Abstract
Lrp5/6 are crucial coreceptors for Wnt/β-catenin signaling, a pathway biochemically distinct from noncanonical Wnt signaling pathways. Here, we examined the possible participation of Lrp5/6 in noncanonical Wnt signaling. We found that Lrp6 physically interacts with Wnt5a, but that this does not lead to phosphorylation of Lrp6 or activation of the Wnt/β-catenin pathway. Overexpression of Lrp6 blocks activation of the Wnt5a downstream target Rac1, and this effect is dependent on intact Lrp6 extracellular domains. These results suggested that the extracellular domain of Lrp6 inhibits noncanonical Wnt signaling in vitro. In vivo, Lrp6−/− mice exhibited exencephaly and a heart phenotype. Surprisingly, these defects were rescued by deletion of Wnt5a, indicating that the phenotypes resulted from noncanonical Wnt gain-of-function. Similarly, Lrp5 and Lrp6 antisense morpholino-treated Xenopus embryos exhibited convergent extension and heart phenotypes that were rescued by knockdown of noncanonical XWnt5a and XWnt11. Thus, we provide evidence that the extracellular domains of Lrp5/6 behave as physiologically relevant inhibitors of noncanonical Wnt signaling during Xenopus and mouse development in vivo.
INTRODUCTION
Wnts are extracellular lipoglycoproteins that activate several downstream signaling pathways depending on cellular context. The best defined pathways include the canonical Wnt/β-catenin pathway and the noncanonical Wnt/planar cell polarity (PCP) pathway. These two pathways regulate distinct biological processes. Certain components of Wnt signaling machinery are, based on current evidence, believed to be dedicated to only one of these two paths. Such components include Wnt ligands, receptors/coreceptors, and cytoplasmic components, in which Wnt1/Wnt3a, Lrp5/6 (XLRP5, and XLRP6 in Xenopus) and axin/APC/GSK3 are usually associated with the canonical Wnt pathway (Clevers, 2006) and Wnt5a/Wnt-11, Vangl1/2, Celsr1, and Rho/Rho kinase/c-Jun NH2-terminal kinase are associated with the noncanonical Wnt pathway (Seifert and Mlodzik, 2007).
Deficiency in the core components of the vertebrate Wnt/PCP pathway results in defects in embryonic development that are different from the defects found in Wnt/β-catenin pathway mutants (van Amerongen and Berns, 2006; Seifert and Mlodzik, 2007). The Wnt/PCP pathway is usually associated with the regulation of cell polarity and/or cell migration. In line with this, both gain-of-function (GOF) and loss-of-function (LOF) in Wnt/PCP often produce similar/identical phenotypes (Fanto and McNeill, 2004; Klein and Mlodzik, 2005; Schambony and Wedlich, 2007).
It was suggested that membrane proteins and proteoglycans that act as Wnt coreceptors, e.g., Lrp5/6, Ror1/2, and Knypek (Wehrli et al., 2000; Topczewski et al., 2001; Oishi et al., 2003; Mikels and Nusse, 2006; Schambony and Wedlich, 2007), may be the factors deciding the predominant direction of Wnt signaling. A large body of evidence suggests that Lrp5 and Lrp6 are crucial coreceptors for Wnt/β-catenin signaling (Pinson et al., 2000; Tamai et al., 2000; Wehrli et al., 2000). However, a recent report by Tahinci et al. (2007) suggests that the intracellular part of Lrp6 can also act as an inhibitor of noncanonical Wnt signaling in Xenopus. Yet, it is not clear whether there is a general role of endogenous Lrp5/6 as an inhibitor of noncanonical Wnt signaling, and how Lrp5/6 achieves its inhibitory action.
Here, we performed in vitro and in vivo experiments, both in Xenopus and mouse, to further define the involvement of endogenous Lrp5/6 in noncanonical Wnt signaling. We show that Wnt5a physically interacts with Lrp6, and overexpression of Lrp6 inhibits the activity of the Rho GTPase Rac1. Moreover, Lrp5 and/or Lrp6 deficiency in Xenopus and mouse caused noncanonical Wnt gain of function (GOF) defects, which could be rescued by ablation of noncanonical Wnts. These data provide for the first time the evidence that extracellular parts of Lrp5/6 can sequester noncanonical Wnt ligands and act as physiologically relevant inhibitors of noncanonical Wnt signaling in multiple organs of Xenopus and mouse, including the heart and neural tube, during vertebrate development.
MATERIALS AND METHODS
Tissue Culture
SN4741 cells were obtained from Dr. J. H. Son (Son et al., 1999). B1A and B1A overexpressing hemagglutinin (HA)-Wnt5a (HA-Wnt5a-B1A) fibroblasts were a kind gift of Jan Kitajewski (Columbia University, New York, NY; Shimizu et al., 1997). SN4741 and human embryonic kidney (HEK) 293 cells were grown and treated as described previously (Schulte et al., 2005; Bryja et al., 2007b).
Wnt3a and Wnt5a (R&D Systems, Minneapolis, MN) were tested for activity using previously established protocols (Bryja et al., 2007a,b). Concentrations required for maximal activity (Dvl shift) varied from batch to batch.
Western Blotting, Rac1 Activity Assay and Immunoglobulin G (IgG) Pull-Down
Immunoblotting and sample preparation were done as published previously (Bryja et al., 2007a). When required, signal intensity was quantified using Scion Image densitometry software (Scion, Frederick, MD). Antibody details are given in Supplemental Material. Activity of Rac1, Rho, and Cdc42 was analyzed essentially as published previously (Unterseher et al., 2004). Briefly, glutathione transferase (GST)-p21-activated kinase (PAK)-CDC42/Rac interactive binding domain (CRIB), GST-Wiskott-Aldrich syndrome protein (WASP)-CRIB, GST-RHOtekin recombinant proteins were coupled to glutathione-Sepharose beads for detection of activated RAC-1, CDC42, and RHO A, respectively. Cells were washed with ice-cold phosphate-buffered saline (PBS) and subsequently allowed to lyse in ice-cold lysis buffer (10 mM Tris-Cl, pH 7.5, 110 mM NaCl, 1 mM EDTA, 10 mM MgCl2, 1% Triton X-100, 0.1% SDS, 20 mM β-glycerophosphate, 1 mM dithiothreitol, and complete protease inhibitors (Roche Molecular Biochemicals, Indianapolis, IN) for 5 min. Crude cell lysates were spun down in chilled tubes at 14,000 rpm for 5 min at 4°C. Supernatants (5% saved as input) were supplemented with bait proteins coupled to glutathione-Sepharose beads, and tubes were incubated rotating end-over-end at 4°C for 15 min. Beads were washed three times with washing buffer (lysis buffer without SDS and protease inhibitors) on ice and subsequently mixed with 2× Laemmli buffer. Each sample was boiled (5 min) before loading on SDS-polyacrylamide gel electrophoresis (PAGE).
For analysis of the interaction of Lrp6 and Wnt5a by IgG pull-down, B1A and HA-Wnt5a-B1A cells were transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) with a vector encoding the extracellular part of Lrp6 fused with human Fc fragment (Lpr6N-Fc; Tamai et al., 2000). After 2 d, culture media were collected and supplemented with 0.5% NP-40, and remaining cells were extracted for 15 min in ice-cold lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM sodium chloride, 0.5% NP-40, 1 mM EDTA, 1× protease inhibitor cocktail [Roche Diagnostics]) to generate samples of conditioned media and cell lysate, respectively. Then samples were cleared by centrifugation at 15,000 × g for 5 min at 4°C and incubated with protein G-coupled Sepharose beads (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) overnight. Next day, beads were washed with lysis buffer five times, mixed with 2× Laemmli buffer, and subjected to SDS-PAGE.
Frog Handling, Microinjections, and Keller Explants
Embryos were obtained by in vitro fertilization, cultured, and injected as described previously (Unterseher et al., 2004). Embryos were injected at the four-cell stage in both dorsal blastomeres or at the eight-cell stage in one dorsal blastomere. If not indicated otherwise, injection amounts of plasmids were 60 pg of mLRP5 or mLRP6; 5 pg of constitutively active (ca) RhoA; 10 pg of ca Rac 1; 100 pg of β-catenin, XWnt5a, dominant-negative (dn) RhoA, or dn Rac 1; 20 pg of XWnt-11; and 100 pg of β-galactosidase; MOs were 0.8 pmol (all MOs: GeneTools, Philomath, OR; sequences are given in Supplemental Material). Keller open face explants for analysis of convergent extension movements were prepared at stage 10.5 and cultured, imaged, and scored as described previously (Unterseher et al., 2004). Statistical evaluation was performed using Student's t test. Whole mount in situ hybridizations were carried out using the digoxigenin/alkaline phosphatase detection system (Roche Molecular Biochemicals) as described previously (Hollemann et al., 1999).
Mouse Strains and Genotyping
Lrp6 (Pinson et al., 2000) and Wnt5a (Yamaguchi et al., 1999a) mutant mice were housed, bred, and treated in accordance with the ethical approval for animal experimentation granted by Stockholms Norra Djurförsöks Etiska Nämnd. The genotyping was performed by polymerase chain reaction and is described in detail in Supplemental Material.
Whole-Mount in Situ Hybridization (ISH) of Mouse Embryos
The original cDNA clones described in the literature were used as templates for the generation of cRNA probes. Details are available upon request. Whole-mount in situ hybridization was performed as described previously (Wilkinson and Nieto, 1993). Embryos were photographed on a stereoscope (Leica, Wetzlar, Germany) or an Axiophot (Carl Zeiss, Jena, Germany) compound microscope. Unless indicated otherwise, at least three mutant embryos were examined with each probe, and all yielded similar results.
Heart Analysis in Mouse
Before embedding, embryos were fixed in 4% paraformaldehyde overnight, and then they were incubated 12 h at 4°C in 15% sucrose in PBS. Embryos at stage embryonic day (E) 10.5 were embedded in 7.5% gelatin/sucrose and at stage E14.5 in Tissue-Tek OCT (Labonord, Villeneuve d'Ascq, France). Ten- to 16-μm-thick sections were obtained using a cryostat. Slides were washed 2 × 10 min in PBS at 37°C and stained for 30 min in 0.5% eosin solution (Labonord), progressively dehydrated to 100% ethanol, and mounted into Cytoseal (Richard Allan Scientific, Kalamazoo, MI).
RESULTS
Lrp6 Interacts with Wnt5a and Blocks Noncanonical Wnt Signaling in Vitro
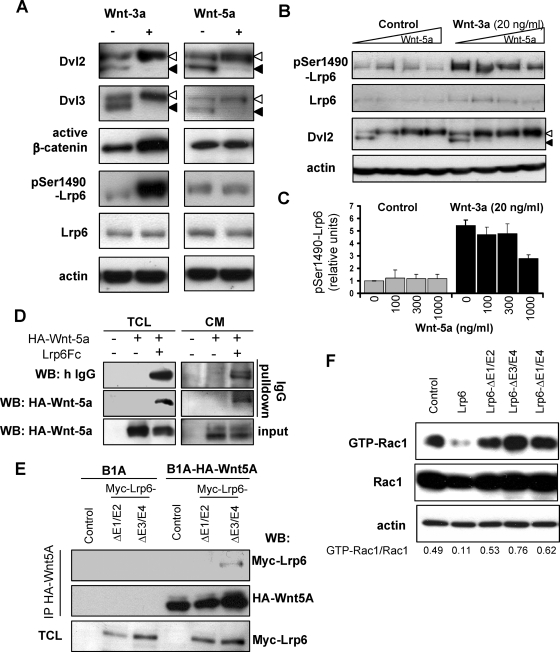
To analyze the involvement of Lrp6 in the noncanonical Wnt pathway, we treated SN4741 cells with Wnt5a and used Wnt3a (a canonical Wnt) for comparison. Treatment with either Wnt (100 ng/ml) led to the phosphorylation of Dvl2 and Dvl3, detected by a mobility shift of the protein on SDS-PAGE, as shown previously (Gonzalez-Sancho et al., 2004; Schulte et al., 2005). Although both Wnt3a and Wnt5a induced Dvl phosphorylation, only Wnt3a induced β-catenin activation and Lrp6 phosphorylation at Ser1490 (Tamai et al., 2004), as assessed by antibodies recognizing active β-catenin (ABC; the form of β-catenin dephosphorylated on Ser37 and Thr41; van Noort et al., 2002) or pSer1490-Lrp6 (Figure 1A). These data demonstrated the ability of Wnt3a, but not Wnt5a, to induce Wnt/β-catenin signaling via phosphorylation of Lrp6.
Figure 1.
Wnt5a can bind Lrp6, inhibit Lrp6 phosphorylation and Lrp6 inhibits Rac1 activation. (A) SN4741 cells were stimulated with 100 ng/ml Wnt3a or Wnt5a. Stimulation with either of these led to the phosphorylation of Dvl2 and Dvl3 (indicated by open arrowheads), as assessed by a mobility shift in a Western blot. Only Wnt3a led to an increase in active β-catenin and phosphorylation of Lrp6 at Ser1490. (B) Wnt5a inhibits Wnt3a-induced phosphorylation of Lrp6 at Ser1490, but not Dvl2 phosphorylation, in a dose-dependant manner. Total Lrp6 did not significantly change by Wnt treatment. Actin was used as loading control. The level of Lrp6 phosphorylation from three independent experiments is quantified in C. (D) Lrp6 Fc can associate with HA-Wnt5a. Lrp6 Fc was overexpressed in HA-Wnt5a expressing B1A fibroblasts (B1A fibroblasts were used as a control). Total cell lysates (TCL) or conditioned media (CM) were subjected to IgG pull-down, and HA-Wnt5a was detected only in samples also expressing Lrp6-Fc. (E) Myc-tagged Lrp6 mutants lacking either E1 and E2 (Myc-Lrp6ΔE1-E2) or E3 and E4 (Myc-Lrp6ΔE3-E4) were overexpressed in B1A, or B1A cells stably expressing HA-tagged Wnt5a. Cells lysates were immunoprecipitated using antibody directed against HA-tag. Expression of Myc-Lrp6 and HA-Wnt5a in immunoprecipitates was determined by Western blotting. (F) HEK cells were transfected with Myc-Rac1 and indicated Lrp6 constructs. The activation of Rac1 was determined using Rac1 activation assay, and Western blot for Myc-Rac1. The signal ratio for GTP-Rac1/Rac1 was quantified and demonstrates that only full length Lrp6, but not mutants in any of the extracellular domains of Lrp6, inhibited Rac1 activity.
Wnt-induced phosphorylation of Lrp6 at Ser1490 was shown to recruit axin to Lrp6 and promote further downstream signaling to β-catenin (Tamai et al., 2004; Davidson et al., 2005; Zeng et al., 2005). Wnt5a failed to activate Lrp6 and the β-catenin pathway in SN4741 cells (Figure 1A), although it induced phosphorylation of Dvl. Such activation of Dvl was shown to be Lrp6-independent (Gonzalez-Sancho et al., 2004). Based on this finding, it was expected that Wnt5a should not interfere with Wnt3a-induced phosphorylation of Lrp6. However, in Wnt3a-treated (20 ng/ml) SN4741 cells, Wnt5a efficiently reduced, in a dose-dependent manner, theWnt3a-induced phosphorylation of Lrp6 at Ser1490 (Figure 1, B and C). These data suggested that Wnt5a directly or indirectly interfered with the phosphorylation of Lrp6 induced by Wnt3a. We therefore examined whether Wnt5a could bind or physically interact with Lrp6.
To explore this possibility, we overexpressed the extracellular part of Lrp6 fused to the Fc fragment of human IgG (Lrp6N-Fc; Tamai et al., 2000) in B1A fibroblasts and in B1A fibroblasts overexpressing HA-tagged Wnt5a (HA-Wnt5a; Shimizu et al., 1997). Cell lysates and serum free-conditioned media were subjected to hFc (IgG) pull-down by incubation with protein G-Sepharose beads and subsequent Western blotting. As shown in Figure 1D, after IgG pull-down HA-Wnt5a was present only in the samples expressing Lrp6-Fc but not in any of the control conditions. Extracellular domains E1 and E2 (YWTD EGF repeats), but not E3 and E4, of Lrp6 are required for binding of Wnt5a to Lrp6 (Figure 1E) as shown previously for Wnts activating the Wnt/β-catenin pathway (Mao et al., 2001). These results demonstrated that the extracellular part of Lrp6 can physically interact with Wnt5a. Because recombinant Wnt5a can induce the small Rho GTPase Rac1 (Andersson et al., 2008), a downstream component of the Wnt/PCP pathway, we next tested whether activity of Rac1 was Lrp6 dependent. As we show in Figure 1F, the overexpression of Lrp6 reduced the activity of Rac1 (Figure 1F). Importantly, Lrp6 mutants lacking either the Wnt-5a binding (ΔE1-E2; Figure 1E), or Dkk1 binding (ΔE3-E4) or the entire extracellular domain (ΔE1-E4) of Lrp6 (Mao et al., 2001) were not sufficient to reduce the activity of Rac1. These findings suggested that extracellular domains of Lrp5/6 may work as inhibitors of noncanonical Wnts and prompted us to test this hypothesis in vivo.
XLRP5 Is Essential for Convergent Extension Movements in Xenopus
In vertebrate embryos, β-catenin–independent Wnt pathways regulate several developmental processes, including convergent extension (CE) movements in the gastrulating Xenopus embryo. To determine the importance of Lrp5 and Lrp6 in Xenopus CE in vivo, we modulated the levels of Lrp5/6 by injecting mRNAs encoding mouse Lrp5 or Lrp6 or antisense morpholino-oligonucleotides directed against XLRP5 and XLRP6 (XLRP5 MO and XLRP6 MO, Supplemental Figure S1A) and examined Keller explants of the dorsal marginal zone. The scheme of timing of injections performed in Xenopus embryos and subsequent analysis is shown in Supplemental Figure S1B.
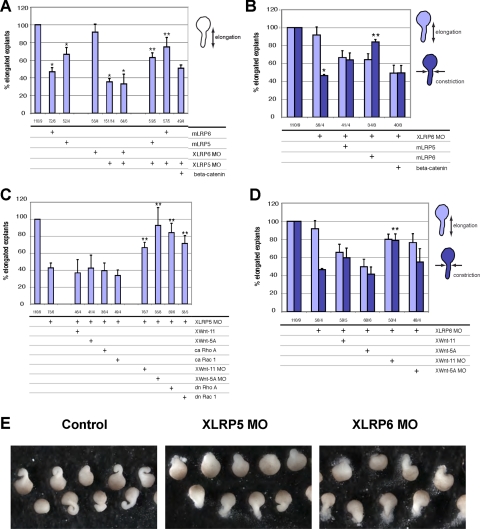
We show in Figure 2A, in agreement with Tahinci et al. (2007) that XLRP5 MO strongly affected explant elongation, whereas XLRP6 MO had less effect. However, depletion of XLRP6 affected explant constriction (Figure 2B), which indicated defective noncanonical Wnt signaling (Unterseher et al., 2004; Schambony and Wedlich, 2007). Coinjection of both XLRP MOs blocked elongation similarly to XLRP5 MO alone. As expected, overexpression of mLrp5 or mLrp6 also affected explant elongation similarly (Figure 2A). Coinjection of XLRP5 MO or XLRP6 MO with mLRP5 or mLRP6 induced a partial rescue of explant elongation and constriction, respectively (Figure 2, A and B), indicating partial redundancy of LRP5/6 and demonstrating that the effects of XLRP MOs were specific. Importantly, the effects of XLRP5 depletion seem to be β-catenin independent because coinjection of β-catenin RNA with XLRP5 MO did not rescue the phenotype (Figure 2A).
Figure 2.
Lrp5/6 are crucial regulators of convergent extension (CE) movements in Xenopus. (A and B) Injection of Lrp5 or Lrp6 mRNA or XLRP5/6 MOs all inhibit convergent extension of Keller explants from stage 10.5 that are rescued by mLrp5/6 but cannot be rescued by β-catenin coinjection (*, significant difference from control; **, significant rescue of MO, p > 0.95). (C and D) The CE defects induced by XLRP5 MO or XLRP6 MO are not rescued by Wnt5a or Wnt11 overexpression or constitutively active (ca) RhoA and Rac1. However, down-regulation of noncanonical signaling by XWnt5a or XWnt11 MO rescued XLRP5 and XLRP6 depletion phenotypes. XLRP5 MO induced inhibition of elongation was also rescued by dn RhoA and Rac1 (**, significant rescue of MO; p > 0.95). (E) Typical morphology of Keller explants injected with XLRP5 and XLRP6 MOs. The numbers under the graphs indicate number of injected embryos/number of independent experiments.
It should be noted that, when injected into the dorsal marginal zone, XLRP5 MO induced other defects, which are associated with a β-catenin LOF, e.g., ventralization. We scored these defects by calculating the dorso-anterior index (Kao and Elinson, 1988) and found that XLRP5 MO-induced ventralization was rescued by coinjection of mLrp5 and to the same extent also by β-catenin (Supplemental Figure S1C), indicating that these phenotypes are the result of defective canonical Wnt signaling caused by XLRP5 depletion.
To identify the molecular mechanism responsible for the observed phenotypes of XLRP5 knockdown in Xenopus embryos, we performed a set of rescue experiments. We hypothesized that if the XLRP5 knockdown caused an increase in noncanonical Wnt signaling, as our in vitro experiments suggested, negative but not positive regulators of noncanonical Wnt signaling should rescue the phenotype. Coinjection of XWnt5a, XWnt11, and ca forms of known downstream effectors of Wnt/PCP pathway—ca RhoA and ca Rac1 (Habas et al., 2003) did not rescue the phenotype (Figure 2C). Instead, inhibition of noncanonical Wnt pathways by knockdown of XWnt5a or XWnt11 or dn forms of Rac1 or RhoA were able to rescue XLRP5 MO. In XLRP6 MO, we observed that coinjection of XWnt-11 MO but not XWnt-5a MO restored constriction (Figure 2D). These and the previous experiments suggest that the depletion of XLRP5/6 induced a noncanonical Wnt GOF phenotype.
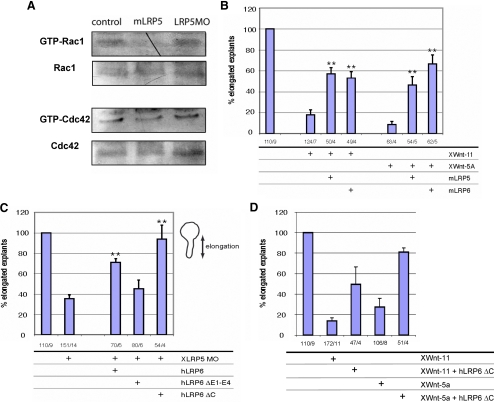
These results indicate that Lrp5/6 interact with and sequester noncanonical Wnts, partially preventing their physiological activity and thereby inhibiting noncanonical signaling. To test the possibility that Lrp5/6 overexpression directly blocks the activity of small GTPases, we measured the activity of Rac1 and Cdc42, two GTPases that have been shown to be under the control of noncanonical Wnt signaling in Xenopus embryos (Habas et al., 2003; Penzo-Mendez et al., 2003; Kim and Han, 2005; Schambony and Wedlich, 2007). As we show in Figure 3A, overexpression of XLRP5 negatively regulates the activity of Rac1 and Cdc42. The XLRP5 MOs have a weak positive effect, which, especially in Cdc42, might be due to its effects on total Rac1/Cdc42. We were unable to detect significant differences in the activity of RhoA (data not shown). These results provide biochemical support for the rescue experiments and demonstrate that Lrp5 affects noncanonical Wnt signaling via modulation of the activity of small GTPases.
Figure 3.
(A) Embryos injected with mLRP5 or XLRP5 MO were lysed and analyzed for the activity of small GTPases Rac1 and Cdc42. (B) XWnt5a- or XWnt11-induced CE defects can be partially rescued by coinjection of Lrp5 or Lrp6 (**, significant rescue of XWnt-11 and XWnt-5a, respectively; p > 0.95). (C) Effects of FL and mutant hLRP6 on CE defects induced by XLRP5 MOs. hLRP6 and also hLRP6 lacking cytoplasmic domain (hLRP6ΔC) can efficiently rescue XLRP5 MO defects, whereas hLRP6 lacking the extracellular domains E1-E4 cannot (**, significant rescue of MO; p > 0.95). (D) Lrp6 lacking cytoplasmic domain (hLRP6ΔC) can rescue elongation defects caused by overexpression of XWnt-11 or XWnt-5a. The numbers under the graphs indicate number of injected embryos/number of independent experiments.
To further test our model, we overexpressed XWnt11 and XWnt5a, which resulted in a strong inhibition of explant elongation by noncanonical GOF (Figure 3B). This inhibition of explant elongation by XWnt-11 or XWnt-5a overexpression was rescued by coinjection of either mLrp5 or mLrp6 (Figure 3A). To test which LRP-domains are required for its function in CE, we attempted to rescue the stronger XLRP5 knockdown phenotype with hLrp6 deletion mutants (Figure 3C). The mutant lacking the extracellular domains (ΔE1-E4; Mao et al., 2001) failed to rescue XLRP5 MO, whereas the mutant without cytoplasmic domain (Lrp6 ΔC; Tamai et al., 2004) efficiently improved CE defects induced by XLRP5 MOs. Importantly, Lrp6 lacking the intracellular domain (Lrp6 ΔC) was sufficient to rescue elongation defects caused by overexpression of XWnt-11 or XWnt-5a (Figure 3D). This data confirmed that Lrp5/6, and specifically the extracellular domains, in addition to playing a role in the Wnt/β-catenin pathway, are physiologically relevant inhibitors of noncanonical Wnt signaling.
XWnt11 MO Rescues the Heart Phenotype Induced by XLRP5/6 MO in Xenopus embryos
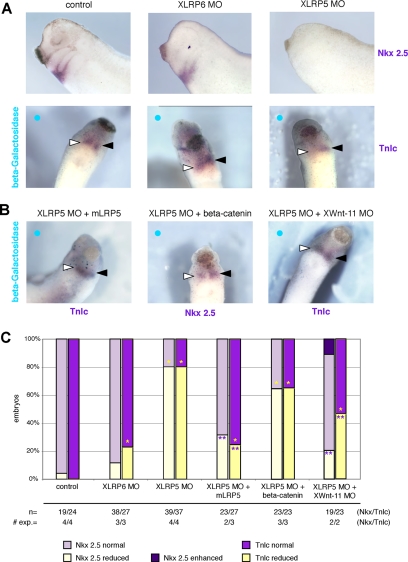
Noncanonical Wnts are known to regulate not only CE movements but also heart development in Xenopus (Pandur et al., 2002). To analyze the role of Lrp5/6 in heart development we investigated the expression of cardiac marker genes in embryos injected with XLRP MOs targeted to the presumptive cardiac mesoderm in eight–cell-stage embryos to avoid convergent extension and primary-axis defects (Supplemental Figure S1B). XLRP5 MO, and to a lesser extent XLRP6 MO, caused a down-regulation of the cardiac markers Nkx 2.5 and troponin (Figure 4A), two defects also observed in the XWnt-11 knockdown (Pandur et al., 2002). However, heart development is regulated by both canonical and noncanonical Wnt signaling (Brade et al., 2006) and both pathways could be potentially affected by XLRP5 depletion. To better distinguish the contribution of each pathway and to demonstrate specificity of the observed defects, we performed a set of rescue experiments. The cardiac markers Troponin and Nkx 2.5 were rescued by coinjection of mLRP5, which demonstrates the specificity of XLRP5 MO. However, only a partial rescue was observed after coinjection of β-catenin (Figure 4, B and C), suggesting that the XLRP5 knockdown phenotype is at least partially β-catenin independent. To test whether this XLRP5 MO cardiac phenotype is contributed to by noncanonical Wnt signaling, as shown previously for CE movements, we coinjected XWnt11 MO. We observed a partial rescue of the XLRP5 MO cardiac phenotype by XWnt11 MO (Figure 4, B and C), suggesting that XLRP5 MO induces a noncanonical Wnt GOF as shown for CE movements above. In summary, these data demonstrate that endogenous XLRP5/6 regulates not only canonical Wnt signaling but also CE movements and heart development, two processes driven by noncanonical Wnt signaling in Xenopus. Furthermore, our results suggest that the inhibition of noncanonical Wnt signaling by Lrp5/6 is achieved by an interaction of Lrp5/6 with noncanonical Wnts, which may reduce the availability of noncanonical Wnts for signaling.
Figure 4.
XLRP5/6 regulate heart development. (A) Knockdown of XLrp5 and XLrp6 affects heart development. Embryos injected with XLRP5 and XLRP6 MO in one or both dorsal blastomeres were analyzed by whole mount ISH for the expression of heart markers Nkx2.5 and Troponin lc (Tnlc) at stage 28. Injected side shown by blue circle in single-blastomere injections, open triangle shows Tnlc/Nkx2.5 on injected side, closed triangle shows TnIc/Nkx2.5 on uninjected side. (B) XLRP5 MO-induced reduction in heart markers can be rescued with mLrp5 and XWnt-11 MO (Tnlc) and only partially rescued with β-catenin overexpression (Nkx2.5). (C) Quantification of ISH analysis of cardiac markers (*, significantly differs from β-galactosidase controls; p > 0.95); **, significantly differs from XLRP5MO; p > 0.95).
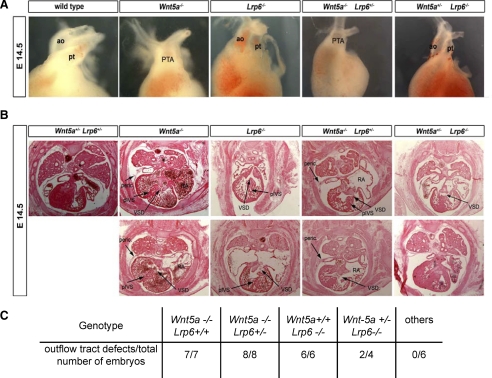
Lrp6-deficient Mice Display Heart Malformations That Are Partially Rescued by Deletion of Wnt5a
Based on our analysis of Wnt signaling in vitro and in vivo, in Xenopus embryos, we hypothesized that Lrp5 or Lrp6 should also interact with noncanonical Wnts and regulate heart development in mice. To test this hypothesis, we analyzed heart morphology at E10.5 and E14.5 in Lrp6 and Wnt5a mutant mice (Yamaguchi et al., 1999a; Pinson et al., 2000). At E10.5, no obvious defects in heart morphology were detected, although Lrp6−/− hearts were a little bit smaller (Supplemental Figure S2), likely reflecting the overall smaller size of Lrp6 mutant embryos (Pinson et al., 2000). However, at E14.5 major outflow tract deformities were observed in either Lrp6 or Wnt5a mutants (Figure 5, A and B). Wnt5a mutants exhibited persistent truncus arteriosus (PTA) and the right atrium was expanded, whereas in Lrp6 mutants the great arteries were separated, resulting in a transposition of the great arteries.
Figure 5.
Lrp6 mutant mice exhibit heart defects, which can be rescued by Wnt-5a heterozygosity. (A) Heart morphology in Lrp6 and Wnt5a mutants at E14.5. Heart of E14.5 wild type, Lrp6−/− and Wnt5a−/− mouse embryos was dissected and the morphology was analyzed. Representative examples are shown. ao, aorta; pt, pulmonary artery; PTA, persistent truncus arteriosus. (B) Hearts of embryos (E14.5) with indicated genotypes were sectioned and stained with hematoxylin/eosin. Typical heart defects are indicated by arrows. peric., pericardium; pIVS, porous intraventricular septum; VSD, ventricular septal defects; RA, right atrium. (C) Quantification of arterial pole deformities of embryos in individual genotypes.
Analysis of Wnt5a single mutants revealed not only defects in intraventricular septation, with PTA, but also ventricular septal defects (VSD). Defects in the closure of the intraventricular septum (IVS) were observed in two of three analyzed embryos. The myocardium in the Wnt5a mutants was abnormally thin, the right atria were expanded, and the pericardium showed an abnormal morphology compared with wild type. In Lrp6 single mutants, we also observed VSD, the septum seemed porous, and the myocardium abnormally thin. No pericardial deformities were found in the Lrp6 mutants. In summary, at E14.5, both Wnt5a and Lrp6 single mutants displayed arterial pole defects. Similar defects were observed in other mutants of the noncanonical signaling pathway, such as that for Vang-like 2 factor (Henderson et al., 2006) and Dvl2 mutants (Hamblet et al., 2002).
These data suggest that both Lrp6 and Wnt5a are necessary for proper heart development in mouse. Based on the previous results we concluded that the heart phenotype of Lrp6 mutants could in part be due to an excess of noncanonical signaling. We therefore decided to analyze the effect of removing one allele of Wnt5a at a later stage of heart development (E14.5) in Lrp6 mutants, expecting that an excess of noncanonical signaling in Lrp6 mutants may be mitigated by reducingWnt5a.
Strikingly, analysis of Lrp6 null mice in which one allele of Wnt5a had been removed led to a partial or complete rescue of the heart defects seen in Lrp6 single mutants. Specifically, two of four compound Wnt5a+/− Lrp6−/− embryos did not display any heart defects at E14.5 (Figure 5C), and the heart defects in the two remaining compound Wnt5a+/− Lrp6−/− embryos showed a marked improvement in myocardium and IVS, and the phenotype was less severe than that in Lrp6−/− mice (Figure 5B). E14.5 Wnt5a−/− Lrp6+/− embryos showed identical heart defects to Wnt5a−/− Lrp6+/+ (Figure 5, A and B). Our analysis of heart development in mice thus supports and extends our findings obtained in Xenopus. Together, these data indicate that Lrp6 deficiency causes noncanonical Wnt pathway GOF phenotypes and that Lrp6 can inhibit noncanonical Wnt signaling in vivo. Furthermore these findings underline the importance of Wnt/PCP signaling in heart development and particularly in outflow tract morphogenesis.
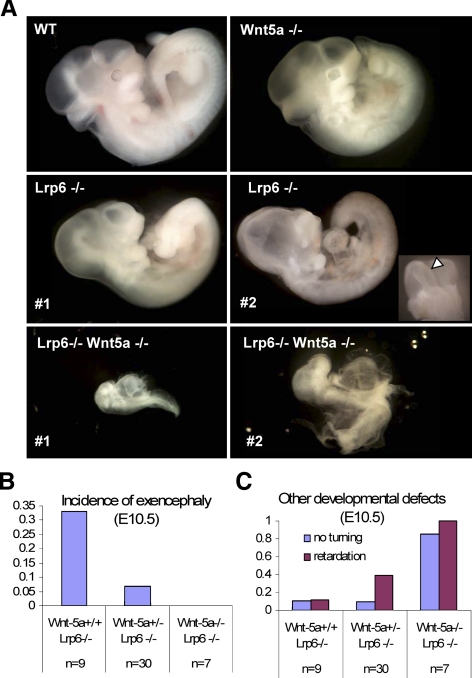
Deletion of Wnt5a Completely Rescues Exencephaly in Lrp6 Mutant Mice
To define in more detail the functional interaction between Lrp6- and Wnt5a-driven pathways in vivo and to ascertain whether that interaction is more widespread than anticipated previously, we studied the neural phenotype of compound Wnt5a and Lrp6 mutants. Both Wnt5a and Lrp6 deficiencies are embryonal lethal and we thus crossed Wnt5a+/− mice with Lrp6+/− mice to generate double heterozygous Wnt5a+/− Lrp6+/− animals. These double heterozygous animals were born with the expected Mendelian frequency (25.13%, expected 25%), and we did not notice any gross morphological, fertility, or behavioral defects in comparison with parental Wnt5a+/− or Lrp6+/− mutants. A small proportion (<5%) of Wnt5a +/− Lrp6+/− mice showed tail deformities, which were also present at comparable frequency in Lrp6+/− mice. After crossing of double-heterozygous mice, we recovered Lrp6−/−Wnt5a−/− embryos at E10.5 at the expected frequency. All Lrp6−/−Wnt5a−/− double null embryos were severely developmentally delayed (Figure 6A), and we did not obtain any Wnt5a/Lrp6 double knockout embryos at E12.5.
Figure 6.
Analysis of Wnt5a;Lrp6 double null mice at E10.5: Wnt5a deficiency rescues exencephaly but promotes general retardation of Lrp6−/−embryos. (A) Wnt5a and Lrp6 single mutants occur as described previously at E10.5, with some Lrp6 embryos exhibiting exencephaly (arrow), compound Wnt5a−/− Lrp6−/− display developmental delay and fail to undergo turning. (B) Loss of Wnt5a in Lrp6 null mice rescued exencephaly. Exencephaly was observed in 30% of Lrp6 null mutants, this frequency was partially rescued by loss of one allele of Wnt5a and completely rescued by loss of both Wnt5a alleles. (C) Developmental delay and a failure to undergo turning was only observed in 10% of Lrp6−/− mice but was exacerbated by loss of Wnt-5a and occurred in almost in 100% of the Wnt5a−/− Lrp6−/− double knockout mice.
We observed exencephaly (neural tube completely open in cephalic region) in ∼30% of Wnt5a+/+Lrp6−/− embryos at E10.5 (Figure 6A, open arrow) and in 25% of Lrp6−/− embryos at E12.5 (n = 20). Neural tube closure defects such as exencephaly are usually associated with aberrant CE movements and are observed in several other mutants of the Wnt/PCP pathway components such as Dvl2, Vangl2, and Wnt5a-deficient mice (Torban et al., 2004; Wang et al., 2006; Qian et al., 2007). Previous studies directly proved the importance of Wnt5a for neural tube closure in mouse (Qian et al., 2007) and demonstrated that similar defects can also be caused by GOF of noncanonical Wnt ligands (Shariatmadari et al., 2005). Interestingly, the proportion of Lrp6 mutants with exencephaly can be efficiently reduced by Wnt5a heterozygosity and in the double Wnt5a/Lrp6-deficient embryos we observed a complete rescue of exencephaly (Figure 6B). This observation is in good agreement with our in vitro findings, analyses of CE in Xenopus and heart development in Xenopus and mouse. Thus, our results provide evidence that Lrp5/6 deficiency results in noncanonical Wnt GOF defects and attributes Lrp5/6 a role as an inhibitor of noncanonical Wnt signaling in multiple vertebrate systems.
Wnt5a and Lrp6 Cooperate in Early Mouse Embryonic Development
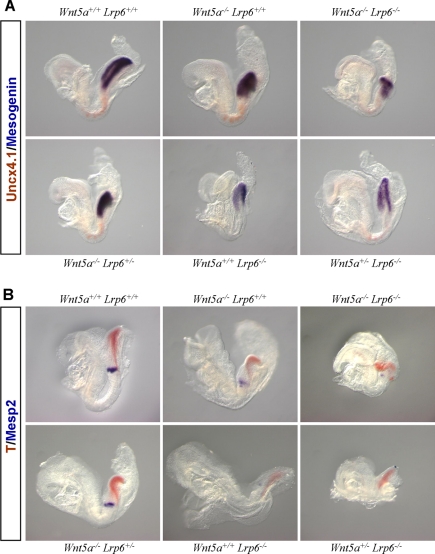
Although it is known that Lrp5/6 is required as a coreceptor for canonical Wnt signaling and we hereby report that Lrp6 can inhibit noncanonical Wnt signaling, we also found evidence that these are not the only two modalities of interaction between Lrp6 and Wnt5a. We noticed that some of the compound Wnt5a/Lrp6 mutants displayed intermediate phenotypes at E10.5, and so we analyzed allelic combinations of Wnt5a and Lrp6. The analyzed phenotypic features included general developmental delay (defined as an embryo <40% size of WT littermates) and a lack of embryo turning, which usually takes place at E9.0. At E10.5, Lrp6−/−Wnt5a−/− embryos showed a complex phenotype that included a developmental delay/lack of turning with almost complete penetrance. A similar phenotype, but less penetrant, was observed in Lrp6−/−Wnt5a+/− embryos (Figure 6C). These data suggest that in addition to Wnt/PCP pathway defects (heart deformities and exencephaly), which were rescued by Wnt-5a deficiency, other mechanistically unrelated interactions exist between Lrp6- and Wnt5a-driven pathways during early morphogenesis. We hypothesized that such defects could result from the combination of defects in the Wnt/β-catenin pathway of the Lrp6−/− mutants and the Wnt/PCP pathway of Wnt5a−/− mutants resulting in additive or synergistic effects in other developmental processes such as embryonic turning. Analysis of embryos with various combinations of Lrp6 and Wnt5a null alleles at E8.5 (Figure 7) confirmed that Lrp6 deficiency resulted in decreased levels of genes directly or indirectly regulated by β-catenin, such as mesogenin (msgn), brachyury (T), and Mesp2 (Yamaguchi et al., 1999b). The expression of these genes is abolished in Wnt3a mutants, and in conditional β-catenin LOF mutants (Dunty et al., 2008). In contrast, Wnt5a−/− embryos did not show any alterations in the expression of β-catenin target genes but showed smaller somites as a result of affected CE movements (Figure 7A). Compound Lrp6−/−Wnt5a−/− mutants showed an additive phenotype, with no evidence for further perturbation of β-catenin target gene expression (in comparison to the Lrp6 null) or of somite condensation (in comparison to the Wnt5a null). Thus, our analysis suggests that the early phenotype of Wnt5a; Lrp6 double mutants (growth retardation and lack of embryo turning) results from defects in the regulation of common biological process by the Wnt/β-catenin and Wnt/PCP pathways, rather than a direct interaction between the two signaling pathways.
Figure 7.
Analysis of β-catenin target genes in Wnt5a, Lrp6 compound mutants: Wnt-5a deficiency does not affect the expression of β-catenin target genes and Lrp6 deficiency does not affect the somite compression. (A) Embryos with indicated genotype were analyzed by two-color whole-mount ISH at E8.5. Somite marker Uncx4.1 is in red and β-catenin target gene mesogenin is in purple. (B) The expression of β-catenin target gene brachyury (T) is in red and a marker of segmentation clock Mesp2 is shown in purple.
DISCUSSION
It is well documented that Lrp5/6 are coreceptors necessary for Wnt signal transduction toward β-catenin (Pinson et al., 2000; Tamai et al., 2000; Wehrli et al., 2000). Here, we demonstrate an additional role for the extracellular domain of Lrp5/6 as an inhibitor of noncanonical Wnt signal transduction. Based on a series of experiments in vitro and in vivo, in Xenopus and mouse, we suggest that Lrp5/6 is a physiologically relevant inhibitor of noncanonical Wnt signaling, which acts by interacting with and reducing the availability of noncanonical Wnts for signaling. This conclusion is based on the following critical pieces of evidence: 1) Wnt5a physically interacts with Lrp6; 2) Lrp6 overexpression blocks activation of Rac1 in vitro; 3) Lrp5 and/or Lrp6 deficiency in Xenopus or mouse results in β-catenin–independent defects in CE and heart development; 4) these defects can be partially rescued by depletion of noncanonical Wnt ligands both in Xenopus and in mouse; and 5) Lrp6 deficiency in mice results in exencephaly, which is completely rescued by deletion of Wnt5a.
Can our findings in XLRP5/6 depleted Xenopus or Lrp6-deficient mouse embryos be explained simply as β-catenin lack-of-function phenotypes influenced by the loss of Wnt5a and/or Wnt11 rather than Lrp5/6 regulating noncanonical Wnt signaling? First, although inhibition of β-catenin signaling has been reported to block CE in Xenopus (Kuhl et al., 2001), we were not able to rescue XLRP5/6 MO phenotypes with β-catenin in Keller explants but rather rescued the phenotype with dn Rac1 or dn RhoA, suggesting that the phenotype is caused by a noncanonical GOF. In contrast, the overexpression of XWnt-8 (Kuhl et al., 2001) and β-catenin (Schambony, unpublished observation) has no negative effect on CE, but we observed CE defects after overexpression of mLrp5 and mLrp6. Consistently, overexpression of hLrp6 ΔE1-E4, which acts as a constitutive activator of canonical Wnt-signaling, had no negative effect on CE (data not shown). Moreover, the effects of XLRP5 MO on dorsal axis formation, which is a well-defined β-catenin-dependent process, were almost abolished by coinjection of β-catenin. Thus, our results suggest that the mechanisms of CE disruption by blocking β-catenin signaling and by XLRP5/6 knockdown are distinct. Second, the analysis of Wnt5a mutant mice either alone or in combination with Lrp6 deletions did not show any alteration (either negative or positive) in the level of β-catenin target genes. Moreover, Wnt5a did not activate canonical Wnt signaling in vitro. Thus, Wnt5a deficiency or treatment does not affect β-catenin target genes despite the fact that selected β-catenin target genes are expressed in the Wnt5a expression domain (Yamaguchi et al., 1999a). Wnt5a has also been shown to interfere with the β-catenin pathway, but such inhibition requires activation of canonical Wnt signaling and/or specific cellular and receptor contexts (He et al., 1997; Tao et al., 2005; Mikels and Nusse, 2006; Bryja et al., 2007b; Kofron et al., 2007). Thus, our results support the idea that deletion of Lrp6 or knockdown of Lrp5 results not only in β-catenin-dependent phenotypes, as described previously, but also in a disinhibition of noncanonical Wnt signaling that results in diverse noncanonical GOF phenotypes such as defects in CE movements, cardiac outflow tract morphogenesis, and neural tube closure.
During cardiac development, canonical Wnt-signaling is required in the very early phase of cardiac precursor specification in the mesoderm (Naito et al., 2006; Ueno et al., 2007), followed by an inhibition of the same pathway by DKK1 and crescent (Marvin et al., 2001; Schneider and Nimpf, 2003) and activation of noncanonical pathways by Wnt-11 as shown by GOF and LOF (Eisenberg and Eisenberg, 1999; Pandur et al., 2002; Garriock et al., 2005; Ueno et al., 2007). Why does the increase in noncanonical Wnt signaling upon XLRP5 knockdown result in a loss of cardiac marker genes? Because both GOF and LOF of noncanonical Wnt signaling result in impaired cell polarity, deficits in migration of cardiac precursors to the anterior ventral side of the embryo would alter the exposure of cardiac precursors to inductive signals and therefore result in the loss of cardiac markers. This interpretation is also consistent with a recently proposed model of cardiac precursor migration and specification (Eisenberg and Eisenberg, 2006) and the regulation of cell–cell adhesion by noncanonical Wnts (Brade et al., 2006).
It is believed that Lrp5/6 provides specificity to Wnt signaling by directing signaling to the β-catenin pathway (Mikels and Nusse, 2006). Although not empirically tested, binding specificity for canonical Wnts was thought to be a part of that mechanism (He et al., 2004). The physical interaction of Lrp6 and Wnt5a that we report seems to contradict that view. Our experiments demonstrate that Wnt5a can bind the extracellular soluble domain of Lrp6, although Wnt5a (in contrast to Wnt3a) cannot induce Ser1490-phosphorylation of Lrp6 or β-catenin activation. It is not clear whether the interaction between Lrp5/6 and Wnt5a is direct or mediated by other protein/proteoglycans such as the Wnt/PCP pathway component Knypek (Topczewski et al., 2001), which can bind Dkk1 (Caneparo et al., 2007), a high-affinity ligand for Lrp5/6 (Mao et al., 2001). Thus, our data reveal another level of complexity in Wnt signaling because Lrp5/6 binds a larger array of Wnts than previously anticipated, but only a subset of these ligands can activate specific Lrp6 phosphorylation and downstream signaling to β-catenin.
Our functional in vivo studies suggest that Lrp5/6 (in Xenopus) and Lrp6 (in mouse) are negative regulators of noncanonical Wnt signaling. When this study was prepared for publication, Tahinci et al. (2007) reported that a stretch of 36 amino acids from the cytoplasmic domain of Lrp6 can block CE in Xenopus. Our data support the fact that Lrp5/6 inhibit CE via activation of noncanonical Wnt signaling, and they suggest that an additional mechanism of inhibition exists. Our experiments showed that mutations in the extracellular domain strongly interfere with the ability of Lrp6 to either block Rac1 or rescue CE defects caused by XLRP5 knockdown, whereas the membrane-tethered extracellular domain of Lrp6 (Lrp6ΔC) behaved as full-length Lrp6. This suggests that the mechanism of Lrp5/6 function in noncanonical Wnt signaling involves formation of complexes on the cell surface, which sequester noncanonical Wnts ligands and prevent their interaction with signaling receptors such as Frizzleds. The effects of Lrp6 on Rac1 activation require both E1+E2 Wnt-binding domains, and E3+E4 Dkk1-binding domains, pointing out the importance of physical interaction of noncanonical Wnts and Lrp5/6 for the function of Lrp5/6 in noncanonical signaling. This suggests that extracellular complexes organized by Lrp5/6 and inhibiting noncanonical Wnt signaling contain several components, e.g., Wnts, Dkk, and/or membrane glycoproteins such as Knypek (Caneparo et al., 2007).
Our data support a model, where the availability of noncanonical Wnts and the degree of noncanonical Wnt signaling seems to be determined under physiological conditions by the molecular ratio of noncanonical Wnts and free Lrp5/6 available for binding. Indeed, developmental defects caused by the excess of noncanonical Wnt5a and Wnt11 were efficiently rescued by overexpression of Lrp5 or Lrp6, and developmental defects caused by the reduction of Wnt5a or Wnt11 were rescued by knockdown of Lrp5. In agreement with our model, a recent study by Caneparo and colleagues demonstrated that the Lrp5/6 ligand, Dkk1, can act as a β-catenin–independent positive regulator of Wnt/PCP pathway in vivo (Caneparo et al., 2007). Importantly, we hereby demonstrate that the interaction between Lrp5/6 and noncanonical Wnt ligands is physiologically important and regulates normal development.
In summary, we provide several lines of evidence, both in vitro and in vivo, in Xenopus and mouse that define Lrp5 and Lrp6 as general inhibitors of the noncanonical Wnt signaling pathway. Moroever, our results indicate that Lrp5/6 receptors, by binding canonical or noncanonical Wnts, determine not only the level of Wnt signaling through Wnt/β-catenin but also through noncanonical branches of Wnt signaling.
Supplementary Material
ACKNOWLEDGMENTS
We thank Andrew McMahon (Harvard University, Boston, MA) and William Skarnes (Sanger Institute, Cambridge, United Kingdom) for providing Wnt5a and Lrp6 mutant mice. We also thank Jan Kitajewski (Columbia University, New York, NY) for HA-Wnt5a-B1A cells; Dr. J. H. Son (Columbia University, New York, NY) for SN4741 cells; Christoph Niehrs (German Cancer Research Center, Heidelberg, Germany) for vectors encoding hLrp6 and its extracellular deletions; Mikhail Semenov and Xi He (Harvard Medical School) for vectors encoding mLrp5, mLrp6, Lrp6ΔC, and Lrp6-Fc; and Paul Krieg (University of Arizona, Tucson, AZ) for Xenopus Nkx2.5 and Troponin ISH probes. We would also like to thank Michael Kühl (University of Ulm, Ulm, Germany), Sigolene Meilhac (Pasteur Institute, Paris, France), and the Arenas laboratory for critical reading of the manuscript and valuable discussions and Cecilia Olsson, Annika Käller, Claudia Winter, Caroline Berger, and C. Cimper for excellent technical and secretarial assistance. V. B. and M. E. were financed by the “EuroStemCell” project. This work was supported by European Molecular Biology Organization Installation Grant, Academy of Sciences of the Czech Republic (AVOZ50040507 and AVOZ50040702) and Ministry of Education, Youth and Sports of the Czech Republic (MSM0021622430) to V. B.; Swedish Foundation for Strategic Research, Swedish Royal Academy of Sciences, Knut and Alice Wallenberg Foundation, European Commission (Eurostemcell), Swedish Medical Research Council and Karolinska Institutet to E. A.; the Pasteur Institute, the Centre National de la Recherche Scientifique, the European Union Integrated Project Heart Repair to M. B., and the German Research Foundation (Scha965/2-3) to A. S.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-07-0711) on December 3, 2008.
REFERENCES
- Andersson E. R., Prakash N., Cajanek L., Minina E., Bryja V., Bryjova L., Yamaguchi T. P., Hall A. C., Wurst W., Arenas E. Wnt5a regulates ventral midbrain morphogenesis and the development of A9–A10 dopaminergic cells in vivo. PLoS ONE. 2008;3:e3517. doi: 10.1371/journal.pone.0003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade T., Manner J., Kuhl M. The role of Wnt signalling in cardiac development and tissue remodelling in the mature heart. Cardiovasc. Res. 2006;72:198–209. doi: 10.1016/j.cardiores.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Bryja V., Schulte G., Arenas E. Wnt-3a utilizes a novel low dose and rapid pathway that does not require casein kinase 1-mediated phosphorylation of Dvl to activate β-catenin. Cell Signal. 2007a;19:610–616. doi: 10.1016/j.cellsig.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Bryja V., Schulte G., Rawal N., Grahn A., Arenas E. Wnt-5a induces Dishevelled phosphorylation and dopaminergic differentiation via a CK1-dependent mechanism. J. Cell Sci. 2007b;120:586–595. doi: 10.1242/jcs.03368. [DOI] [PubMed] [Google Scholar]
- Caneparo L., Huang Y. L., Staudt N., Tada M., Ahrendt R., Kazanskaya O., Niehrs C., Houart C. Dickkopf-1 regulates gastrulation movements by coordinated modulation of Wnt/beta catenin and Wnt/PCP activities, through interaction with the Dally-like homolog Knypek. Genes Dev. 2007;21:465–480. doi: 10.1101/gad.406007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo-Branco G., Wagner J., Rodriguez F. J., Kele J., Sousa K., Rawal N., Pasolli H. A., Fuchs E., Kitajewski J., Arenas E. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc. Natl. Acad. Sci. USA. 2003;100:12747–12752. doi: 10.1073/pnas.1534900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Davidson G., Wu W., Shen J., Bilic J., Fenger U., Stannek P., Glinka A., Niehrs C. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- Dunty W. C., Jr, Biris K. K., Chalamalasetty R. B., Taketo M. M., Lewandoski M., Yamaguchi T. P. Wnt3a/beta-catenin signaling controls posterior body development by coordinating mesoderm formation and segmentation. Development. 2008;135:85–94. doi: 10.1242/dev.009266. [DOI] [PubMed] [Google Scholar]
- Eisenberg C. A., Eisenberg L. M. WNT11 promotes cardiac tissue formation of early mesoderm. Dev. Dyn. 1999;216:45–58. doi: 10.1002/(SICI)1097-0177(199909)216:1<45::AID-DVDY7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Eisenberg L. M., Eisenberg C. A. Wnt signal transduction and the formation of the myocardium. Dev. Biol. 2006;293:305–315. doi: 10.1016/j.ydbio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Fanto M., McNeill H. Planar polarity from flies to vertebrates. J. Cell Sci. 2004;117:527–533. doi: 10.1242/jcs.00973. [DOI] [PubMed] [Google Scholar]
- Garriock R. J., D'Agostino S. L., Pilcher K. C., Krieg P. A. Wnt11-R, a protein closely related to mammalian Wnt11, is required for heart morphogenesis in Xenopus. Dev. Biol. 2005;279:179–192. doi: 10.1016/j.ydbio.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sancho J. M., Brennan K. R., Castelo-Soccio L. A., Brown A. M. Wnt proteins induce dishevelled phosphorylation via an LRP5/6-independent mechanism, irrespective of their ability to stabilize beta-catenin. Mol. Cell. Biol. 2004;24:4757–4768. doi: 10.1128/MCB.24.11.4757-4768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R., Dawid I. B., He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblet N. S., Lijam N., Ruiz-Lozano P., Wang J., Yang Y., Luo Z., Mei L., Chien K. R., Sussman D. J., Wynshaw-Boris A. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–5838. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- He X., Saint-Jeannet J. P., Wang Y., Nathans J., Dawid I., Varmus H. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275:1652–1654. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- He X., Semenov M., Tamai K., Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Henderson D. J., Phillips H. M., Chaudhry B. Vang-like 2 and noncanonical Wnt signaling in outflow tract development. Trends Cardiovasc. Med. 2006;16:38–45. doi: 10.1016/j.tcm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Hollemann T., Panitz F., Pieler T. In situ hybridization techniques with Xenopus embryos. In: Richter J. D., editor. A Comparative Methods Approach to the Study of Oocytes and Embryos. Oxford, United Kingdom: Oxford University Press; 1999. [Google Scholar]
- Kao K. R., Elinson R. P. The entire mesodermal mantle behaves as Spemann's organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev. Biol. 1988;127:64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- Kim G. H., Han J. K. JNK and ROKalpha function in the noncanonical Wnt/RhoA signaling pathway to regulate Xenopus convergent extension movements. Dev. Dyn. 2005;232:958–968. doi: 10.1002/dvdy.20262. [DOI] [PubMed] [Google Scholar]
- Klein T. J., Mlodzik M. Planar cell polarization: an emerging model points in the right direction. Annu. Rev. Cell Dev. Biol. 2005;21:155–176. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- Kofron M., Birsoy B., Houston D., Tao Q., Wylie C., Heasman J. Wnt11/beta-catenin signaling in both oocytes and early embryos acts through LRP6-mediated regulation of axin. Development. 2007;134:503–513. doi: 10.1242/dev.02739. [DOI] [PubMed] [Google Scholar]
- Kuhl M., Geis K., Sheldahl L. C., Pukrop T., Moon R. T., Wedlich D. Antagonistic regulation of convergent extension movements in Xenopus by Wnt/beta-catenin and Wnt/Ca2+ signaling. Mech. Dev. 2001;106:61–76. doi: 10.1016/s0925-4773(01)00416-6. [DOI] [PubMed] [Google Scholar]
- Mao B., Wu W., Li Y., Hoppe D., Stannek P., Glinka A., Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Marvin M. J., Di Rocco G., Gardiner A., Bush S. M., Lassar A. B. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels A. J., Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito A. T., Shiojima I., Akazawa H., Hidaka K., Morisaki T., Kikuchi A., Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc. Natl. Acad. Sci. USA. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I., et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Pandur P., Lasche M., Eisenberg L. M., Kuhl M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418:636–641. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- Penzo-Mendez A., Umbhauer M., Djiane A., Boucaut J. C., Riou J. F. Activation of Gbetagamma signaling downstream of Wnt-11/Xfz7 regulates Cdc42 activity during Xenopus gastrulation. Dev. Biol. 2003;257:302–314. doi: 10.1016/s0012-1606(03)00067-8. [DOI] [PubMed] [Google Scholar]
- Pinson K. I., Brennan J., Monkley S., Avery B. J., Skarnes W. C. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Qian D., Jones C., Rzadzinska A., Mark S., Zhang X., Steel K. P., Dai X., Chen P. Wnt5a functions in planar cell polarity regulation in mice. Dev. Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambony A., Wedlich D. Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev. Cell. 2007;12:779–792. doi: 10.1016/j.devcel.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Schneider W. J., Nimpf J. LDL receptor relatives at the crossroad of endocytosis and signaling. Cell Mol Life Sci. 2003;60:892–903. doi: 10.1007/s00018-003-2183-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte G., Bryja V., Rawal N., Castelo-Branco G., Sousa K. M., Arenas E. Purified Wnt-5a increases differentiation of midbrain dopaminergic cells and dishevelled phosphorylation. J. Neurochem. 2005;92:1550–1553. doi: 10.1111/j.1471-4159.2004.03022.x. [DOI] [PubMed] [Google Scholar]
- Seifert J. R., Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat. Rev. Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- Shariatmadari M., Peyronnet J., Papachristou P., Horn Z., Sousa K. M., Arenas E., Ringstedt T. Increased Wnt levels in the neural tube impair the function of adherens junctions during neurulation. Mol. Cell Neurosci. 2005;30:437–451. doi: 10.1016/j.mcn.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Julius M. A., Giarre M., Zheng Z., Brown A. M., Kitajewski J. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ. 1997;8:1349–1358. [PubMed] [Google Scholar]
- Son J. H., Chun H. S., Joh T. H., Cho S., Conti B., Lee J. W. Neuroprotection and neuronal differentiation studies using substantia nigra dopaminergic cells derived from transgenic mouse embryos. J. Neurosci. 1999;19:10–20. doi: 10.1523/JNEUROSCI.19-01-00010.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahinci E., Thorne C. A., Franklin J. L., Salic A., Christian K. M., Lee L. A., Coffey R. J., Lee E. Lrp6 is required for convergent extension during Xenopus gastrulation. Development. 2007;134:4095–4106. doi: 10.1242/dev.010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai K., Semenov M., Kato Y., Spokony R., Liu C., Katsuyama Y., Hess F., Saint-Jeannet J. P., He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- Tamai K., Zeng X., Liu C., Zhang X., Harada Y., Chang Z., He X. A mechanism for Wnt coreceptor activation. Mol. Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- Tao Q., Yokota C., Puck H., Kofron M., Birsoy B., Yan D., Asashima M., Wylie C. C., Lin X., Heasman J. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Topczewski J., Sepich D. S., Myers D. C., Walker C., Amores A., Lele Z., Hammerschmidt M., Postlethwait J., Solnica-Krezel L. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev. Cell. 2001;1:251–264. doi: 10.1016/s1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Torban E., Kor C., Gros P. Van Gogh-like2 (Strabismus) and its role in planar cell polarity and convergent extension in vertebrates. Trends Genet. 2004;20:570–577. doi: 10.1016/j.tig.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Ueno S., Weidinger G., Osugi T., Kohn A. D., Golob J. L., Pabon L., Reinecke H., Moon R. T., Murry C. E. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterseher F., Hefele J. A., Giehl K., De Robertis E. M., Wedlich D., Schambony A. Paraxial protocadherin coordinates cell polarity during convergent extension via Rho A and JNK. EMBO J. 2004;23:3259–3269. doi: 10.1038/sj.emboj.7600332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R., Berns A. Knockout mouse models to study Wnt signal transduction. Trends Genet. 2006;22:678–689. doi: 10.1016/j.tig.2006.10.001. [DOI] [PubMed] [Google Scholar]
- van Noort M., Meeldijk J., van der Zee R., Destree O., Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J. Biol. Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- Wang J., Hamblet N. S., Mark S., Dickinson M. E., Brinkman B. C., Segil N., Fraser S. E., Chen P., Wallingford J. B., Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli M., Dougan S. T., Caldwell K., O'Keefe L., Schwartz S., Vaizel-Ohayon D., Schejter E., Tomlinson A., DiNardo S. Arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Nieto M. A. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T. P., Bradley A., McMahon A. P., Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999a;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T. P., Takada S., Yoshikawa Y., Wu N., McMahon A. P. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev. 1999b;13:3185–3190. doi: 10.1101/gad.13.24.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Tamai K., Doble B., Li S., Huang H., Habas R., Okamura H., Woodgett J., He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.