity. When a short temporal gap (100 ms) was interposed between the response (limb lowering) and shock onset, subjects failed to learn. In contrast, delaying shock offset by the same amount had no effect. Importantly, by delaying shock offset, subjects received more shock (a longer burst), implying that extending shock duration had little effect on learning. Similarly, in Experiment 4 we found that both single pulses and 80 ms shock bursts produced a comparable learning deficit. In both cases, what mattered most was the onset of stimulation, rather than when it terminated.
The impact of sequential shock onsets depends on temporal spacing. If the gap between shocks is too short (0.2 s; 5 Hz), or too long (10 s; 0.5 Hz), intermittent shock does not produce a learning deficit. Interestingly, because our variable ISI has a range of 0.2–3.8 s, some shocks occurred close in time (0.2 s apart). Because the second shock in such a pair occurred within an interval that is too short to produce a learning deficit, the second shock should have little effect; only those shocks that occur further apart should contribute to the induction of the learning deficit. Given this, one might wonder whether fixed and variable spacing have a differential effect because only the latter yielded shocks spaced more than 2 s apart. We know that this cannot account for the effect of variable spaced shocks because shocks spaced evenly at 2 s induce a deficit when administered for just 6 min; with limited training, both variable and fixed spaced shocks have a comparable effect. Because both shock schedules produce a deficit when given for 6 min, further training with fixed spaced must reduce the deficit because it engages an additional, restorative, process.
Elsewhere, we have shown that continuous AC shock does not induce a learning deficit (Crown et al., 2002a). Similarly, a continuous string of DC shock pulses did not inhibit subsequent learning; only intermittent shock produced a deficit. Again, these results imply that the induction of the learning deficit is tied to the onset of nociceptive stimulation and that this event is most effective when the stimuli are applied in a variable fashion.
Fixed Spaced and Controllable Shock have Comparable Effects
We have previously shown that instrumental training can both prevent, and reverse, the learning deficit induced by uncontrollable stimulation. The results of Experiments 9 and 10 imply that extended training with fixed spacing can also reverse the deficit. Other new data suggest that prior exposure to fixed-spaced shock engages a protective effect that blocks the induction of the learning deficit (Baumbauer et al., 2008), and that this protective effect lasts at least 24 hrs. Interestingly, administration of an NMDA antagonist (MK-801) prior to training with fixed-spaced shock blocks the development of this protective effect and inhibiting protein synthesis immediately after fixed-spaced shock disrupts its consolidation. Taken together, these results imply that fixed-spaced shock has a beneficial effect because it evokes a form of NMDAR-mediated plasticity.
Another consequence of instrumental training is that it fosters learning when subjects are later tested with a higher response criterion. We have recently shown that pretreatment with fixed-spaced shock also fosters subsequent learning (Huie et al., 2008). Further, and in contrast to the learning deficit, this effect became stronger when shock intensity was reduced. This would appear to suggest that the benefits of fixed-spaced shock are not tied to prolonged C-fiber activity. This finding resembles an earlier observation—that increasing shock intensity can undermine instrumental learning (Grau et al., 1998). In both cases, these effects may emerge because the concurrent intense activation of C-fibers may engage inhibitory processes that undermine learning. Further work is being conducted to explore this hypothesis.
We have also shown that uncontrollable stimulation impacts recovery after a contusion injury (Grau et al., 2004). In these experiments, subjects receive a contusion in the lower thoracic region and recovery is monitored for the next 3–6 weeks. Just 6 min of variable/uncontrollable intermittent tailshock 24 hr after injury impairs recovery and increases tissue loss. Nociceptive stimulation has no effect if given in a controllable fashion. The present results suggest that introducing a form of temporal predictability by means of fixed spacing could also lessen the adverse effect of shock treatment.
Neurobiological Mechanisms
Our long-term goal is to describe spinal phenomena at both a functional and neurobiological level (Grau and Joynes, 2005). Addressing these issues requires a detailed map of the circumstances that generate our behavioral effects (the eliciting conditions; Killeen, 2001). The present results provide some important details that will serve to guide, and constrain, future studies. First, as discussed above, the results reinforce the view that the induction of the learning deficit is tied to intense C-fiber stimulation (Baumbauer et al., 2007b; Hook et al., 2008; Ferguson et al., 2006). A second implication stems from work demonstrating that single pulses and burst stimulation result in different patterns of neurotransmitter release. Lever et al. (2001) showed that both bursting and single pulses elicit comparable release of substance P. However, the release of BDNF was greater after shock bursts. The fact that both types of stimuli elicited substance P release is consistent with our claim that the induction of the deficit depends on C-fiber activity and the observation that both shock pulses and bursts produced a comparable deficit. The observation that BDNF release was greater with shock bursts also has implications, for other studies suggest that controllable (but not uncontrollable) stimulation up-regulates BDNF expression within the spinal cord (Gómez-Pinilla et al., 2007). Further, pretreatment with a drug that inhibits BDNF action at the receptor level blocks the beneficial effects of controllable stimulation (Gómez-Pinilla et al., 2007). Conversely, pretreatment with exogenous BDNF has an effect that emulates the benefits of controllability, inhibiting the induction of the deficit and restoring the capacity for learning in previously shocked rats (Huie et al., 2006). These observations suggest that the benefits of controllable stimulation may depend, in part, on the use of shock bursts that promote BDNF synthesis and release. Similarly, fixed spaced shock bursts may have a greater beneficial effect than single pulses, an effect that may be mediated by BDNF. Studies are under way to examine these possibilities.
A third way in which the present results inform our understanding of the underlying neurobiology stems from a comparison to prior studies using electrophysiological procedures to study spinal LTP/LTD. Prior studies have established that high frequency stimulation (50 Hz and greater) produces LTP while lower frequency stimuli (< 3 Hz) can yield LTD. Because high frequency stimulation (3.6–360 s) did not produce a learning deficit, our data would appear to argue against an interpretation of the deficit in terms of a LTP-like effect. This is important because diffuse over-excitation could disrupt learning by saturating spinal plasticity (Baumbauer et al., 2007b; Ferguson et al., 2006; Moser et al., 1999). The frequencies that generated a lasting deficit fell, instead, in the range typically used to induce LTD. This outcome, in combination with data demonstrating that a pharmacological manipulation known to generate LTD (pretreatment with a mGlu agonist) produces a lasting learning deficit (Ferguson et al., 2006), suggests that uncontrollable shock may disrupt instrumental learning because it produces a diffuse form of LTD that inhibits selective adaptive response modifications. However, the story here is complicated by recent electrophysiological studies examining the relative impact of variable versus fixed spaced shock. Electrophysiologists typically apply DC shock pulses with a fixed spacing and, under these conditions, extended exposure (900 pulses over 15 min [1 Hz]) favors the induction of spinal LTD. If, however, the pulses are applied in a variable fashion (a procedure thought to emulate some naturalistic conditions), LTP is observed (Perrett et al., 2001). Interestingly, it is not known whether the emergence of these differences depends on the duration of stimulus exposure. Perhaps, as reported here, both patterns of shock pulses would have similar electrophysiological consequences and induce LTD if fewer pulses were given; LTD may only emerge after extended training. For present purposes, what is critical is that these observations suggest again that the deficit could be linked to a form of LTP (saturation-like effect). Conversely, the benefits of fixed spaced shock (and perhaps, controllability) might reflect an opponent-like LTD that serves to re-balance synaptic weights and allow selective (LTP-dependent) response modifications. What this cannot explain, however, is why high frequency stimulation (that should induce a robust LTP) does not produce a learning deficit. It also remains unclear why the induction of our behavioral effects depends on stimulus onset (the initial pulse/action potentials), and why these onsets must occur at a relatively low frequency. Whatever the answer, it is worth noting that these stimulus conditions do fall within normal physiological ranges, an issue sometimes raised as a criticism of studies using high frequency stimulation to induce LTP.
Timing Outside the Brain
Researchers examining the encoding of temporal relations typically emphasize the role of brain structures, such the cerebellum (Ivry, 1993; Mauk et al., 2000; Meck, 2005; Ohyama et al., 2003; Raymond et al., 1996). We have shown that variable and fixed spaced stimuli have divergent effects at the level of the spinal cord and, in this way, our results imply that spinal neurons are sensitive to temporal relations. There is a sense in which this was already known, for it is well established that neurons within the lumbosacral spinal cord are capable of organizing stepping behavior, a coordinated act that requires a central pattern generator (Cazalets et al., 1995; de Leon et al., 1998; Edgerton et al., 1992; Rossignol et al., 1998; Tillakaratne et al, 2002). Rhythmic behavior of this type depends on a kind of neural pace maker, and the observation that spinal neurons can support repetitive, regularly spaced, steps (as well as rhythmic scratching) suggests a timing mechanism is present. Interestingly, the frequencies we have shown impact spinal cord plasticity fall within the frequency range of stepping behavior (de Leon et al., 1994; Roy et al., 1991). Because spinal neurons are “tuned” to this frequency range, and programmed to oscillate in a regular fashion, fixed spaced stimuli may be encoded as within a natural range and adaptively coupled with pre-existing circuits (Timberlake, 1999; Timberlake and Lucas, 1989). Further, because stepping does not depend on C-fiber activity, the benefits of regular stimulation should be evident at lower shock intensities (as noted above). Conversely, variable stimulation would seem antagonistic to rhythmic neural activity and, as a consequence, have a disruptive effect. If these hypotheses have merit, regular stimulation could potentially benefit recovery after a spinal injury. However, care should be taken to avoid presenting stimuli in an unpredictable, or uncontrollable, fashion at an intensity that engages intense C-fiber activity.
Abbrevations
- AC
alternating current
- ANCOVA
Analysis of covariance
- ANOVA
Analysis of variance
- BDNF
brain-derived neurotrophic factor
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- DC
direct current
- GABA
γ-aminobutyric acid
- ISI
Interstimulus interval
- LTP
Long-term potentiation
- LTD
Long-term depression
- N
Newton
- NMDA
N-methyl-D-aspartate
- T2
second thoracic vertebrae
- Tukey’s HSD
Tukey’s Honestly Significant Difference test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azkue JJ, Liu XG, Zimmermann M, Sandkühler J. Induction of long-term potentiation of C fibre-evoked spinal field potentials requires recruitment of group I, but not group II/III metabotropic glutamate receptors. Pain. 2003;106:373–379. doi: 10.1016/j.pain.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Baumbauer KM, Young EE, Hoy KC, Abood A, Joynes RL. Administration of a Ca2+/calmodulin-dependent protein kinase II (CaMKII) inhibitor reverses the noncontingent shock learning deficit observed in spinal rats. Behav Neurosci. 2007;121:570–578. doi: 10.1037/0735-7044.121.3.570. [DOI] [PubMed] [Google Scholar]
- Baumbauer KM, Young EE, Hoy KC, Joynes RL. Intrathecal administration of neurokinin 1 and neurokinin 2 receptor antagonists undermines the savings effect in spinal rats trained in an instrumental learning paradigm. Behav Neurosci. 2007a;121:186–199. doi: 10.1037/0735-7044.121.1.186. [DOI] [PubMed] [Google Scholar]
- Baumbauer KM, Young EE, Hoy KC, Joynes RL. Neurokinin receptors modulate the impact of uncontrollable stimulation on adaptive spinal plasticity. Behav Neurosci. 2007b;121:1082–1094. doi: 10.1037/0735-7044.121.5.1082. [DOI] [PubMed] [Google Scholar]
- Baumbauer KM, Hughes AJ, Huie JR, Hoy KC, Grau JW. Abstract Viewer/Itinerary Planner. Washington, D.C.: Society for Neuroscience; 2008. Evidence of timing in the absence of supraspinal input: Fixed space stimulation protects against the detrimental effects of uncontrollable tailshock. [Google Scholar]
- Beggs AL, Steinmetz JE, Patterson MM. Classical conditioning of a flexor nerve response in spinal cats: Effects of tibial nerve CS and a differential conditioning paradigm. Behav Neurosci. 1985;99:496–508. doi: 10.1037//0735-7044.99.3.496. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerger AA, Eisenstein EM, Reep RL. The yoked control in instrumental avoidance conditioning: An empirical and methodological analysis. Physiol Psychol. 1981;9:351–353. [Google Scholar]
- Cazalets JR, Borde M, Clarac F. Localization and organization of the central pattern generator for hindlimb locomotion in the newborn rat. J Neurosci. 1995;15:4943–4951. doi: 10.1523/JNEUROSCI.15-07-04943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church RM. Systematic effect of random error in the yoked control design. Psychol Bull. 1964;62:122–131. doi: 10.1037/h0042733. [DOI] [PubMed] [Google Scholar]
- Church RM. The yoked control design. In: Archer T, Nilsson L, editors. Aversion, avoidance, and anxiety: Perspectives on aversively motivated behavior. Hillsdale, NJ: Erlbaum; 1989. –403.pp. 415 [Google Scholar]
- Coderre TJ, Katz J. Peripheral and central hyperexcitability: Differential signs and symptoms in persistent pain. Behav Brain Sci. 1997;20:404–419. doi: 10.1017/s0140525x97251484. [DOI] [PubMed] [Google Scholar]
- Crown ED, Grau JW. Preserving and restoring behavioral potential within the spinal cord using an instrumental training paradigm. J Neurophysiol. 2001;86:845–855. doi: 10.1152/jn.2001.86.2.845. [DOI] [PubMed] [Google Scholar]
- Crown ED, Ferguson AR, Joynes RL, Grau JW. Instrumental learning within the spinal cord: IV. Induction and retention of the behavioral deficit observed after noncontingent shock. Behav Neurosci. 2002a;116:1032–1051. doi: 10.1037//0735-7044.116.6.1032. [DOI] [PubMed] [Google Scholar]
- Crown ED, Ferguson AR, Joynes RL, Grau JW. Instrumental learning within the spinal cord: II. Evidence for central mediation. Physiol Behav. 2002b;77:259–267. doi: 10.1016/s0031-9384(02)00859-4. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Extensor- and flexor-like modulation within motor pools of the rat hindlimb during treadmill locomotion and swimming. Brain Res. 1994;654:241–250. doi: 10.1016/0006-8993(94)90485-5. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol. 1998;79:1329–1340. doi: 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Goddard GV. Long-term potentiation of the perforant path-granule cell synapse in the rat hippocampus. Brain Res. 1975;86:205–215. doi: 10.1016/0006-8993(75)90697-6. [DOI] [PubMed] [Google Scholar]
- Durkovic RG. Classical conditioning, sensitization, and habituation in the spinal cat. Physiol Behav. 1975;14:297–304. doi: 10.1016/0031-9384(75)90037-2. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, De Leon RD, Tillakaratne N, Recktenwald MR, Hodgson JA, Roy RR. Use-dependent plasticity in spinal stepping and standing. Adv Neurol. 1997;72:233–247. [PubMed] [Google Scholar]
- Edgerton VR, Roy RR, Hodgson JA, Prober RJ, de Guzman CP, de Leon R. Potential of adult mammalian lumbosacral spinal cord to execute and acquire improved locomotion in the absence of supraspinal input. J Neurotrauma. 1992;9:S119–S128. [PubMed] [Google Scholar]
- Fanselow MS. Conditioned fear-induced opiate analgesia: A competing motivational state theory of stress analgesia. Ann NY Acad Sci. 1986;46:740–754. doi: 10.1111/j.1749-6632.1986.tb14617.x. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Crown ED, Grau JW. Nociceptive plasticity inhibits adaptive learning in the spinal cord. Neurosci. 2006;141:421–431. doi: 10.1016/j.neuroscience.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Patton BC, Bopp AC, Meagher MW, Grau JW. Brief exposure to a mild stressor enhances morphine conditioned place preference in male rats. Psychopharmacology. 2004;175:47–52. doi: 10.1007/s00213-004-1780-3. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Washburn SN, Crown ED, Grau JW. GABA-A activation is involved in noncontingent shock inhibition of instrumental conditioning in spinal rats. Behav Neurosci. 2003;117:799–812. doi: 10.1037/0735-7044.117.4.799. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F, Huie JR, Ying Z, Ferguson AR, Crown ED, Baumbauer KM, Edgerton VR, Grau JW. BDNF and Learning: Evidence that Instrumental Training Promotes Learning within the Spinal Cord by Up-Regulating BDNF Expression. Neurosci. 2007;148:893–906. doi: 10.1016/j.neuroscience.2007.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, Barstow DG, Joynes RL. Instrumental learning within the spinal cord: I. Behavioral Properties. Behav Neurosci. 1998;112:1366–1386. doi: 10.1037//0735-7044.112.6.1366. [DOI] [PubMed] [Google Scholar]
- Grau JW, Crown ED, Ferguson AR, Washburn SN, Hook MA, Miranda RC. Instrumental learning within the spinal cord: Underlying mechanisms and implications for recovery after injury. Behav Cogn Neurosci Rev. 2006;5:191–239. doi: 10.1177/1534582306289738. [DOI] [PubMed] [Google Scholar]
- Grau JW, Hyson RL, Maier SF, Madden J, Barchas JD. Long-term stress-induced analgesia and activation of the opiate system. Science. 1981;213:1409–1411. doi: 10.1126/science.7268445. [DOI] [PubMed] [Google Scholar]
- Grau JW, Joynes RL. A neural-functionalist approach to learning. Int J Comp Psychol. 2005;18:1–22. [Google Scholar]
- Grau JW, Washburn SN, Hook MA, Ferguson AR, Crown ED, Garcia G, Bolding KA, Miranda RC. Uncontrollable stimulation undermines recovery after spinal cord injury. J Neurotrauma. 2004;21:1795–1817. doi: 10.1089/neu.2004.21.1795. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: A dual-process theory. Psychol Rev. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Handwerker HO, Kilo S, Reeh PW. Unresponsive afferent nerve fibers in the sural nerve of the rat. J Physiol. 1991;435:229–242. doi: 10.1113/jphysiol.1991.sp018507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng C, de Leon RD. The rodent lumbar spinal cord learns to correct errors in hindlimb coordination caused by viscous force perturbations during stepping. J Neurosci. 2007;27:8558–8562. doi: 10.1523/JNEUROSCI.1635-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero JF, Laird JMA, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Hodgson JA, Roy RR, de Leon R, Dobkin B, Edgerton VR. Can the mammalian lumbar spinal cord learn a motor task? Med Sci Sports Exerc. 1994;26:1491–1497. [PubMed] [Google Scholar]
- Hohmann AG, Neely MH, Piña J, Nackley AG. Neonatal chronic hind paw inflammation alters sensitization to intradermal capsaicin in adult rats: a behavioral and immunocytochemical study. J Pain. 2005;6:798–808. doi: 10.1016/j.jpain.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Hook MA, Huie JR, Grau JW. Peripheral inflammation undermines the plasticity of the isolated spinal cord. Behav Neurosci. 2008;122:233–249. doi: 10.1037/0735-7044.122.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huie JR, Hoy KC, Miranda RC, Grau JW. 2006 Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience; 2006. BDNF facilitates learning in the rat spinal cord. Program No. 555.17. 2006. Online. [Google Scholar]
- Huie JR, Baumbauer KM, Hughes AJ, Grau JW. Abstract Viewer/Itinerary Planner. Washington, D.C.: Society for Neuroscience; 2006. Evidence of timing in the absence of supraspinal input: Fixed space stimulation enables future learning. [Google Scholar]
- Ivry R. Cerebellar involvement in the explicit representation of temporal information. Ann NY Acad Sci. 1993;682:214–230. doi: 10.1111/j.1749-6632.1993.tb22970.x. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Ferguson AR, Crown ED, Patton BC, Grau JW. Instrumental learning within the spinal cord: V. Evidence the behavioral deficit observed after noncontingent nociceptive stimulation reflects an intraspinal modification. Behav Brain Res. 2003;141:159–170. doi: 10.1016/s0166-4328(02)00372-8. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Grau JW. Mechanisms of Pavlovian conditioning: Role of protection from habituation in spinal conditioning. Behav Neurosci. 1996;110:1375–1387. doi: 10.1037//0735-7044.110.6.1375. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Grau JW. Instrumental learning with the spinal cord: III. Prior exposure to noncontingent shock induces a behavioral deficit that is blocked by an opioid antagonist. Neurobiol Learn Mem. 2004;82:35–51. doi: 10.1016/j.nlm.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Janjua K, Grau JW. Instrumental learning within the spinal cord: VI. The NMDA receptor antagonist, AP5, disrupts acquisition and maintenance of an acquired flexion response. Behav Brain Res. 2004;154:431–438. doi: 10.1016/j.bbr.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Killeen PR. The four causes of behavior. Curr Dir Psychol Sci. 2001;10:136–140. doi: 10.1111/1467-8721.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in the rat foot. J Neurophysiol. 1993;69:1684–1699. doi: 10.1152/jn.1993.69.5.1684. [DOI] [PubMed] [Google Scholar]
- Lever IJ, Bradbury EJ, Cunningham JR, Adelson DW, Jones MG, McMahon SB, Marvizón JCG, Malcangio M. Brain-derived neurotrophic factor is released in the dorsal horn by distinctive patterns of afferent fiber stimulation. J Neurosci. 2001;21:4469–4477. doi: 10.1523/JNEUROSCI.21-12-04469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain. 1999;79:75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]
- Liu XG, Morton CR, Azuke JJ, Zimmermann M, Sandkühler J. Long-term depression of C-fibre-evoked spinal field potentials by stimulation of primary afferent A delta-fibres in the adult rat. E J Neurosci. 1998;10:3069–3075. doi: 10.1046/j.1460-9568.1998.00310.x. [DOI] [PubMed] [Google Scholar]
- Liu XG, Sandkühler J. Long-term potentiation of C fiber-evoked potentials in the rat spinal dorsal horn is prevented by spinal N-methyl-D-aspartic acid receptor blockage. Neurosci Lett. 1995;191:43–46. doi: 10.1016/0304-3940(95)11553-0. [DOI] [PubMed] [Google Scholar]
- Liu XG, Sandkühler J. Characterization of long-term potentiation of C-fiber-evoked potentials in spinal dorsal horn of adult rat: Essential role of NK1 and NK2 receptors. J of Neurophysiol. 1997;78:1973–1982. doi: 10.1152/jn.1997.78.4.1973. [DOI] [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. Progressive tactile hypersensitivity: an inflammation-induced incremental increase in the excitability of the spinal cord. Pain. 1996;67:97–106. doi: 10.1016/0304-3959(96)03105-3. [DOI] [PubMed] [Google Scholar]
- Maier SF. Determinants of the nature of environmentally induced hypoalgesia. Behav Neurosci. 1989;103:131–143. doi: 10.1037//0735-7044.103.1.131. [DOI] [PubMed] [Google Scholar]
- Maier SF, Seligman MEP. Learned helplessness: Theory and evidence. Exp Psychol. 1976;105:3–46. [Google Scholar]
- Malenka RC. Synaptic plasticity in the hippocampus: LTP and LTD. Cell. 1994;78:535–538. doi: 10.1016/0092-8674(94)90517-7. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Medina JF, Nores WL, Ohyama T. Cerebellar function: coordination, learning, or timing? Curr Biol. 2000;10:R522–R555. doi: 10.1016/s0960-9822(00)00584-4. [DOI] [PubMed] [Google Scholar]
- Meagher MW, Ferguson AR, Crown ED, McLemore S, King TE, Sieve AN, Grau JW. Shock-induced hyperalgesia: IV. Generality. J Exp Psychol Anim Behav Process. 2001;27:219–238. [PubMed] [Google Scholar]
- Meck WH. Neuropsychology of timing and time perception. Brain and Cogn. 2005;58:1–8. doi: 10.1016/j.bandc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Mendell LM. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966;16:316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Murphy M, Mauck MD. What the cerebellum computes. Trends Neurosci. 2003;26:222–227. doi: 10.1016/S0166-2236(03)00054-7. [DOI] [PubMed] [Google Scholar]
- Overmier JB, Seligman ME. Effect of inescapable shock upon subsequent escape and avoidance responding. J Comp Physiol Psychol. 1967;63:28–33. doi: 10.1037/h0024166. [DOI] [PubMed] [Google Scholar]
- Patton BC, Hook MA, Crown ED, Ferguson AR, Grau JW. The behavioral deficit observed following noncontingent shock in spinalized rats is prevented by the protein synthesis inhibitor cycloheximide. Behav Neurosci. 2004;118:653–658. doi: 10.1037/0735-7044.118.3.653. [DOI] [PubMed] [Google Scholar]
- Perrett SP, Dudek SM, Eagleman D, Montague PR, Friedlander MJ. LTD induction in adult visual cortex: Role of stimulus timing and inhibition. J Neurosci. 2001;21:2308–2319. doi: 10.1523/JNEUROSCI.21-07-02308.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randić M, Jiang MC, Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci. 1993;13:5228–5241. doi: 10.1523/JNEUROSCI.13-12-05228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG, Mauk MD. The cerebellum: a neuronal learning machine? Science. 1996;272:1126–1131. doi: 10.1126/science.272.5265.1126. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Chau C, Brustein E, Giroux N, Bouyer L, Barbeau H, Reader TA. Pharmacological activation and modulation of the central pattern generator for locomotion in the cat. Ann NY Acad Sci. 1998;860:346–359. doi: 10.1111/j.1749-6632.1998.tb09061.x. [DOI] [PubMed] [Google Scholar]
- Roy RR, Hutchinson DL, Pierotti DJ, Hodgson JA, Edgerton VR. EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J App Physiol. 1991;70:2522–2529. doi: 10.1152/jappl.1991.70.6.2522. [DOI] [PubMed] [Google Scholar]
- Salter MW. The neurobiology of central sensitization. J Musculoskelet Pain. 2002;10:23–33. [Google Scholar]
- Sanders KH, Zimmermann M. Mechanoreceptors in rat glabrous skin: Redevelopment of function after nerve crush. J Neurophysiol. 1986;44:644–659. doi: 10.1152/jn.1986.55.4.644. [DOI] [PubMed] [Google Scholar]
- Sandkühler J, Chen JG, Cheng G, Randić M. Low-frequency stimulation of afferent Adelta-fibers induces long-term depression at primary afferent synapses with substantia gelatinosa neurons in the rat. J Neurosci. 1997;17:6483–6491. doi: 10.1523/JNEUROSCI.17-16-06483.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouenborg J. Functional and topographical properties of field potentials evoked in the rat dorsal horn by cutaneous C-fiber stimulation. J Physiol. 1984;356:169–192. doi: 10.1113/jphysiol.1984.sp015459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouenborg J, Sjölund BH. Activity evoked by A- and C- afferent fibers in rat dorsal horn neurons and its relation to a flexion reflex. J Neurophysiol. 1983;50:1108–1121. doi: 10.1152/jn.1983.50.5.1108. [DOI] [PubMed] [Google Scholar]
- Segal RL, Wolf SL. Operant conditioning of spinal stretch reflexes in patients with spinal cord injuries. Exp Neurol. 1994;130:202–213. doi: 10.1006/exnr.1994.1199. [DOI] [PubMed] [Google Scholar]
- Seligman MEP, Maier SF. Failure to escape traumatic shock. J Exp Psychol. 1967;74:1–9. doi: 10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- Spencer WA, Thompson RF, Neilson DRJ. Response decrement of the flexion response in the acute spinal cat and transient restoration by strong stimuli. J Neurophysiol. 1966;29:221–239. doi: 10.1152/jn.1966.29.2.221. [DOI] [PubMed] [Google Scholar]
- Terman GW, Shavit Y, Lewis JW, Cannon JT, Liebeskind JC. Intrinsic mechanisms of pain inhibition: Activation by stress. Science. 1984;226:1270–1277. doi: 10.1126/science.6505691. [DOI] [PubMed] [Google Scholar]
- Tillakaratne NJ, de Leon RD, Hoang TX, Roy RR, Edgerton VR, Tobin AJ. Use-dependent modulation of inhibitory capacity in the feline lumbar spinal cord. J Neurosci. 2002;22:3130–3143. doi: 10.1523/JNEUROSCI.22-08-03130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake W. Biological Behaviorism. In: O’Donohue W, Kitchener R, editors. Handbook of behaviorism. New York: Academic Press; 1999. pp. 243–284. [Google Scholar]
- Timberlake W, Lucas GA. Behavior systems and learning: From misbehavior to general principles. In: Klein SB, Mowrer RR, editors. Contemporary learning theories: Instrumental conditioning theory and the impact of biological constraints on learning. Hillsdale, NJ: Erlbaum; 1989. pp. 237–275. [Google Scholar]
- Toth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ. Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci. 2000;20:8279–8289. doi: 10.1523/JNEUROSCI.20-22-08279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD, Woolf CJ. Muscle but not cutaneous C-afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. J Physiol. 1984;356:443–458. doi: 10.1113/jphysiol.1984.sp015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD, Woolf CJ. The brief and the prolonged facilitatory effects of unmyelinated afferent input on the rat spinal cord are independently influenced by peripheral nerve section. Neurosci. 1986;17:1199–1205. doi: 10.1016/0306-4522(86)90087-4. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Mayer DJ. Multiple endogenous opiate and nonopiate analgesic systems: Evidence of their existence and clinical implications. Ann NY Acad Sci. 1986;467:273–299. doi: 10.1111/j.1749-6632.1986.tb14635.x. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Lee C, Carp JS. Operantly conditioned plasticity in spinal cord. Ann NY Acad Sci. 1991;627:338–348. doi: 10.1111/j.1749-6632.1991.tb25936.x. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, King AE. Physiology and morphology of multireceptive neurons with C-afferent fiber inputs in the deep dorsal horn of the rat lumbar spinal cord. J Neurophysiol. 1987;58:460–479. doi: 10.1152/jn.1987.58.3.460. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Hua XY, Kalcheva I, Nozaki-Taquchi N, Marsala M. The spinal biology in humans and animals of pain states generated by persistent small afferent input. Proc Natl Acad Sci U S A. 1999;96:7680–7686. doi: 10.1073/pnas.96.14.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EE, Baumbauer KM, Elliot A, Joynes RL. Neonatal hindpaw injury disrupts acquisition of an instrumental response in adult spinal rats. Behav Neurosci. 2007;12:1095–1100. doi: 10.1037/0735-7044.121.5.1095. [DOI] [PubMed] [Google Scholar]