Abstract
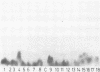
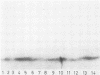
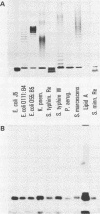
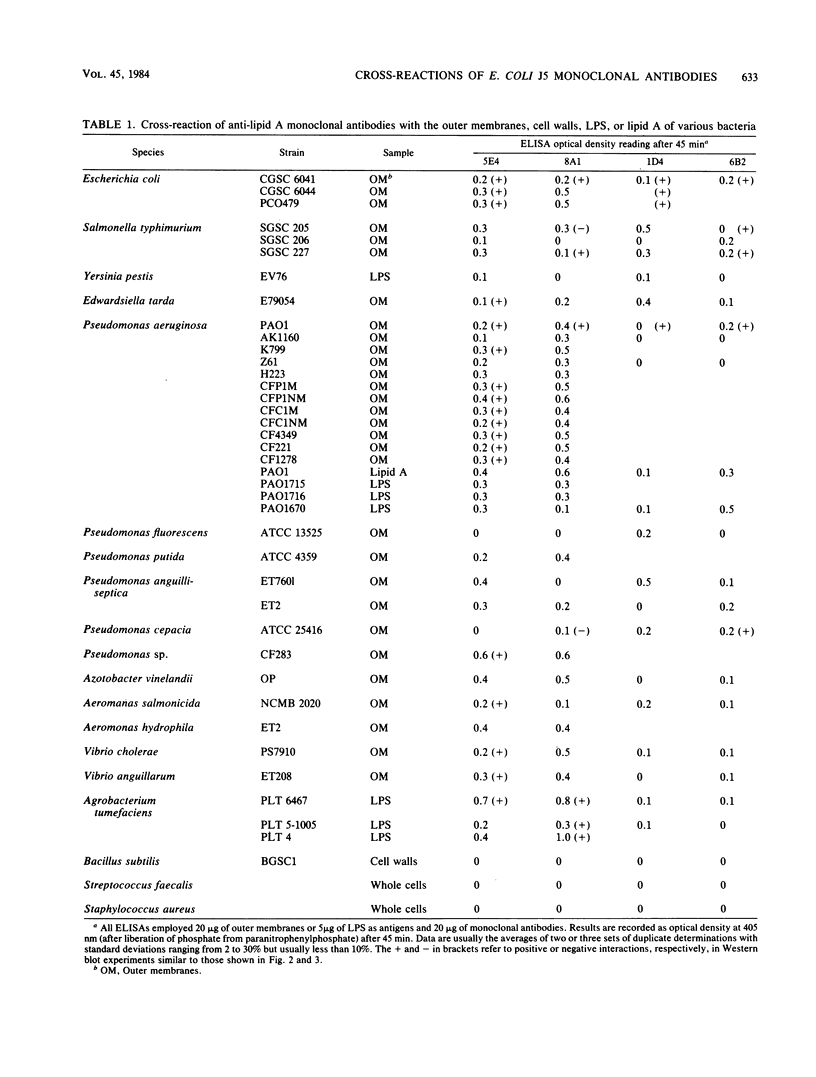
Four monoclonal antibodies against Escherichia coli J5 were studied. Each of these monoclonal antibodies reacted with purified lipopolysaccharides from E. coli J5, the deep rough mutant Salmonella minnesota Re595, Agrobacterium tumefaciens, and Pseudomonas aeruginosa PAO1 as well as with the purified lipid A of P. aeruginosa. Enzyme-linked immunosorbent assays using the outer membranes from a variety of gram-negative bacteria demonstrated that these lipid A-specific monoclonal antibodies interacted with between 84 and 97% of the gram-negative bacterial species tested. One of the monoclonal antibodies, 5E4, was shown to interact with 34 of the 35 outer membrane or lipopolysaccharide antigens tested. Immunoenzymatic staining of Western electrophoretic blots of separated P. aeruginosa outer membrane components was used to demonstrate that antibody 5E4 interacted with a similar fast-migrating band, corresponding to rough lipopolysaccharide, from all 17 serotype strains and all 14 clinical isolates of P. aeruginosa. Similarly, iodinated goat anti-mouse immunoglobulin was used to detect the binding of monoclonal antibody 8A1 to a fast-migrating band on Western electrophoretic blots of purified lipopolysaccharides from Klebsiella pneumoniae and both smooth and rough strains of E. coli, Salmonella typhimurium, and S. minnesota. These results suggest considerable conservation of single antigenic sites in the lipid A of gram-negative bacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley S. G. Cellular and molecular mechanisms of action of bacterial endotoxins. Annu Rev Microbiol. 1979;33:67–94. doi: 10.1146/annurev.mi.33.100179.000435. [DOI] [PubMed] [Google Scholar]
- Braude A. I., Ziegler E. J., McCutchan J. A. Antiserum treatment of gram-negative bacteremia. Schweiz Med Wochenschr. 1978 Dec 2;108(48):1872–1876. [PubMed] [Google Scholar]
- Chester I. R., Meadow P. M., Pitt T. L. The relationship between the O-antigenic lipopolysaccharides and serological specificity in strains of Pseudomonas aeruginosa of different O-serotypes. J Gen Microbiol. 1973 Oct;78(2):305–318. doi: 10.1099/00221287-78-2-305. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Charnetzky W. T., Hurlbert R. F., Hancock R. E. Effects of growth temperature, 47-megadalton plasmid, and calcium deficiency on the outer membrane protein porin and lipopolysaccharide composition of Yersinia pestis EV76. Infect Immun. 1983 Dec;42(3):1092–1101. doi: 10.1128/iai.42.3.1092-1101.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Chan L., Darveau R. P., Speert D. P., Pier G. B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983 Oct;42(1):170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Wieczorek A. A., Mutharia L. M., Poole K. Monoclonal antibodies against Pseudomonas aeruginosa outer membrane antigens: isolation and characterization. Infect Immun. 1982 Jul;37(1):166–171. doi: 10.1128/iai.37.1.166-171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstra H., Dankert J. Antigenic cross-reactivity of major outer membrane proteins in enterobacteriaceae species. J Gen Microbiol. 1979 Apr;111(2):293–302. doi: 10.1099/00221287-111-2-293. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Kuzio J., Angus B. L., Hancock R. E. Chemical and chromatographic analysis of lipopolysaccharide from an antibiotic-supersusceptible mutant of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1982 Feb;21(2):310–319. doi: 10.1128/aac.21.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Mutharia L. M., Hancock R. E. Surface localization of Pseudomonas aeruginosa outer membrane porin protein F by using monoclonal antibodies. Infect Immun. 1983 Dec;42(3):1027–1033. doi: 10.1128/iai.42.3.1027-1033.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutharia L. M., Nicas T. I., Hancock R. E. Outer membrane proteins of Pseudomonas aeruginosa serotype strains. J Infect Dis. 1982 Dec;146(6):770–779. doi: 10.1093/infdis/146.6.770. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- O'Connor C. G., Ashman L. K. Application of the nitrocellulose transfer technique and alkaline phosphatase conjugated anti-immunoglobulin for determination of the specificity of monoclonal antibodies to protein mixtures. J Immunol Methods. 1982 Oct 29;54(2):267–271. doi: 10.1016/0022-1759(82)90068-0. [DOI] [PubMed] [Google Scholar]
- Pennington J. E., Menkes E. Type-specific vs. cross-protective vaccination for gram-negative bacterial pneumonia. J Infect Dis. 1981 Dec;144(6):599–603. doi: 10.1093/infdis/144.6.599. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Wollenweber H. W., Zähringer U., Lüderitz O. Lipid A, the lipid component of bacterial lipopolysaccharides: relation of chemical structure to biological activity. Klin Wochenschr. 1982 Jul 15;60(14):705–709. doi: 10.1007/BF01716559. [DOI] [PubMed] [Google Scholar]
- Schmidt G., Fromme I., Mayer H. Immunochemical studies on core lipopolysaccharides of Enterobacteriaceae of different genera. Eur J Biochem. 1970 Jun;14(2):357–366. doi: 10.1111/j.1432-1033.1970.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Stanley C., Lew A. M., Steward M. W. The measurement of antibody affinity: a comparison of five techniques utilizing a panel of monoclonal anti-DNP antibodies and the effect of high affinity antibody on the measurement of low affinity antibody. J Immunol Methods. 1983 Nov 11;64(1-2):119–132. doi: 10.1016/0022-1759(83)90390-3. [DOI] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Mascagni P., Nashed M. A., Anderson L., Raetz C. R. Fatty acyl derivatives of glucosamine 1-phosphate in Escherichia coli and their relation to lipid A. Complete structure of A diacyl GlcN-1-P found in a phosphatidylglycerol-deficient mutant. J Biol Chem. 1983 Jun 25;258(12):7379–7385. [PubMed] [Google Scholar]
- Tanamoto K., Homma J. Y. Essential regions of the lipopolysaccharide of Pseudomonas aeruginosa responsible for pyrogenicity and activation of the proclotting enzyme of horseshoe crabs. Comparison with antitumor, interferon-inducing and adjuvant activities. J Biochem. 1982 Mar;91(3):741–746. doi: 10.1093/oxfordjournals.jbchem.a133760. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Wollenweber H. W., Broady K. W., Lüderitz O., Rietschel E. T. The chemical structure of lipid A. Demonstration of amide-linked 3-acyloxyacyl residues in Salmonella minnesota Re lipopolysaccharide. Eur J Biochem. 1982 May;124(1):191–198. doi: 10.1111/j.1432-1033.1982.tb05924.x. [DOI] [PubMed] [Google Scholar]
- Young L. S., Stevens P., Ingram J. Functional role of antibody against "core" glycolipid of Enterobacteriaceae. J Clin Invest. 1975 Oct;56(4):850–861. doi: 10.1172/JCI108164. [DOI] [PMC free article] [PubMed] [Google Scholar]