Abstract
Alcoholic fatty liver is a potentially pathologic condition which can progress to steatohepatitis, fibrosis, and cirrhosis if alcohol consumption is continued. Alcohol exposure may induce fatty liver by increasing NADH/NAD+ ratio, increasing sterol regulatory element-binding protein-1 (SREBP-1) activity, decreasing peroxisome proliferator-activated receptor-α (PPAR-α) activity, and increasing complement C3 hepatic levels. Alcohol may increase SREBP-1 activity by decreasing the activities of AMP-activated protein kinase and sirtuin-1. Tumor necrosis factor-α (TNF-α) produced in response to alcohol exposure may cause fatty liver by up-regulating SREBP-1 activity, whereas betaine and pioglitazone may attenuate fatty liver by down-regulating SREBP-1 activity. PPAR-α agonists have potentials to attenuate alcoholic fatty liver. Adiponectin and interleukin-6 may attenuate alcoholic fatty liver by up-regulating PPAR-α and insulin signaling pathways while down-regulating SREBP-1 activity, and suppressing TNF-α production. Recent studies show that paracrine activation of hepatic cannabinoid receptor 1 by hepatic stellate cell-derived endocannabinoids also contributes to the development of alcoholic fatty liver. Furthermore, oxidative modifications and inactivation of the enzymes involved in the mitochondrial and/or peroxisomal β-oxidation of fatty acids could contribute to fat accumulation in the liver.
Keywords: alcohol, fatty liver, NADH/NAD+, oxidative stress, cytokines, adipokines, oxidation of fatty acids, signaling pathways
Introduction
Alcoholic liver disease (ALD) is a major cause of morbidity and mortality in the world. In the initial stage of the disease, triglycerides accumulate in hepatocytes leading to the development of fatty liver (steatosis), which is a reversible condition. If alcohol consumption is continued, steatosis can progress to steatohepatitis, fibrosis, cirrhosis, and even hepatocellular carcinoma especially in the presence of other co-morbidity factors (Lieber, 2004) such as hepatitis virus infection (Rigamonti et al., 2003; Otani et al., 2005), diabetes (Hassan et al., 2002), and exposure to smoking (Kuper et al., 2000) or drugs (McClain et al., 1980; Seeff et al., 1986). Alcoholic fatty liver has been considered as a benign condition for a long time; however, increasing evidence suggests that it is a potentially pathologic condition. This is based on the following biochemical features associated with fatty liver developed in response to chronic administration of Lieber DeCarli ethanol liquid diet (up to 36% ethanol-derived calories) to rodents: 1) induction of hepatic cytochrome P4502E1 (CYP2E1) (Koop and Tierney, 1990; Lieber, 1999; Ohnishi and Lieber, 1977; Roberts et al., 1994 and 1995); 2) activation of NADPH oxidase (Kono et al., 2000) and xanthine oxidase (Nordmann et al., 1992); 3) increased hepatic levels of toxic lipid peroxidation products such as 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA) (Aleynik and Lieber, 2003; Bykov et al., 2006; Sampey et al., 2003; Stewart et al., 2007b); 4) increased deposition of iron in the liver (Valerio et al., 1996); 5) hepatic mitochondrial dysfunction and glutathione (GSH) depletion (Bailey and Cunningham, 2002; Garcia-Ruiz et al., 1995; Zhao et al., 2002); 6) hepatic S-adenosylmethionine (SAM) depletion (Aleynik and Lieber, 2003; Lu et al., 2000) with elevated homocysteine (Barak et al., 2000; Blasco et al., 2005; Ji and Kaplowitz, 2003); 7) increased hepatic expression of tumor necrosis factor-α (TNF-α) mRNA and protein levels (Lin et al., 1998; Pritchard et al., 2007); 8) elevated serum alanine aminotransferase (ALT) levels (Bykov et al., 2006; Gillis and Nagy, 1997; Pritchard et al., 2007; Valerio et al., 1996); 9) induction of hepatocyte apoptosis (Higuchi et al., 2001; Ishii et al., 2003); 10) increased hepatic levels of cellular fibronectin and alpha smooth muscle actin (α-SMA) (Gillis and Nagy, 1997); and 11) increased insulin resistance (He et al., 2007) and plasminogen activator inhibitor-1 (Bergheim et al., 2006).
Induction of CYP2E1 activity, increased lipid peroxidation, increased deposition of iron, and depletion of SAM and GSH are markers of oxidative stress, which has been implicated in the pathogenesis of ALD (Caro and Cederbaum, 2004; French et al., 1997). TNF-α is a pro-inflammatory cytokine and an elevated level of serum ALT is a marker of hepatic injury. A serum AST/ALT ratio greater than 2 is considered as an indicator for alcoholic liver diseases while the ratios less than 2 are for non-alcoholic fatty liver diseases (see review by Levitsky and Mailliard, 2004). Induction of apoptosis and increased hepatic level of cellular fibronectin are early responses to injury. Increased levels of α-SMA suggest hepatic stellate cell activation, which may result in excess of collagen deposition and subsequent fibrosis, reflecting the progressive conditions of more severe liver diseases with inflammation. In addition, alcoholic fatty liver is associated with increased hepatic levels of free fatty acids which can induce CYP2E1(Lieber, 2004) and increase the production of interleukin-8, an inflammatory chemokine and a potent attractant for neutrophils (Joshi-Barve et al., 2007). Excess hepatic fat accumulation could result from increased fatty acid synthesis, decreased fatty acid oxidation, increased transportation of fatty acids from the peripheral organs to the liver, and/or blunted export of fatty acids from the liver. This report describes the underlying molecular mechanisms of alcoholic fatty liver.
A. Role of reducing equivalents (NADH)
In the liver, alcohol is primarily metabolized by cytosolic alcohol dehydrogenase (ADH) to acetaldehyde, which is further metabolized to acetate by mitochondrial aldehyde dehydrogenase (ALDH2) (Zakhari and Li, 2007). Both enzymes use NAD+ as a cofactor, producing a reducing equivalent NADH in both steps. Increased production of NADH was implicated in the disruption of many dehydrogenase-related reactions in the cytoplasm and mitochondria [i.e. the tricarboxylic acid cycle (TCA) and β-oxidation of fatty acids], thereby suppressing energy supply and fatty acid oxidation, which in turn results in alcoholic fatty liver (see reviews by Crabb, 1993; Grunnet and Kondrup, 1986; Fromenty et al., 1997; Lieber, 1991). Furthermore, excess amounts of acetate produced from the ethanol metabolism could be used for fatty acid and cholesterol biosynthesis, contributing to alcoholic fatty liver. Since fat infiltration into the liver persists despite normalization of NADH/NAD+ ratio after extended ethanol exposure in baboons (Salaspuro et al., 1981), this redox mechanism seems insufficient to explain the formation of fatty liver. However, NADH may contribute in the initiation of hepatic fat infiltration in response to alcohol exposure. A similar result was also observed in rats where addition of methylene blue to the ethanol liquid diet restored the ethanol-mediated redox changes but not fat contents (measured by total lipids and hepatic triacylglycerol) (Ryle et al., 1985). In this study, methylene blue did not affect the weight gain, liver weight, ethanol intake, or serum ethanol concentration in the two groups of ethanol-fed rats (with and without methylene blue), further supporting the conclusion.
B. Role of sterol regulatory element-binding proteins (SREBPs)
SREBPs are a family of transcription factors that regulate the enzymes responsible for the synthesis of cholesterol, fatty acids, and triglycerides in liver and other tissues. There are three isoforms of SREBP: SREBP-1a, SREBP-1c and SREBP-2. SREBP-1a and 1c are alternatively spliced forms of the SREBP protein encoded by the same gene. SREBP-1 and SREBP-2 are involved in regulating fatty acid and cholesterol synthesis, respectively. SREBPs are synthesized as precursors (∼125 kDa) bound to the endoplasmic reticulum and nuclear envelope. Upon activation, SREBPs are released from the membrane into the nucleus as a mature protein (∼68 kDa) by a sequential two-step cleavage process (Brown and Goldstein, 1999).
The connection between SREBP and fatty liver was recognized when researchers observed massive fatty liver in transgenic mice over-expressing human SREBP-1a (Shimano et al., 1996; Shimano et al., 1997). These findings were corroborated when absence of SREBP-1 was shown to ameliorate fatty liver in ob/ob obese mice (Yahagi et al., 2002). The role of SREBP-1 in alcohol-induced fat accumulation has been investigated using in vitro as well as in vivo systems. In hepatoma cell lines-expressing cytosolic ADH and mitochondrial ALDH2, ethanol exposure significantly increased the SREBP-regulated transcription via elevated levels of the mature SREBP-1 protein (You et al., 2002). The effect of ethanol on SREBP-1 appears to be mediated through its metabolism to acetaldehyde. This fact is based on the following observations: acetaldehyde enhanced the level of SREBP-1 in HepG2 cells; lack of effect of ethanol on SREBP-1 production in CV-1 cells devoid of ADH; inhibition of ethanol oxidation with 4-methylpyrazole, an inhibitor of ADH, blocked the effect of ethanol; and inhibition of acetaldehyde oxidation with cynamide, an inhibitor of ALDH2, enhanced the effect of ethanol. These in vitro results were further confirmed in an animal model where C57BL/6J mice were fed an ethanol liquid diet (27.5% ethanol-derived calories) for 4 weeks. In this model, the level of active (mature) form of hepatic SREBP-1 was significantly elevated in ethanol-exposed mice, and this increase was associated with increased gene expression of the lipogenic enzymes as well as accumulation of triglycerides in the mouse liver exposed to an ethanol liquid diet compared to pair-fed control mice (You et al., 2002). Taken together, these results suggest that acetaldehyde produced from ethanol metabolism can increase the synthesis of the mature SREBP-1 protein which enhances hepatic lipogenesis, thereby leading to the development of fatty liver. Further studies by these researchers showed that the effect of ethanol on SREBP-regulated transcriptional activation was mediated partly through inhibition of AMP-activated protein kinase (AMPK) activity (You et al., 2004).
The role of SREBP-1 was also investigated in an intragastric ethanol infusion model of alcoholic liver injury (Ji and Kaplowitz, 2003). Administration of ethanol for 6 weeks significantly increased the levels of SREBP protein and its mRNA in the mouse liver. These changes were associated with fatty liver, necroinflammation, and apoptosis. Administration of betaine (trimethylglycine) ameliorated fatty liver and inhibited SREBP up-regulation, thus further confirming the role of SREBP in the development of alcoholic fatty liver (Ji and Kaplowitz, 2003). Using the same intragastric ethanol infusion model of alcoholic liver injury, pioglitazone (an anti-diabetic agent) was shown to down-regulate SREBP-1 in rat liver and primary cultured hepatocytes (Tomita et al., 2004). This effect of pioglitazone was associated with decreased triglyceride synthesis and prevention of alcoholic fatty liver in rats. Taken together, these findings suggest that elevated SREBP plays a critical role in alcoholic fatty liver and that inhibiting SREBP activity may be a viable strategy to attenuate alcoholic fatty liver.
Compared to the well-established role of TNF-α in alcoholic liver inflammation, its contribution in developing alcoholic fatty liver (steatosis) has been less characterized. However, in mice, TNF-α expression has been shown to increase in alcoholic fatty liver (Lin et al., 1998; Pritchard et al., 2007) and absence of its receptor (TNF-α R1) activity prevents the development of alcoholic fatty liver (Ji et al., 2004; Yin et al., 1999). In addition, TNF-α has been shown to increase mRNA expression of SREBP-1c in the liver of mice (Endo et al., 2007) and to stimulate the maturation of SREBP-1 protein in human hepatocytes (Lawler et al., 1998). Thus TNF-α may also contribute to the development of alcoholic fatty liver by up-regulating the SREBP activity.
C. Role of AMP-activated protein kinase (AMPK)
AMPK is known to act as a key metabolic “master switch” by phosphorylating the target enzymes involved in lipid metabolism in many tissues including the liver (see review by Long and Zierath, 2006). It can promote fatty acid oxidation by inactivation of acetyl-CoA carboxylase (ACC) (Hardie et al., 1998; Winder and Hardie, 1999) and activation of malonyl Co-A decarboxylase (MCD). ACC is a rate limiting enzyme for fatty acid biosynthesis in the liver and other tissues (Abu-Elheiga et al., 2001). It catalyzes the conversion of acetyl-CoA to malonyl-CoA, which is a precursor for the synthesis of fatty acids, as well as a potent inhibitor of carnitine palmitoyltransferase-1 (CPT-1). CPT-1 plays an important role in the transport of fatty acids from cytoplasm into mitochondria where fatty acids are metabolized via mitochondrial β-oxidation pathway. MCD is known to degrade malonyl-CoA. Thus AMPK-induced inhibition of ACC and activation of MCD can result in decreased synthesis and increased degradation of malonyl-CoA, respectively. Subsequently, the decreased concentration of cellular malonyl-CoA can relieve inhibition of mitochondrial CPT-1, leading to increased transport of fatty acids into the mitochondria and subsequent oxidation.
In addition to directly regulating the activity of the lipid metabolizing enzymes, AMPK also modulates SREBP-1 activity. Metformin, an activator of AMPK and anti-diabetic drug, blunted lipid accumulation in the liver of insulin resistant ob/ob obese mice, partly, by decreasing SREBP-1 protein level (Lin et al., 2000). Metformin-induced activation of AMPK also led to decreased levels SREBP-1c mRNA and protein as well as its lipogenic target genes in rat hepatocytes (Zhou et al., 2001). Furthermore, overexpression of adiponectin, a known activator of AMPK, significantly reduced SREBP-1c expression in mouse liver (Shklyaev et al., 2003). Thus AMPK activation may attenuate fatty liver by blunting the SREBP-1 activation (see Figure 1).
Figure 1.
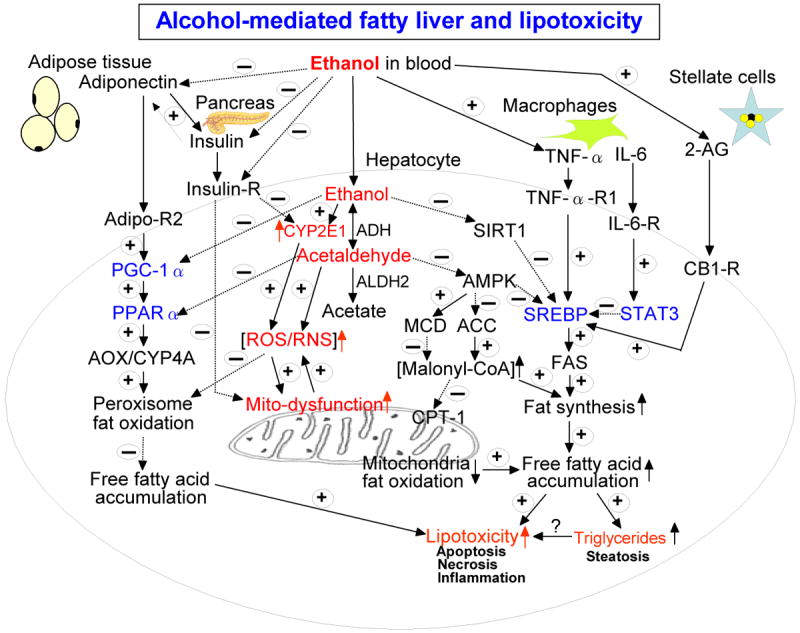
Schematic diagram of the major pathways of alcoholic fatty liver and lipotoxicity. Besides the major pathways indicated in this diagram, ethanol intake is also known to promote fat transport into the liver from peripheral tissues while ethanol inhibits fat export from the liver. Adiponectin also activates AMPK, resulting in decreased SREBP and ACC activities while it suppresses functions of stellate cells. TNFα decreases the level of adiponectin. These cross-talk exist between various factors, resulting in different levels of fat accumulation in the liver. The positive signs with solid lines represent activation and/or up-regulation of the down-stream targets while the negative signs with broken lines indicate the opposite effects. Transcriptional factors are shown in blue color while the molecules/conditions promoting oxidative/nitrosative stress are marked in red color. Abbreviations used: ADH, alcohol dehydrogenase; ALDH2, mitochondrial aldehyde dehydrogenase; CYP2E1, ethanol-inducible cytochrome P450 2E1; ROS, reactive oxygen species; RNS, reactive nitrogen species; insulin-R, insulin receptor in hepatocytes; Sirt1, sirtuin1; AMPK, AMP-activated protein kinase; ACC, acyl-CoA carboxylase; MCD, malonyl-CoA decarboxylase; CPT-1, carnitine palmitate transferase-1; TNFα-R1, TNFα receptor 1 in hepatocytes; SREBP, sterol regulatory element binding protein; FAS, fatty acid synthase; IL-6, interleukin-6; IL-6-R, IL-6 receptor; 2-AG, 2-arachidonoylglycerol; STAT3, signal transducer and activator of transcription 3; CB-1-R, endocannabinoid receptor 1; Adipo-R2, adiponectin receptor 2 in hepatocytes; PGC-1α, peroxisome proliferator activator receptor γ co-activator protein α; PPARα, peroxisome proliferator activator receptor α; AOX, acyl-CoA oxidase; CYP4A, cytochrome P450 4A.
The information discussed above provided a rationale basis for researchers to further investigate the role of AMPK in the development of alcoholic fatty liver (You et al., 2004). In rat hepatoma cell lines (H4IIEC3 and McA-RH7777) expressing ADH and ALDH2, considerable levels of AMPK basal activity and constitutively-expressed SREBP-1 protein are detected in ethanol naïve untreated cells. Addition of ethanol inhibits the AMPK activities while it elevates SREBP-1 mRNA and promotes production of mature SREBP-1 in both cells. Both metformin and 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) (two activators of AMPK) significantly suppressed ethanol-induced transcription of SREBP-regulated genes and blunted the ability of ethanol to increase the levels of mature SREBP-1 protein. These results were corroborated when over-expression of a constitutively active form of AMPK blocked the effect of ethanol, whereas co-expression of a dominant-negative form of AMPK augmented the effect. In addition, ethanol inhibited AMPK activity, increased the activity of ACC, and suppressed the oxidation of palmitic acid in hepatic cells. Finally, ethanol feeding (27.5% ethanol-derived calories with 12% fat-derived calories) resulted in decreased hepatic AMPK activity while it increased ACC activity and elevated malonyl-CoA content in the mouse liver. Similar results of ethanol-mediated inhibition of AMPK activity with increased hepatic fat accumulation have been reported by two other laboratories. AMPK activity was inhibited in ethanol-exposed rats (36% ethanol-derived calories and 35% fat-derived calories) (Garcia-Villafranca et al., 2008) and micropigs (40% ethanol-derived calories and 30% fat-derived calories) (Esfandiary et al., 2007), respectively. These results indicate that AMPK may also contribute to the development of alcoholic fatty liver through regulating the SREBP-1 activation and mitochondrial fatty acid oxidation (see Fig. 1).
D. Role of mammalian sirtuin-1 (SIRT-1)
SIRT-1, a longevity factor and NAD+-dependent protein deacetylase, is emerging as a metabolic master regulator. SIRT-1 is known to bind to SREBP-1, resulting in its inactivation via deacetylation. Recently, researchers have investigated the role of SIRT-1 in the development of alcoholic fatty liver (Lieber et al., 2008; You et al., 2008a; You et al., 2008b). In rat hepatoma cells (H4IIEC3), expressing moderate levels of ADH and ALDH2, SIRT-1 was shown to deacetylate SREBP-1, which led to its decreased transcriptional activity (You et al., 2008b). Ethanol exposure reduced the level of SIRT-1 content and thus blocked the SIRT-1-induced deacetylation of SREBP-1, resulting in elevated levels of the acetylated active nuclear form of SREBP-1c in ethanol-exposed mice, leading to increased fat synthesis. In addition, ethanol-induced transcription of SREBP-1 regulated genes was suppressed by a SIRT-1 agonist, resveratrol. Furthermore, ectopic expression of wild type SIRT-1 plasmid abolished the effect of ethanol, whereas co-expression of deacetylase-defective SIRT-1 (H363Y) mutant plasmid augmented the ethanol effect on the transcriptional activation of SREBP-regulated genes. These results suggest that ethanol-induced SREBP-1 is, in part, mediated through SIRT-1 inhibition (see Fig. 1).
E. Role of peroxisome proliferator-activated receptor-alpha (PPAR-α)
PPAR-α is a member of the nuclear hormone receptor super family (see review by Yu et al, 2003). Upon dimerization with retinoid X receptors (RXR), PPAR-α regulates transcription of a set of genes containing the peroxisome proliferator response elements (PPRE), which are involved in oxidation, transport, and export of free fatty acids. These include membrane transporters such as CPT-1, apolipoprotein genes, and several components of the mitochondrial and peroxisomal fatty acid β-oxidation pathways (Yu et al., 2003). In addition, MCD, which controls the levels of malonyl-CoA, is positively regulated by PPAR-α (Lee et al., 2004). PPAR-α is activated by its binding to free fatty acids and this occurs when intracellular concentration of the free fatty acids rises. Using PPAR-α null mice, it was shown that PPAR-α modulates constitutive expression of the genes for several enzymes involved in the mitochondrial and peroxisomal β-oxidation of fatty acids (Aoyama et al., 1998). The PPAR-α regulated enzymes involved in fatty acid oxidation include acyl-CoA oxidase (AOX), 3-hydroxyacyl-CoA dehydrogenase, multifunctional β-oxidation protein (3-ketoacyl-CoA thiolase), acyl-CoA synthase, MCD, cytochrome P450 4A (CYP4A), CPT-1, etc (Aoyama et al., 1998; Hardwick 2008; Knight et al., 2005). Therefore, activation of these enzymes by endogenous or synthetic PPAR-α agonists is expected to accelerated degradation of various fatty acids, resulting in attenuation of alcoholic fatty liver.
The connection between PPAR-α and fatty liver became apparent when researchers observed fatty liver in aging PPAR-α knockout mice (Costet et al., 1998). In addition, PPAR-α-null mice chronically fed a high fat diet or fasted for 24 hours exhibited massive lipid accumulation in their livers (Kersten et al., 1999). Furthermore, PPAR-α knockout mice were more sensitive to the development of steatohepatitis when fed a methionine- and choline-deficient diet (Ip et al., 2003). These results suggest that PPAR-α deficiency promotes the development of fatty liver.
The role of PPAR-α in alcoholic fatty liver has been investigated in cultured hepatocytes as well as in ethanol-fed rodents. Ethanol inhibited PPAR-α activation of a reporter gene in H4IIEC3 hepatoma cells expressing the alcohol metabolizing enzymes but not in CV-1 cells which lack these enzymes (Galli et al., 2001). Ethanol also inhibited the ability of Wy14,643 (a PPAR-α agonist) to activate the reporter gene in the hepatoma cell lines or cultured rat hepatocytes. This effect of ethanol was attenuated by the ADH inhibitor 4-methypyrazole but augmented by the ALDH inhibitor cynamide, implicating acetaldehyde as a causal factor (Galli et al., 2001). Furthermore, in vitro experiments by these investigators revealed that ethanol metabolism blocked transcriptional activation of PPAR-α partly due to impairment of its ability to bind DNA.
In mice, chronic ethanol feeding (27.5% ethanol-derived calories) decreased RXR-α protein levels, inhibited DNA binding of the PPAR-α/RXR-α heterodimer, decreased the levels of mRNA for several PPAR-α regulated genes in the liver, and resulted in the development of fatty liver (Fischer et al., 2003). Simultaneous administration of WY14,643 restored the DNA binding activity of PPAR-α/RXR-α, induced mRNA levels of several PPAR-α target genes, stimulated the fatty acid oxidation in liver homogenates, and prevented fatty liver in ethanol-fed animals (Fischer et al., 2003). These results suggest that metabolism of alcohol (ethanol) to acetaldehyde contributes to the development of fatty liver by blocking the function of PPAR-α, which is partly caused by its impaired ability to bind DNA.
The important role of PPAR-α in alcoholic fatty liver was further confirmed by the following in vivo results. Down-regulation of PPAR-α activity and fatty liver were observed following intragastric ethanol infusion to the rat (Nanji et al., 2004). Treatment with clofibrate, a PPAR-α activating ligand, abolished fatty liver and necroinflammatory injury observed in ethanol-exposed rats. Similarly, in mice, interleukin-6 treatment ameliorated alcoholic fatty liver which was in part attributed to up-regulation of PPAR-α and increased mitochondrial β-oxidation of fatty acids (Hong et al., 2004). The protective role of PPAR-α against alcoholic steatosis was also demonstrated in ethanol-fed PPAR-α knockout mice, which were much more sensitive to develop alcoholic fatty liver and injury (hepatomegaly, necroinflammation, fibrosis, etc) relative to ethanol-fed wild type mice (Nakajima et al., 2004). All these results obtained from in vitro and in vivo experiments strongly indicate important roles of PPAR-α and its down-stream target genes in preventing alcoholic fatty liver and injury.
F. Role of adiponectin
Adiponectin is a peptide hormone exclusively secreted from adipose tissues (Berg et al., 2002). The structure of adiponectin closely resembles that of TNF-α (Shapiro and Scherer, 1998), although these two proteins exhibit completely opposite effects (Maeda et al., 2002). Two adiponectin receptors (AdipoR1 and AdipoR2) have been cloned and adipoR2 is predominantly expressed in the liver (Yamauchi et al., 2003). Chronic ethanol administration significantly decreased plasma adiponectin levels and this was associated with the development of fatty liver in mice (Xu et al., 2003). Adiponectin administration to these mice attenuated alcoholic fatty liver by: 1) increasing CPT-1 activity and thus enhancing fatty acid oxidation; 2) decreasing the activity of two key enzymes – ACC and fatty acid synthase - involved in hepatic fatty acid synthesis; and 3) suppressing hepatic production of TNF-α (Xu et al., 2003). Adiponectin has been shown to increase PPAR-α ligand activity in hepatocytes (Yamauchi et al., 2002; 2003; and 2001), which may have been responsible for increased CPT-1 activity by adiponectin as reported by Xu and colleagues (2003). In addition, adiponectin has been shown to up-regulate hepatic activity of AMPK (Yamauchi et al., 2002), which likely decreases SREBP-1 activity (discussed above in the AMPK section). Thus adiponectin may attenuate alcoholic fatty liver by up-regulating PPAR-α and CPT-1 while down-regulating the two key enzymes in fat biosynthesis such as ACC and fatty acid synthase. This subject has been recently reviewed (see reviews by Rogers et al., 2008; Sozio and Crabb, 2008; You and Crabb, 2004).
G. Role of interleukin-6
Chronic alcoholic liver disease is associated with elevation of the serum and hepatic IL-6 levels, suggesting that elevated IL-6 may contribute to the pathogenesis of alcoholic liver injury (Colmenero et al., 2007; Hill et al., 1992; Khoruts et al., 1991; Sheron et al., 1991). However, accumulating evidence suggests that elevation of IL-6 may play a compensatory role in protecting against alcoholic liver injury. In fact, IL-6-deficient mice are more susceptible to alcohol-induced steatosis and liver injury, while treatment with IL-6 ameliorates alcohol-induced fatty liver and prevents mortality associated with alcoholic fatty liver transplants (El-Assal et al., 2004; Hong et al., 2002; Hong et al., 2004; Sun et al., 2003). Treatment with the IL-6 inducer ME3738 ameliorates alcohol-induced fatty liver disease in rats (Fukumura et al., 2007). The protective role of IL-6 in alcoholic fatty liver disease is likely mediated via (1) inhibition of CYP2E1, oxidative stress, lipid peroxidation, mitochondrial permeability transition, (2) restoration of ATP, (3) induction of antiapoptotic proteins Bcl-2/Bcl-xL, (4) decreasing TNF-α, and (5) induction/activation of PPARα (El-Assal et al., 2004; Hakkkola et al., 2003; Hong et al., 2002; Hong et al., 2004; Sun et al., 2003). The hepatoprotective function of IL-6 is mediated mainly through activation of signal transducer and activator of transcription 3 (STAT3). Consistent (or in parallel) with elevation of the serum and hepatic IL-6 levels, activation of STAT3 is detected in human patients suffering from alcoholic hepatitis and cirrhosis (Horiguchi et al., 2007; Larrea et al., 2006). The studies from cell-type specific STAT3 knockout mice suggest that STAT3 in hepatocytes plays an important role in inhibiting fatty acid synthesis but promoting inflammation, while STAT3 in macrophages/neutrophils inhibits inflammation during alcoholic liver injury (Horiguchi et al., 2008). The anti-lipogenic effect of STAT3 in hepatocytes is mediated, at least in part, via inhibiting of SREBP1 gene expression (Horiguchi et al., 2008).
H. Role of complement system
The complement system consists of more than 35 soluble and cell-bound proteins and it is involved in the activities of both innate immunity and acquired immunity. Activation of the complement system also contributes to the pathogenesis of a variety of liver disorders including alcoholic liver injury (Qin and Gao, 2006). Activation of the complement pathways results in the activation of C3, which in turn, leads to lysis of target and infected cells. Recently, researchers have investigated the role of complement C3 in the development of alcoholic fatty liver. In rats, chronic ethanol-induced fatty liver was associated with hepatic deposition of C3 (Jarvelainen et al., 2002). These results were further confirmed in a recent study in which ethanol feeding to wild-type mice resulted in increased C3a levels in plasma with development of fatty liver (Pritchard et al., 2007). Conversely, in C3 knockout mice, fatty liver was absent or significantly reduced after chronic or acute alcohol exposure (Bykov et al., 2006; Pritchard et al., 2007). These studies with C3 knockout mice suggest that C3 contributes to ethanol-induced fatty liver. The mechanisms by which C3 knockout mice are protected against ethanol-induced fat infiltration are not clear. A hepatic gene expression and lipid analysis study showed that ethanol exposure increased serum and liver adiponectin levels and down-regulated transcripts of the lipogenic enzymes in C3 knockout mice (Bykov et al., 2007). These studies suggest that complement C3 may promote ethanol-induced hepatic steatosis by decreasing adiponectin and increasing transcripts of the lipogenic enzymes.
I. Role of endocannabinoids
Endocannabinoids are lipid mediators that play important roles in a wide variety of functions both in the central nervous system and in peripheral organs via interacting with cannabinoid receptor 1 and 2 (CB1-R and CB2-R) (Kunos et al., 2008). CB1 receptors are expressed at high levels in the brain but are also present at low levels in peripheral tissues, including the liver. CB2 receptors are expressed mainly in immune cells and hematopoietic cells (Pacher et al., 2006). Accumulating evidence suggests that endocannabinoids, via acting on CB1-R and CB2-R, are involved in the pathogenesis of a variety of liver disorders including alcoholic and nonalcoholic fatty liver disease (Kunos and Osei-Hyiaman, 2008; Pacher et al., 2006). High fat diet or alcohol feeding induces fatty liver and increases the hepatic expression of CB1 receptors and upregulates the endocannabinoids in the liver. Blocking CB1 receptors with an antagonist or disruption of the CB1 gene ameliorates high fat diet- and alcohol-induced fatty liver (Jeong et al., 2008; Osei-Hyiaman et al., 2005; Osei-Hyiaman et al., 2008). Clinical studies also show that daily cannabis use correlates with the severity of steatosis in patients with chronic hepatitis C (Hezode et al., 2008). Collectively, these studies suggest that the endocannabinoid system, activated by alcohol feeding, can contribute to the pathogenesis of alcoholic fatty liver and injury. The mechanisms underlying the role of endocannabinoids in alcoholic fatty liver disease have been extensively investigated by Jeong et al. (2008). These studies show that chronic alcohol consumption stimulates hepatic stellate cells to produce 2-arachidonoylglycerol (2-AG), which then interacts with the hepatocyte CB1 receptor and induces expression of lipogenic genes including SREBP-1c and FAS (Jeong et al., 2008) (see Fig. 1).
J. Role of insulin
Insulin, a major hormone produced and secreted from the pancreas beta cells, regulates glucose homeostasis and lipid metabolism in the target tissues including the adipose tissue, skeletal muscles and liver. Hepatocytes are known to contain insulin receptors (IR), involved in phosphorylation of Tyr residues of its substrates (IRS-1 and IRS-2) by insulin receptor tyrosine kinases (see review by Fritsche et al., 2008). Insulin also modulates liver growth and proliferation through modulating the PI3K-Akt dependent signal transduction pathway (see reviews by Kim and Novak, 2007; de la Monte et al., 2008) while supporting the energy supply by activating the pyruvate dehydrogenase complex in the mitochondrial TCA cycle (see review by Patel and Roche, 1990). In addition, insulin is known to decrease the CYP2E1 content by destabilizing its mRNA (de Waziers et al., 1995; Woodcroft et al., 2002), possibly leading to reduced ROS production from CYP2E1-mediated reactions. However, chronic and binge ethanol exposures promote apoptosis and necroinflammation of pancreatic beta cells resulting in decreased insulin production and secretion (see review by Pandol and Raraty, 2007; Shin et al., 2002; Singh et al., 1986). Chronic and binge ethanol exposure also interrupts the normal insulin signaling pathways in various target organs including the liver, contributing to decreased proliferation of hepatocytes and insulin resistance with fatty liver in animals and humans (de la Monte et al., 2008; He et al., 2007; Kang et al., 2007b; Mohr et al., 1998; Shelmet et al., 1988). Because of ethanol-mediated suppression of insulin production, secretion and signaling pathways, the CYP2E1 level would remain elevated. Consequently, insulin-mediated glucose/lipid metabolism should be altered in ethanol-exposed tissues. These results may provide one reason why ethanol-exposed animals or human alcoholics produce greater amounts of ROS from elevated CYP2E1 and thus may be more prone to develop type II diabetes with insulin resistance and fatty liver than non-drinkers (Beulens et al, 2005; Carlsson et al., 2003; Sotaniemi et al., 1985). Therefore, it is possible that ethanol-related ROS/RNS may activate stress-activated protein kinases including JNK, which is known to phopshorylate and inhibit IRS-1, as observed in multiple forms of insulin resistance (Houstis et al., 2006) or in obese mice (Diehl 2004; Solinas et al., 2006). These results are consistent with the roles of CYP2E1 and JNK1 in causing insulin resistance in mouse models of nonalcoholic steatohepatitis (Schattenberg et al., 2006 and 2005). Although these models of JNK-mediated insulin resistance were not studied in the models of alcoholic fatty liver, similar mechanisms are expected due to elevation of CYP2E1 and JNK in ethanol-exposed animals. On the other hand, many synthetic agonists of peroxisome proliferator activated receptor γ (PPARγ) such as pioglitzone and rosiglitazone are known to increase insulin sensitivity by activating the insulin signaling pathway, leading to restoration of glucose homeostasis and lipid metabolism accompanied with reduced fatty liver (see reviews by Khashab and Chalasani, 2007; Roden, 2006). Therefore, activation of the insulin signaling pathway by endogenous substances (e.g. adiponectin) or synthetic agents (e.g. pioglitazone) may also contribute to the improved outcomes (i.e. decreased fat accumulation) observed in alcoholic fatty liver and injury (Enomoto et al., 2003; Xu et al., 2003).
K. Role of oxidative inactivation of the enzymes involved in the mitochondrial β-oxidation of fatty acids
Mitochondria play a critical role in energy supply, fat degradation, intermediary metabolism (including the urea cycle), anti-oxidant defense and apoptosis. Mitochondrial fat oxidation becomes important in providing an alternative energy (ketone bodies) when glucose supply is limited (e.g. fasting) or glucose metabolism is altered under certain disease states. It is well-established that mitochondria are a major source of ROS production and a major target of increased oxidative/nitrosative stress. Mitochondrial functions are often suppressed upon exposure to toxic compounds (including ethanol and acetaminophen) and/or in many disease states such as degenerative neurological diseases, aging and cancer (see reviews by Begriche et al., 2003; Hoek et al., 2002; Wallace, 2005). In fact, alcohol can directly inhibit the activities of mitochondrial complexes I and III (Bailey and Cunningham, 2002; Bailey et al., 1999; Cederbaum et al., 1974), resulting in increased ROS production leaked from the mitochondrial respiratory chain. In addition, alcohol is known to induce/activate mitochondrial CYP2E1 (Robin et al., 2005) and inducible NOS (iNOS), which provides excess amounts of nitric oxide (NO) (Barona et al., 2002; McKim et al., 2003). Increased production of ROS and RNS contributes to greater production of more toxic peroxynitrite, which promotes lipid peroxidation, mitochondrial dysfunction, and apoptosis (see review by Pacher et al., 2007). Under increased oxidative/nitrosative stress in ethanol-exposed animals, mitochondrial proteins are oxidatively modified and subjected to spontaneous or proteolytic degradation, resulting in inhibition of their functions and/or decreased protein expression (Suh et al., 2004; Venkatraman et al., 2004b and 2004a). Accumulation of fat and cholesterol observed in alcohol-exposed animals and human alcoholics could result from decreased fat degradation (Cederbaum et al., 1975; Lakshman et al., 1978; Salaspuro, 1981; Vankoningsloo et al., 2005; You et al., 2004). For instance, inactivation of the mitochondrial long-chain 3-hydroxyacyl-CoA dehydrogenase (the C-terminal portion of the α-subunit of mitochondrial trifunctional protein) by genetic mutation leads to maternal acute fatty liver during pregnancy in humans (Ibdah et al., 1999; Sims et al., 1995) and steatosis in heterozygous mice deficient of this enzyme (Ibdah et al., 2005), underscoring an important role of the mitochondrial fat oxidation pathway in overall fat disposal and accumulation. However, the detailed mechanism of suppressed fat oxidation under increased ROS/RNS is poorly understood. To investigate this mechanism, it was hypothesized that increased ROS/RNS following ethanol exposure can oxidatively modify the key enzymes in the β-oxidation of fatty acids, leading to inactivation of these enzymes and thus fat accumulation. Under increased oxidative/nitrosative stress, various amino acid residues such as Cys, His, Lys, Met, Trp, and Tyr can be oxidatively-modified. Oxidative modifications of these amino acid residues in many proteins usually lead to inactivation of their functional activities (Kim et al., 2006a; Moon et al., 2005). For instance, Cys residues can be modified to: S-nitrosylation, S-glutathionylation, disulfide bond, sulfenic acid, sulfinic acid, sulfonic acid and conjugation with reactive metabolites of certain chemicals (e.g. acetaminophen and disulfiram) (Moon et al., 2007; Moon et al., 2006; Moon et al., 2008). In fact, 3-ketoacyl-CoA thiolase involved in the mitochondrial β-oxidation pathway contains two Cys residues (92Cys and 382Cys) and one His (352His) in its active site. The active site Cys residues of 3-ketoacyl-CoA thiolase could have been oxidatively-modified because of its suppressed activity in alcohol-exposed rats (Moon et al., 2006; Song et al., 2008). Furthermore, other enzymes involved in the β-oxidation such as acyl-CoA dehydrogenase, enoyl-CoA hydratase, and β-hydroxy-acyl-CoA dehydrogenase are also oxidatively-modified in alcohol-exposed rats (Moon et al., 2006). Although the activities of these latter enzymes were not measured, their activities are likely inhibited under alcohol-related oxidative stress, based on the general inhibition of the β-oxidation pathway in alcohol-exposed animals (Cederbaum et al., 1975). Because of the similarity between the mitochondrial and peroxisomal fat oxidation pathways, it is likely that the enzymes in the peroxisomal β-oxidation pathway such as acyl-CoA oxidase and 3-ketoacyl-CoA thiolase could be also oxidatively inactivated after alcohol exposure as recently reported (Lu et al., 2008). Reduced fat oxidation, due to oxidative inactivation of the mitochondrial and/or peroxisomal enzymes, could contribute to fat accumulation in the liver.
Addition of long-chain polyunsaturated fatty acids at physiologically-relevant levels decreased the hepatic levels of triglycerides and cholesterol in ethanol-fed rats (36% ethanol-derived calories) compared to those in pair-fed controls (Song et al., 2008). The beneficial effects of polyunsaturated fatty acids are due to prevention of ethanol-mediated oxidative/nitrosative stress and mitochondrial dysfunction. The elevated CYP2E1 and iNOS activities returned to basal levels while the suppressed 3-ketoacy-CoA thiolase activity was restored in rats fed a diet with ethanol and polyunsaturated fatty acids. It is expected that the suppressed rates of fat oxidation in ethanol-exposed tissues could be normalized following treatment with other antioxidants such as SAM and medium-chain triglycerides, based on their inhibitory effects on ethanol-related elevation of CYP2E1 and iNOS activities in alcohol-exposed animals (Dey et al., 2007; Lieber et al., 2007).
L. Future research directions
1) Detailed signaling mechanisms and post-translational modifications
In this short review, we have summarized newly emerging results about alcoholic fatty liver. Despite excellent studies by numerous investigators, more studies are needed to further reveal the detailed mechanisms. For instance, the molecular signaling mechanisms of ethanol-mediated inhibition of AMPK, PGC-1α, or Sirt1 are still unknown, despite the convincing data (Lieber et al., 2008; You et al., 2004 and 2008b). It is unknown if these enzymes/proteins or their upstream signaling proteins (including some transcriptional factors) could be abnormally regulated and/or covalently modified (e.g. protein-adduct formation with significantly elevated acetaldehyde, 4-HNE, and MDA) in alcohol-exposed tissues (Sampey et al., 2003 and 2007b). For instance, 4-HNE significantly suppressed LPS-induced production of IL-6 by preventing the NFkB pathway and suppressing IkBα phosphorylation (Luckey et al., 2002). Therefore, it is expected that 4-HNE, MDA, or acetaldehyde may interrupt the signaling pathways of various factors listed in Fig. 1 at different steps. Alternatively, these signaling proteins could be functionally altered or down-regulated by their post-translational modifications such as oxidation, S-nitrosylation, nitration, acetylation, and/or phosphorylation that are stimulated by ethanol. For example, nitration or phosphorylation of insulin-receptor substrate-1 (IRS-1) is known to interfere with the insulin signaling pathway, contributing to insulin resistance and abnormal glucose/lipid homeostatsis (Nomiyama et al., 2004; Solinas et al., 2006). In addition, PPAR-related receptors could be phosphorylated by various protein kinases including stress-activated protein kinases, resulting in different outcomes in a ligand-dependent or –independent manner (see review by Burns and Vanden Heubel, 2007). Since ethanol intake elevates the levels of peroxynitrite and 4-HNE (a lipid peroxidation product) which can modulate stress-activated proteins kinases (Lee et al., 2002; Sampey et al., 2007a; Shacka et al., 2006; Soh et al., 2000; Song et al., 2001), it is possible that some signaling proteins could be modified by nitration and/or phosphorylation, contributing to different levels and types of fat accumulation. These possibilities need to be studied or considered in interpreting the results.
2) Role of other regulatory factors
Although we have summarized a few key factors in this review, many other factors may also contribute to alcoholic fatty liver. For instance, plasminogen activator inhibitor-1, whose level was elevated by ethanol exposure, also seems important in promoting alcoholic fatty liver in mice (Bergheim et al., 2006). In this model, AMPK activity does not play a crucial role in steatosis induced by acute alcohol exposure. In addition, dietary compositions of carbohydrate (low versus high amounts), fat content (percentage and composition of saturated versus unsaturated fats), and fat chain length (short, medium, and long-chain fatty acids) may affect the levels of adiponectin and other critical factors, contributing to different outcomes of alcoholic fatty liver and injury (Baraona et al., 2002; Lieber et al., 2007; Nanji et al., 1994; Ronis et al., 2004; Song et al., 2008; You et al., 2005; You et al., 2008a). Adipocytes are also known to secret leptin and resistin, which may play a role in alcoholic steatosis by positively and negatively modulating insulin sensitivity/resistance (see review by Bertolani and Marra, 2008). The role of these adipocyte-derived adipokines, interleukin-1, interleukin-8, glucagon, and certain neurotransmitters such as norepinephrine, neuropeptide Y and angiotensin II in alcoholic fatty liver and their controlling mechanisms should be studied in the future.
3) The interplay and feedback cross-talk between different factors
As indicated in Fig. 1, the mechanisms of alcoholic fatty liver are complex and multifactorial. Interaction and feedback cross-talk between many key components and signaling molecules including the transcription factors would make the pathological mechanisms more complicated. For instance, adiponectin, which suppresses fat synthesis by activating AMPK and promotes fat degradation by activating the PGC-1α and PPAR-α, was shown to decrease TNF-α production (Xu et al., 2003). Recent data show that adiponectin suppresses the lipopolysaccharide-stimulated production of TNF-α by both transcriptional and post-transcriptional mechanisms (Park et al., 2008). Paradoxically, TNF-α can suppress the production of adiponectin through activating the JNK (Kim et al., 2005) while it stimulates production of plasminogen-activator inhibitor-1 and IL-6 (Ahn et al., 2007). Furthermore, recent data showed that macrophages negatively affect adipocyte functions, resulting in decreased amounts of adiponectin production, leading to alcoholic fatty liver (Kang et al., 2007b). These results clearly demonstrate complex feedback cross-talk between different cell types and pluripotent soluble factors. Many other examples of cross-talk between insulin versus TNF-α or other factors including epigenetic regulation in different target cells may also exist in alcoholic liver diseases (see reviews by Diehl, 2004; Fritsche et al., 2008; Hoek and Pastorino, 2004; Nieto-Vazquez et al., 2008; Shukla et al., 2008; Wilfred de Alwis and Day, 2007). Therefore, depending on the exposure time (duration), the level of expression, and target tissues of these cellular factors produced after ethanol exposure, different responses may be observed. In addition, different rates of fat accumulation may occur depending on the amount of alcohol (dosage), the pattern (acute versus chronic), the frequency, and the route of administration, all of which affect the cellular signaling responses.
4) Protective role of triglycerides in lipotoxicity and mechanism
By using a specific anti-sense oligonucleotide against diacylglycerol acyltransferase 2 (DGAT2), Diehl and colleagues recently demonstrated a protective role of triglycerides against more severe liver injury in a mouse model of nonalcoholic steatohepatitis (Yamaguchi et al., 2007). In this study, increased accumulation of free fatty acids likely causes more severe liver damage such as increased lipid peroxidation/oxidative stress, lobular necroinflammation and fibrosis. These results are of interest since that progression toward severe lipotoxicity was observed despite the elevated level of serum adiponectin and improvement of insulin resistance in the peripheral tissues. Although the underlying mechanisms for severe hepatic injury by elevated free fatty acids are unclear, increased CYP2E1, ROS and lipid peroxides (4-HNE) seem responsible for the lipotoxicity. It is likely that lipotoxicity caused by free fatty acids could be mediated through activation of the cell-death related JNK, which can be activated by increased oxidative/nitrosative stress (Kamata et al., 2005; Kim et al., 2006b). Alternatively, free fatty acids may sensitize the hepatocytes to TRAIL-mediated cytotoxicity (Maldi et al., 2007 and 2006). The question whether elevated triglycerides (by efficiently disposing the hepatic free fatty acids) prevent severe liver injury such as necroinflammation and fibrosis needs to be carefully evaluated in ethanol-exposed animals and alcoholic humans. Interestingly, one earlier study supports the protective role of triglycerides against more severe liver diseases with necroinflammation (Nakajima et al., 2004). In this study, inflammation, apoptosis and fibrosis were minimally observed in PPAR-α knockout mice when hepatic triglyceride levels reached a highest peak after feeding an ethanol diet for 1 month. However, the severe liver diseases (apoptosis, inflammation, hepatomegaly, and fibrosis with elevated levels of Bax and transforming growth factors) began to be observed when hepatic triglyceride levels started to decline after feeding for 2 months and longer (up to 8 months). The levels of free fatty acid levels and other proteins involved in apoptosis remain to be determined in these mice.
5) Relevance to human conditions
Many of these mechanistic studies were conducted in cultured hepatocyes/hepatoma cells or animal models. Although most of the summarized mechanisms could be applied to understanding of the etiologic mechanisms of alcoholic fatty liver in humans, some cautions should be taken in extrapolating the data obtained from in vitro and in vivo animal studies. For instance, although the data obtained from knockout mice with gene disruption techniques or transgenic mice with over-expression of a target gene provide an important clue for its functional role, the data may not necessarily reflect the human conditions without the supporting biochemical and genetic data in human populations. Therefore, additional genetic studies for many human genes (e.g. single nucleotide polymorphism data, epigenetic studies, gene association and epidemiological analyses, etc) are needed. Several candidate genes associated with fatty liver diseases are listed (see review by Wilfred de Alwis and Day, 2007). Furthermore, the mechanisms for alcoholic steatosis and liver injury in human alcoholics may be more complicated due to individual differences in food intake (e.g. fat and carbohydrate contents with or without dietary supplements), genetic makeup, race, gender, age, and other environmental factors such as bacterial and viral infections, diabetes, body-mass-index (obesity), exercise, smoking, drug exposure/interaction, autoimmune reactions, etc (Day, 2006; Dey and Cederbaum, 2006; Lieber, 2004; Ronis et al., 2004; Stewart et al., 2004; Vidali et al., 2003). Some of these factors (e.g. hepatitis viruses and/or smoking) are expected to synergistically work with alcohol toward accelerating disease processes in the liver and other tissues (see reviews by Morgan et al., 2003; Szabo et al., 2006). Therefore, additive or synergistic relationship between alcohol and other co-morbidity factors (such as viral infections, diabetes and smoking) should be studied. This combinatorial approach may also provide more relevant information to better understand human alcoholic liver diseases.
6) Application of mechanistic studies in understanding alcohol-mediated organ damage in other tissues
It is well established that acute binge ethanol exposure and long-term heavy alcohol intake cause pancreatitis, cardiac myopathy, muscle weakness, adipocyte dysfunction, testis atrophy, and neuropathy with decreased brain volume (structural and cognitive functional declines) (Pfefferbaum et al., 1992 and 1997). Because increased ROS/RNS and lipid peroxidation play important roles in alcohol-mediated liver damage, it is expected that the similar mechanisms likely exist in other tissues. Since several organs depend on liver metabolic functions (e.g. ketogenesis, gluconeogenesis, urea cycle, fatty acid and cholesterol synthesis, etc), understanding of mutual interactions between the liver and other organs seems important. This interaction needs to be further studied for deeper understanding of the pathological mechanisms in other organs of ethanol-exposed animals/humans.
7) Future translational research
Based on the mechanistic studies of alcoholic fatty liver and injury, the efficacies of many substances as preventive and therapeutic agents have been studied. The beneficial agents shown so far against alcoholic liver diseases include: various antioxidants (SAM, betaine, resveratrol, polyenylphosphatidylcholine, medium chain triglycerides, long chain polyunsaturated fatty acids, vitamins, etc); AMPK activator such as metformin; PPAR-α agonists clofibrate and Wy14,643; PPARγ agonists including pioglitazone; CB1 receptor antagonists; TNF-α receptor blocking antibodies; etc. Although the safety issue (toxicity, proper dosage, frequency, etc) has to be checked for each item, special caution should be applied for PPAR-α agonists since PPAR-α activation may cause hepatic cancer in rodents and possibly in humans (see reviews by Gonzalez and Shah, 2008; Yu et al., 2003). In addition, activation of AOX and CYP4A by PPAR-α agonists may produce extra ROS production and lipid peroxidation. For instance, CYP4A-mediated ROS production and lipid peroxidation were demonstrated in CYP2E1 knockout mice fed a methionine and choline-deficient diet (Leclercq et al., 2000). Furthermore, microsomal CYP4A-related metabolism of long chain fatty acids (C≥20) may produce ω and ω-1 dicarboxylic acids, which may inhibit mitochondrial function, leading to increased oxidative stress and toxicity (see review by Yu et al., 2003). Because of these concerns, application of PPAR-α agonists may need additional caution in evaluation of their long-term safety.
M. Summary
Alcoholic fatty liver is a frequently observed potentially pathologic condition which can progress to more severe liver diseases such as necroinflammation and fibrosis/cirrhosis. In this review, we have summarized and integrated the newly emerging factors into a schematic diagram (Fig. 1) that depicts the major pathways in alcoholic fatty liver. As shown in Fig. 1 diagram, hepatic lipid metabolism is regulated by various hormones, chemokines, cytokines and adipokines. Following alcohol exposure, alcoholic fatty liver and injury likely take place through the complex interactions between a variety of cell types, ethanol doses and metabolites, increased oxidative/nitrosative stress, peroxisomal/mitochondrial dysfunction, hormones, paracrine factors, cytokines, transcriptional factors, cell signaling kinases, etc. Besides the major pathways indicated in this diagram, ethanol intake is also known to promote fat transport into the liver from peripheral tissues (e.g. adipose tissues) while ethanol inhibits fat export from the liver (Baraona and Lieber, 1979; Kang et al., 2007a; Lieber, 2004). Because of common features of fat accumulation and disease progression (Diehl et al., 1988; Wilfred de Alwis and Day, 2007), it is expected that similar mechanisms of the major pathways for alcoholic fatty liver (Fig. 1) could also exist in animal models and human patients suffering from nonalcoholic steatohepatitis.
Acknowledgments
This work was supported by the Intramural and Extramural Programs of National Institute on Alcohol Abuse and Alcoholism. We are grateful to the anonymous reviewers' expert comments and constructive criticisms to improve our manuscript. We are also thankful to Dr. James P. Hardwick, Northeastern Ohio University Medical College, for his critical reading and helpful comments.
References
- Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291(5513):2613–6. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- Ahn J, Lee H, Kim S, Ha T. Resveratrol inhibits TNF-alpha-induced changes of adipokines in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2007;364(4):972–7. doi: 10.1016/j.bbrc.2007.10.109. [DOI] [PubMed] [Google Scholar]
- Aleynik SI, Lieber CS. Polyenylphosphatidylcholine corrects the alcohol-induced hepatic oxidative stress by restoring S-adenosylmethionine. Alcohol Alcohol. 2003;38(3):208–12. doi: 10.1093/alcalc/agg066. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha) J Biol Chem. 1998;273(10):5678–84. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- Bailey SM, Cunningham CC. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radic Biol Med. 2002;32(1):11–6. doi: 10.1016/s0891-5849(01)00769-9. [DOI] [PubMed] [Google Scholar]
- Bailey SM, Pietsch EC, Cunningham CC. Ethanol stimulates the production of reactive oxygen species at mitochondrial complexes I and III. Free Radic Biol Med. 1999;27(7-8):891–900. doi: 10.1016/s0891-5849(99)00138-0. [DOI] [PubMed] [Google Scholar]
- Barak AJ, Beckenhauer HC, Kharbanda KK, Tuma DJ. Chronic ethanol consumption increases homocysteine accumulation in hepatocytes. Alcohol. 2001;25(2):77–81. doi: 10.1016/s0741-8329(01)00168-9. [DOI] [PubMed] [Google Scholar]
- Baraona E, Lieber CS. Effects of ethanol on lipid metabolism. J Lipid Res. 1979;20(3):289–315. [PubMed] [Google Scholar]
- Baraona E, Zeballos GA, Shoichet L, Mak KM, Lieber CS. Ethanol consumption increases nitric oxide production in rats, and its peroxynitrite-mediated toxicity is attenuated by polyenylphosphatidylcholine. Alcohol Clin Exp Res. 2002;26(6):883–9. [PubMed] [Google Scholar]
- Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6(1):1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13(2):84–9. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- Bergheim I, Guo L, Davis MA, Lambert JC, Beier JI, Duveau I, Luyendyk JP, Roth RA, Arteel GE. Metformin prevents alcohol-induced liver injury in the mouse: Critical role of plasminogen activator inhibitor-1. Gastroenterology. 2006;130(7):2099–112. doi: 10.1053/j.gastro.2006.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolani C, Marra F. The role of adipokines in liver fibrosis. Pathophysiology. 2008 doi: 10.1016/j.pathophys.2008.05.001. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Beulens JW, Stolk RP, van der Schouw YT, Grobbee DE, Hendriks HF, Bots ML. Alcohol consumption and risk of type 2 diabetes among older women. Diabetes Care. 2005;28(12):2933–8. doi: 10.2337/diacare.28.12.2933. [DOI] [PubMed] [Google Scholar]
- Blasco C, Caballería J, Deulofeu R, Lligoña A, Parés A, Lluis JM, Gual A, Rodés J. Prevalence and mechanisms of hyperhomocysteinemia in chronic alcoholics. Alcohol Clin Exp Res. 2005;29(6):1044–8. doi: 10.1097/01.alc.0000169265.36440.ee. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci USA. 1999;96(20):11041–8. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns KA, Vanden Heuvel JP. Modulation of PPAR activity via phosphorylation. Biochim Biophys Acta. 2007;1771(8):952–60. doi: 10.1016/j.bbalip.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykov I, Jauhiainen M, Olkkonen VM, Saarikoski ST, Ehnholm C, Junnikkala S, Vakeva A, Lindros KO, Meri S. Hepatic gene expression and lipid parameters in complement C3(-/-) mice that do not develop ethanol-induced steatosis. J Hepatol. 2007;46(5):907–14. doi: 10.1016/j.jhep.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Bykov I, Junnikkala S, Pekna M, Lindros KO, Meri S. Complement C3 contributes to ethanol-induced liver steatosis in mice. Ann Med. 2006;38(4):280–6. doi: 10.1080/07853890600664608. [DOI] [PubMed] [Google Scholar]
- Carlsson S, Hammar N, Grill V, Kaprio J. Alcohol consumption and the incidence of type 2 diabetes: a 20-year follow-up of the Finnish twin cohort study. Diabetes Care. 2003;26(10):2785–90. doi: 10.2337/diacare.26.10.2785. [DOI] [PubMed] [Google Scholar]
- Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- Cederbaum AI, Lieber CS, Beattie DS, Rubin E. Effect of chronic ethanol ingestion on fatty acid oxidation by hepatic mitochondria. J Biol Chem. 1975;250(13):5122–9. [PubMed] [Google Scholar]
- Cederbaum AI, Lieber CS, Rubin E. Effects of chronic ethanol treatment of mitochondrial functions damage to coupling site I. Arch Biochem Biophys. 1974;165(2):560–9. doi: 10.1016/0003-9861(74)90283-5. [DOI] [PubMed] [Google Scholar]
- Colmenero J, Bataller R, Sancho-Bru P, Bellot P, Miquel R, Moreno M, Jares P, Bosch J, Arroyo V, Caballeria J, Gines P. Hepatic expression of candidate genes in patients with alcoholic hepatitis: correlation with disease severity. Gastroenterology. 2007;132(2):687–97. doi: 10.1053/j.gastro.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Costet P, Legendre C, More J, Edgar A, Galtier P, Pineau T. Peroxisome proliferator-activated receptor alpha-isoform deficiency leads to progressive dyslipidemia with sexually dimorphic obesity and steatosis. J Biol Chem. 1998;273(45):29577–85. doi: 10.1074/jbc.273.45.29577. [DOI] [PubMed] [Google Scholar]
- Crabb DW. Recent developments in alcoholism: the liver. Recent Dev Alcohol. 1993;11:207–30. [PubMed] [Google Scholar]
- Day CP. Genes or environment to determine alcoholic liver disease and non-alcoholic fatty liver disease. Liver Int. 2006;26(9):1021–8. doi: 10.1111/j.1478-3231.2006.01323.x. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Yeon JE, Tong M, Longato L, Chaudhry R, Pang MY, Duan K, Wands JR. Insulin resistance in experimental alcohol-induced liver disease. J Gastroenterol Hepatol. 2008 doi: 10.1111/j.1440-1746.2008.05339.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waziers I, Garlatti M, Bouguet J, Beaune PH, Barouki R. Insulin down-regulates cytochrome P450 2B and 2E expression at the post-transcriptional level in the rat hepatoma cell line. Mol Pharmacol. 1995;47(3):474–9. [PubMed] [Google Scholar]
- Dey A, Caro AA, Cederbaum AI. S-adenosyl methionine protects ob/ob mice from CYP2E1-mediated liver injury. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G91–103. doi: 10.1152/ajpgi.00004.2007. [DOI] [PubMed] [Google Scholar]
- Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43(2 Suppl 1):S63–74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- Diehl AM. Obesity and alcoholic liver disease. Alcohol. 2004;34(1):81–7. doi: 10.1016/j.alcohol.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Diehl AM, Goodman Z, Ishak KG. Alcohollike liver disease in nonalcoholics. A clinical and histologic comparison with alcohol-induced liver injury. Gastroenterology. 1988;95(4):1056–62. [PubMed] [Google Scholar]
- El-Assal O, Hong F, Kim WH, Radaeva S, Gao B. IL-6-deficient mice are susceptible to ethanol-induced hepatic steatosis: IL-6 protects against ethanol-induced oxidative stress and mitochondrial permeability transition in the liver. Cell Mol Immunol. 2004;1(3):205–11. [PubMed] [Google Scholar]
- Enomoto N, Takei Y, Hirose M, Konno A, Shibuya T, Matsuyama S, Suzuki S, Kitamura KI, Sato N. Prevention of ethanol-induced liver injury in rats by an agonist of peroxisome proliferator-activated receptor-gamma, pioglitazone. J Pharmacol Exp Ther. 2003;306(3):846–54. doi: 10.1124/jpet.102.047217. [DOI] [PubMed] [Google Scholar]
- Endo M, M T, Seike M, Yoshimatsu H. TNF-alpha induces hepatic steatosis in mice by enhancing gene expression of sterol regulatory element binding protien-1c. Exp Biol Med (Maywood) 2007:614–21. [PubMed] [Google Scholar]
- Esfandiari F, You M, Villanueva JA, Wong DH, French SW, Halsted CH. S-adenosylmethionine attenuates hepatic lipid synthesis in micropigs fed ethanol with a folate-deficient diet. Alcohol Clin Exp Res. 2007;31(7):1231–9. doi: 10.1111/j.1530-0277.2007.00407.x. [DOI] [PubMed] [Google Scholar]
- Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem. 2003;278(30):27997–8004. doi: 10.1074/jbc.M302140200. [DOI] [PubMed] [Google Scholar]
- Fritsche L, Weigert C, Häring HU, Lehmann R. How insulin receptor substrate proteins regulate the metabolic capacity of the liver - Implications for health and disease. Curr Med Chem. 2008;15(13):1316–29. doi: 10.2174/092986708784534956. [DOI] [PubMed] [Google Scholar]
- French SW, Morimoto M, Reitz RC, Koop D, Klopfenstein B, Estes K, Clot P, Ingelman-Sundberg M, Albano E. Lipid peroxidation, CYP2E1 and arachidonic acid metabolism in alcoholic liver disease in rats. J Nutr. 1997;127(5 Suppl):907S–911S. doi: 10.1093/jn/127.5.907S. [DOI] [PubMed] [Google Scholar]
- Fromenty B, Berson A, Pessayre D. Microvesicular steatosis and steatohepatitis: role of mitochondrial dysfunction and lipid peroxidation. J Hepatol. 1997;26 1:13–22. doi: 10.1016/s0168-8278(97)82328-8. [DOI] [PubMed] [Google Scholar]
- Fukumura A, Tsutsumi M, Tsuchishima M, Hayashi N, Fukura M, Yano H, Ozaki K, Takase S. Effect of the inducer of interleukin-6 (ME3738) on rat liver treated with ethanol. Alcohol Clin Exp Res. 2007;31(1 Suppl):S49–53. doi: 10.1111/j.1530-0277.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- Galli A, Pinaire J, Fischer M, Dorris R, Crabb DW. The transcriptional and DNA binding activity of peroxisome proliferator-activated receptor alpha is inhibited by ethanol metabolism. A novel mechanism for the development of ethanol-induced fatty liver. J Biol Chem. 2001;276(1):68–75. doi: 10.1074/jbc.M008791200. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Morales A, Colell A, Ballesta A, Rodes J, Kaplowitz N, Fernandez-Checa JC. Feeding S-adenosyl-L-methionine attenuates both ethanol-induced depletion of mitochondrial glutathione and mitochondrial dysfunction in periportal and perivenous rat hepatocytes. Hepatology. 1995;21(1):207–14. doi: 10.1002/hep.1840210133. [DOI] [PubMed] [Google Scholar]
- García-Villafranca J, Guillén A, Castro J. Ethanol consumption impairs regulation of fatty acid metabolism by decreasing the activity of AMP-activated protein kinase in rat liver. Biochimie. 2008;90(3):460–6. doi: 10.1016/j.biochi.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Gillis SE, Nagy LE. Deposition of cellular fibronectin increases before stellate cell activation in rat liver during ethanol feeding. Alcohol Clin Exp Res. 1997;21(5):857–61. [PubMed] [Google Scholar]
- Gonzalez FJ, Shah YM. PPARalpha: mechanism of species differences and hepatocarcinogenesis of peroxisome proliferators. Toxicology. 2008;246(1):2–8. doi: 10.1016/j.tox.2007.09.030. [DOI] [PubMed] [Google Scholar]
- Grunnet N, Kondrup J. The effect of ethanol on the beta-oxidation of fatty acids. Alcohol Clin Exp Res. 1986;10(6 Suppl):64S–68S. doi: 10.1111/j.1530-0277.1986.tb05182.x. [DOI] [PubMed] [Google Scholar]
- Hakkola J, Hu Y, Ingelman-Sundberg M. Mechanisms of down-regulation of CYP2E1 expression by inflammatory cytokines in rat hepatoma cells. J Pharmacol Exp Ther. 2003;304(3):1048–55. doi: 10.1124/jpet.102.041582. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–55. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- Hardwick JP. Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem Pharmacol. 2008;75(12):2263–75. doi: 10.1016/j.bcp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, Patt YZ. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36(5):1206–13. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- He J, de la Monte S, Wands JR. Acute ethanol exposure inhibits insulin signaling in the liver. Hepatology. 46(6):1791–1800. doi: 10.1002/hep.21904. [DOI] [PubMed] [Google Scholar]
- Hezode C, Zafrani ES, Roudot-Thoraval F, Costentin C, Hessami A, Bouvier-Alias M, Medkour F, Pawlostky JM, Lotersztajn S, Mallat A. Daily cannabis use: a novel risk factor of steatosis severity in patients with chronic hepatitis C. Gastroenterology. 2008;134(2):432–9. doi: 10.1053/j.gastro.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Higuchi H, Adachi M, Miura S, Gores GJ, Ishii H. The mitochondrial permeability transition contributes to acute ethanol-induced apoptosis in rat hepatocytes. Hepatology. 2001;34(2):320–8. doi: 10.1053/jhep.2001.26380. [DOI] [PubMed] [Google Scholar]
- Hill DB, Marsano L, Cohen D, Allen J, Shedlofsky S, McClain CJ. Increased plasma interleukin-6 concentrations in alcoholic hepatitis. J Lab Clin Med. 1992;119(5):547–52. [PubMed] [Google Scholar]
- Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122(7):2049–63. doi: 10.1053/gast.2002.33613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek JB, Pastorino JG. Cellular signaling mechanisms in alcohol-induced liver damage. Semin Liver Dis. 2004;24(3):257–72. doi: 10.1055/s-2004-832939. [DOI] [PubMed] [Google Scholar]
- Hong F, Kim WH, Tian Z, Jaruga B, Ishac E, Shen X, Gao B. Elevated interleukin-6 during ethanol consumption acts as a potential endogenous protective cytokine against ethanol-induced apoptosis in the liver: involvement of induction of Bcl-2 and Bcl-x(L) proteins. Oncogene. 2002;21(1):32–43. doi: 10.1038/sj.onc.1205016. [DOI] [PubMed] [Google Scholar]
- Hong F, Radaeva S, Pan HN, Tian Z, Veech R, Gao B. Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology. 2004;40(4):933–41. doi: 10.1002/hep.20400. [DOI] [PubMed] [Google Scholar]
- Horiguchi N, Ishac EJ, Gao B. Liver regeneration is suppressed in alcoholic cirrhosis: correlation with decreased STAT3 activation. Alcohol. 2007;41(4):271–80. doi: 10.1016/j.alcohol.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi N, Wang L, Mukhopadhyay P, Park O, Jeong WI, Lafdil F, Osei-Hyiaman D, Moh A, Fu XY, Pacher P, Kunos G, Gao B. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 2008;134(4):1148–58. doi: 10.1053/j.gastro.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–8. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- Ibdah JA, Bennett MJ, Rinaldo P, Zhao Y, Gibson B, Sims HF, Strauss AW. A fetal fatty-acid oxidation disorder as a cause of liver disease in pregnant women. N Engl J Med. 1999;340(22):1723–31. doi: 10.1056/NEJM199906033402204. [DOI] [PubMed] [Google Scholar]
- Ibdah JA, Perlegas P, Zhao Y, Angdisen J, Borgerink H, Shadoan MK, Wagner JD, Matern D, Rinaldo P, Cline JM. Mice heterozygous for a defect in mitochondrial trifunctional protein develop hepatic steatosis and insulin resistance. Gastroenterology. 2005;128(5):1381–90. doi: 10.1053/j.gastro.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Ip E, Farrell GC, Robertson G, Hall P, Kirsch R, Leclercq I. Central role of PPARalpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology. 2003;38(1):123–32. doi: 10.1053/jhep.2003.50307. [DOI] [PubMed] [Google Scholar]
- Ishii H, Adachi M, Fernandez-Checa JC, Cederbaum AI, Deaciuc IV, Nanji AA. Role of apoptosis in alcoholic liver injury. Alcohol Clin Exp Res. 2003;27(7):1207–12. [PubMed] [Google Scholar]
- Jarvelainen HA, Vakeva A, Lindros KO, Meri S. Activation of complement components and reduced regulator expression in alcohol-induced liver injury in the rat. Clin Immunol. 2002;105(1):57–63. doi: 10.1006/clim.2002.5267. [DOI] [PubMed] [Google Scholar]
- Jeong WI, Osei-Hyiaman D, Park O, Liu J, Batkai S, Mukhopadhyay P, Horiguchi N, Harvey-White J, Marsicano G, Lutz B, Gao B, Kunos G. Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab. 2008;7(3):227–35. doi: 10.1016/j.cmet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Ji C, Deng Q, Kaplowitz N. Role of TNF-alpha in ethanol-induced hyperhomocysteinemia and murine alcoholic liver injury. Hepatology. 2004;40(2):442–51. doi: 10.1002/hep.20309. [DOI] [PubMed] [Google Scholar]
- Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124(5):1488–99. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- Joshi-Barve S, Barve SS, Amancherla K, Gobejishvili L, Hill D, Cave M, Hote P, McClain CJ. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology. 2007;46(3):823–30. doi: 10.1002/hep.21752. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120(5):649–61. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Kang L, Chen X, Sebastian BM, Pratt BT, Bederman IR, Alexander JC, Previs SF, Nagy LE. Chronic ethanol and triglyceride turnover in white adipose tissue in rats: inhibition of the anti-lipolytic action of insulin after chronic ethanol contributes to increased triglyceride degradation. J Biol Chem. 2007a;282(39):28465–73. doi: 10.1074/jbc.M705503200. [DOI] [PubMed] [Google Scholar]
- Kang L, Sebastian BM, Pritchard MT, Pratt BT, Previs SF, Nagy LE. Chronic ethanol-induced insulin resistance is associated with macrophage infiltration into adipose tissue and altered expression of adipocytokines. Alcohol Clin Exp Res. 2007b;31(9):1581–8. doi: 10.1111/j.1530-0277.2007.00452.x. [DOI] [PubMed] [Google Scholar]
- Khashab M, Chalasani N. Use of insulin sensitizers in NASH. Endocrinol Metab Clin North Am. 2007;36(4):1067–87. doi: 10.1016/j.ecl.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest. 1999;103(11):1489–98. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoruts A, Stahnke L, McClain CJ, Logan G, Allen JI. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology. 1991;13(2):267–76. [PubMed] [Google Scholar]
- Kim BJ, Hood BL, Aragon RA, Hardwick JP, Conrads TP, Veenstra TD, Song BJ. Increased oxidation and degradation of cytosolic proteins in alcohol-exposed mouse liver and hepatoma cells. Proteomics. 2006a;6(4):1250–60. doi: 10.1002/pmic.200500447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Kim JK, Jeon JH, Yoon SR, Choi I, Yang Y. c-Jun N-terminal kinase is involved in the suppression of adiponectin expression by TNF-alpha in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2005;327(2):460–7. doi: 10.1016/j.bbrc.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Kim SK, Novak RF. The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression. Pharmacol Ther. 2007;113(1):88–120. doi: 10.1016/j.pharmthera.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase mediated phosphorylation of Bax leads to its activation, mitochondrial translocation and apoptosis of human hepatoma HepG2 cells. J Biol Chem. 2006b;281(30):21256–65. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- Knight BL, Hebbachi A, Hauton D, Brown AM, Wiggins D, Patel DD, Gibbons GF. A role for PPARalpha in the control of SREBP activity and lipid synthesis in the liver. Biochem J. 2005;389(Pt 2):413–21. doi: 10.1042/BJ20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH, Bradford BU, Holland SM, Thurman RG. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106(7):867–72. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop DR, Tierney DJ. Multiple mechanisms in the regulation of ethanol-inducible cytochrome P450IIE1. Bioessays. 1990;12(9):429–35. doi: 10.1002/bies.950120906. [DOI] [PubMed] [Google Scholar]
- Kunos G, Osei-Hyiaman D. Endocannabinoids and liver disease. IV. Endocannabinoid involvement in obesity and hepatic steatosis. Am J Physiol Gastrointest Liver Physiol. 2008;294(5):G1101–4. doi: 10.1152/ajpgi.00057.2008. [DOI] [PubMed] [Google Scholar]
- Kunos G, Osei-Hyiaman D, Liu J, Godlewski G, Batkai S. Endocannabinoids and the control of energy homeostasis. J Biol Chem. 2008 doi: 10.1074/jbc.R800012200. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuper H, Tzonou A, Kaklamani E, Hsieh CC, Lagiou P, Adami HO, Trichopoulos D, Stuver SO. Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int J Cancer. 2000;85(4):498–502. [PubMed] [Google Scholar]
- Lakshman MR, Gupta AD, Veech RL. Effect of chronic ethanol administration on cholesterol and bile acid synthesis in vivo. Lipids. 1978;13(2):134–6. doi: 10.1007/BF02533255. [DOI] [PubMed] [Google Scholar]
- Larrea E, Aldabe R, Molano E, Fernandez-Rodriguez CM, Ametzazurra A, Civeira MP, Prieto J. Altered expression and activation of signal transducers and activators of transcription (STATs) in hepatitis C virus infection: in vivo and in vitro studies. Gut. 2006;55(8):1188–96. doi: 10.1136/gut.2005.070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler JF, Jr, Yin M, Diehl AM, Roberts E, Chatterjee S. Tumor necrosis factor-alpha stimulates the maturation of sterol regulatory element binding protein-1 in human hepatocytes through the action of neutral sphingomyelinase. J Biol Chem. 1998;273(9):5053–9. doi: 10.1074/jbc.273.9.5053. [DOI] [PubMed] [Google Scholar]
- Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105(8):1067–75. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GY, Kim NH, Zhao ZS, Cha BS, Kim YS. Peroxisomal-proliferator-activated receptor alpha activates transcription of the rat hepatic malonyl-CoA decarboxylase gene: a key regulation of malonyl-CoA level. Biochem J. 2004;378(Pt 3):983–90. doi: 10.1042/BJ20031565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Aroor AR, Shukla SD. Temporal activation of p42/44 mitogen-activated protein kinase and c-Jun N-terminal kinase by acetaldehyde in rat hepatocytes and its loss after chronic ethanol exposure. J Pharmacol Exp Ther. 2002;301(3):908–14. doi: 10.1124/jpet.301.3.908. [DOI] [PubMed] [Google Scholar]
- Levitsky J, Mailliard ME. Diagnosis and therapy of alcoholic liver disease. Semin Liver Dis. 2004;24(3):233–47. doi: 10.1055/s-2004-832937. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Hepatic, metabolic and toxic effects of ethanol: 1991 update. Alcohol Clin Exp Res. 1991;15(4):573–92. doi: 10.1111/j.1530-0277.1991.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Microsomal ethanol-oxidizing system (MEOS): the first 30 years (1968-1998)--a review. Alcohol Clin Exp Res. 1999;23(6):991–1007. [PubMed] [Google Scholar]
- Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34(1):9–19. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Lieber CS, Cao Q, DeCarli LM, Leo MA, Mak KM, Ponomarenko A, Ren C, Wang X. Role of medium-chain triglycerides in the alcohol-mediated cytochrome P450 2E1 induction of mitochondria. Alcohol Clin Exp Res. 2007;31(10):1660–8. doi: 10.1111/j.1530-0277.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- Lieber CS, Leo MA, Wang X, Decarli LM. Effect of chronic alcohol consumption on Hepatic SIRT1 and PGC-1alpha in rats. Biochem Biophys Res Commun. 2008;370(1):44–8. doi: 10.1016/j.bbrc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6(9):998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- Lin HZ, Yang SQ, Zeldin G, Diehl AM. Chronic ethanol consumption induces the production of tumor necrosis factor-alpha and related cytokines in liver and adipose tissue. Alcohol Clin Exp Res. 1998;22(5 Suppl):231S–237S. doi: 10.1097/00000374-199805001-00004. [DOI] [PubMed] [Google Scholar]
- Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116(7):1776–83. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC, Huang ZZ, Yang H, Mato JM, Avila MA, Tsukamoto H. Changes in methionine adenosyltransferase and S-adenosylmethionine homeostasis in alcoholic rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;279(1):G178–85. doi: 10.1152/ajpgi.2000.279.1.G178. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47(5):1483–94. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- Luckey SW, Taylor M, Sampey BP, Scheinman RI, Petersen DR. 4-hydroxynonenal decreases interleukin-6 expression and protein production in primary rat Kupffer cells by inhibiting nuclear factor-kappaB activation. J Pharmacol Exp Ther. 2002;302(1):296–303. doi: 10.1124/jpet.102.033522. [DOI] [PubMed] [Google Scholar]
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8(7):731–7. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56:1124–31. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281(17):12093–101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Kromhout JP, Peterson FJ, Holtzman JL. Potentiation of acetaminophen hepatotoxicity by alcohol. JAMA. 1980;244(3):251–3. [PubMed] [Google Scholar]
- McKim SE, Gabele E, Isayama F, Lambert JC, Tucker LM, Wheeler MD, Connor HD, Mason RP, Doll MA, Hein DW, Arteel GE. Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology. 2003;125(6):1834–44. doi: 10.1053/j.gastro.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Mohr L, Tanaka S, Wands JR. Ethanol inhibits hepatocyte proliferation in insulin receptor substrate 1 transgenic mice. Gastroenterology. 1998;115(6):1558–65. doi: 10.1016/s0016-5085(98)70036-8. [DOI] [PubMed] [Google Scholar]
- Moon KH, Abdelmegeed MA, Song BJ. Inactivation of cytosolic aldehyde dehydrogenase via S-nitrosylation in ethanol-exposed rat liver. FEBS Lett. 2007;581(21):3967–72. doi: 10.1016/j.febslet.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KH, Hood BL, Kim BJ, Hardwick JP, Conrads TP, Veenstra TD, Song BJ. Inactivation of oxidized and S-nitrosylated mitochondrial proteins in alcoholic fatty liver of rats. Hepatology. 2006;44(5):1218–30. doi: 10.1002/hep.21372. [DOI] [PubMed] [Google Scholar]
- Moon KH, Hood BL, Mukhopadhyay P, Rajesh M, Abdelmegeed MA, Kwon YI, Conrads TP, Veenstra TD, Song BJ, Pacher P. Oxidative Inactivation of Key Mitochondrial Proteins Leads to Dysfunction and Injury in Hepatic Ischemia Reperfusion. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.06.048. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KH, Kim BJ, Song BJ. Inhibition of mitochondrial aldehyde dehydrogenase by nitric oxide-mediated S-nitrosylation. FEBS Lett. 2005;579(27):6115–20. doi: 10.1016/j.febslet.2005.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TR, Brenner D, Everhart J, French SW, Fried MW, Gretch DR, Koziel MJ, McClain CJ, Peters MG, Weinman SA, Lucas DL. Hepatitis C and alcohol: fundamental and translational research directions. Alcohol Clin Exp Res. 2003;27(4):726–31. doi: 10.1097/01.ALC.0000062741.58464.19. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Kamijo Y, Tanaka N, Sugiyama E, Tanaka E, Kiyosawa K, Fukushima Y, Peters JM, Gonzalez FJ, Aoyama T. Peroxisome proliferator-activated receptor alpha protects against alcohol-induced liver damage. Hepatology. 2004;40(4):972–80. doi: 10.1002/hep.20399. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Dannenberg AJ, Jokelainen K, Bass NM. Alcoholic liver injury in the rat is associated with reduced expression of peroxisome proliferator-alpha (PPARalpha)-regulated genes and is ameliorated by PPARalpha activation. J Pharmacol Exp Ther. 2004;310(1):417–24. doi: 10.1124/jpet.103.064717. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Zhao S, Sadrzadeh SMH, Waxman DJ. Markedly enhanced cytochrome P450 2E1 induction and lipid peroxidation is associated with severe liver injury in fish-oil-ethanol fed rats. Alcohol Clin Exp Res. 1994;18(5):1280–5. doi: 10.1111/j.1530-0277.1994.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Nieto-Vazquez I, Fernandez-Veledo S, Kramer DK, Vila-Bedmar R, Garcia-Guerra L, Lorenzo M. Insulin resistance associated to obesity: the link TNF-alpha. Arch Physiol Biochem. 2008;114(3):183–94. doi: 10.1080/13813450802181047. [DOI] [PubMed] [Google Scholar]
- Nomiyama T, Igarashi Y, Taka H, Mineki R, Uchida T, Ogihara T, Choi JB, Uchino H, Tanaka Y, Maegawa H, Kashiwagi A, Murayama K, Kawamori R, Watada H. Reduction of insulin-stimulated glucose uptake by peroxynitrite is concurrent with tyrosine nitration of insulin receptor substrate-1. Biochem Biophys Res Commun. 2004;320(3):639–47. doi: 10.1016/j.bbrc.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Nordmann R, Ribiere C, Rouach H. Implication of free radical mechanisms in ethanol-induced cellular injury. Free Radic Biol Med. 1992;12(3):219–40. doi: 10.1016/0891-5849(92)90030-k. [DOI] [PubMed] [Google Scholar]
- Ohnishi K, Lieber CS. Reconstitution of the microsomal ethanol-oxidizing system. Qualitative and quantitative changes of cytochrome P-450 after chronic ethanol consumption. J Biol Chem. 1977;252(20):7124–31. [PubMed] [Google Scholar]
- Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115(5):1298–305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI, Bátkai S, Marsicano G, Lutz B, Buettner C, Kunos G. Hepatic CB(1) receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008 doi: 10.1172/JCI34827. Aug 1 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani K, Korenaga M, Beard MR, Li K, Qian T, Showalter LA, Singh AK, Wang T, Weinman SA. Hepatitis C virus core protein, cytochrome P450 2E1, and alcohol produce combined mitochondrial injury and cytotoxicity in hepatoma cells. Gastroenterology. 2005;128(1):96–107. doi: 10.1053/j.gastro.2004.10.045. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58(3):389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandol SJ, Raraty M. Pathobiology of alcoholic pancreatitis. Pancreatology. 2007;7(2-3):105–14. doi: 10.1159/000104235. [DOI] [PubMed] [Google Scholar]
- Park PH, Huang H, McMullen MR, Mandal P, Sun L, Nagy LE. Suppression of lipopolysaccharide-stimulated tumor necrosis factor-alpha production by adiponectin is mediated by transcriptional and post-transcriptional mechanisms. J Biol Chem. 2008 doi: 10.1074/jbc.M802787200. Aug 4 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MS, Roche TE. Molecular biology and biochemistry of pyruvate dehydrogenase complexes. FASEB J. 1990;4(14):3224–33. doi: 10.1096/fasebj.4.14.2227213. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16(6):1078–89. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21(3):521–9. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Pritchard MT, McMullen MR, Stavitsky AB, Cohen JI, Lin F, Medof ME, Nagy LE. Differential contributions of C3, C5, and decay-accelerating factor to ethanol-induced fatty liver in mice. Gastroenterology. 2007;132(3):1117–26. doi: 10.1053/j.gastro.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XB, Gao B. The complement system in liver diseases. Cell Mol Immunol. 2006;3(5):333–40. [PubMed] [Google Scholar]
- Rigamonti C, Mottaran E, Reale E, Rolla R, Cipriani V, Capelli F, Boldorini R, Vidali M, Sartori M, Albano E. Moderate alcohol consumption increases oxidative stress in patients with chronic hepatitis C. Hepatology. 2003;38(1):42–9. doi: 10.1053/jhep.2003.50275. [DOI] [PubMed] [Google Scholar]
- Roberts BJ, Shoaf SE, Jeong KS, Song BJ. Induction of CYP2E1 in liver, kidney, brain and intestine during chronic ethanol administration and withdrawal: evidence that CYP2E1 possesses a rapid phase half-life of 6 hours or less. Biochem Biophys Res Commun. 1994;205(2):1064–71. doi: 10.1006/bbrc.1994.2774. [DOI] [PubMed] [Google Scholar]
- Roberts BJ, Song BJ, Soh Y, Park SS, Shoaf SE. Ethanol induces CYP2E1 by protein stabilization. Role of ubiquitin conjugation in the rapid degradation of CYP2E1. J Biol Chem. 1995;270(50):29632–5. doi: 10.1074/jbc.270.50.29632. [DOI] [PubMed] [Google Scholar]
- Robin MA, Sauvage I, Grandperret T, Descatoire V, Pessayre D, Fromenty B. Ethanol increases mitochondrial cytochrome P450 2E1 in mouse liver and rat hepatocytes. FEBS Lett. 2005;579(30):6895–902. doi: 10.1016/j.febslet.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Roden M. Mechanisms of disease: hepatic steatosis in type 2 diabetes-pathogenesis and clinical relevance. Nat Clin Pract Endocrinol Metab. 2006;2(6):335–48. doi: 10.1038/ncpendmet0190. [DOI] [PubMed] [Google Scholar]
- Rogers CQ, Ajmo JM, You M. Adiponectin and alcoholic fatty liver disease. IUBMB Life. 2008 doi: 10.1002/iub.124. Aug 15 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Hakkak R, Korourian S, Albano E, Yoon S, Ingelman-Sundberg M, Lindros KO, Badger TM. Alcoholic liver disease in rats fed ethanol as part of oral or intragastric low-carbohydrate liquid diets. Exp Biol Med (Maywood) 2004;229(4):351–60. doi: 10.1177/153537020422900410. [DOI] [PubMed] [Google Scholar]
- Ryle PR, Chakraborty J, Thomson AD. The effect of methylene blue on the hepatocellular redox state and liver lipid content during chronic ethanol feeding in the rat. Biochem J. 1985;232(3):877–82. doi: 10.1042/bj2320877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaspuro MP, Shaw S, Jayatilleke E, Ross WA, Lieber CS. Attenuation of the ethanol-induced hepatic redox change after chronic alcohol consumption in baboons: metabolic consequences in vivo and in vitro. Hepatology. 1981;1(1):33–8. doi: 10.1002/hep.1840010106. [DOI] [PubMed] [Google Scholar]
- Sampey BP, Carbone DL, Doorn JA, Drechsel DA, Petersen DR. 4-Hydroxy-2-nonenal adduction of extracellular signal-regulated kinase (Erk) and the inhibition of hepatocyte Erk-Est-like protein-1-activating protein-1 signal transduction. Mol Pharmacol. 2007a;71(3):871–83. doi: 10.1124/mol.106.029686. [DOI] [PubMed] [Google Scholar]
- Sampey BP, Korourian S, Ronis MJ, Badger TM, Petersen DR. Immunohistochemical characterization of hepatic malondialdehyde and 4-hydroxynonenal modified proteins during early stages of ethanol-induced liver injury. Alcohol Clin Exp Res. 2003;27(6):1015–22. doi: 10.1097/01.ALC.0000071928.16732.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampey BP, Stewart BJ, Petersen DR. Ethanol-induced modulation of hepatocellular extracellular signal-regulated kinase-1/2 activity via 4-hydroxynonenal. J Biol Chem. 2007b;282(3):1925–37. doi: 10.1074/jbc.M610602200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43(1):163–72. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- Schattenberg JM, Wang Y, Singh R, Rigoli RM, Czaja MJ. Hepatocyte CYP2E1 overexpression and steatohepatitis lead to impaired hepatic insulin signaling. J Biol Chem. 2005;280(11):9887–94. doi: 10.1074/jbc.M410310200. [DOI] [PubMed] [Google Scholar]
- Seeff LB, Cuccherini BA, Zimmerman HJ, Adler E, Benjamin SB. Acetaminophen hepatotoxicity in alcoholics. A therapeutic misadventure. Ann Int Med. 1986;104(3):399–404. doi: 10.7326/0003-4819-104-3-399. [DOI] [PubMed] [Google Scholar]
- Shacka JJ, Sahawneh MA, Gonzalez JD, Ye YZ, D'Alessandro TL, Estévez AG. Two distinct signaling pathways regulate peroxynitrite-induced apoptosis in PC12 cells. Cell Death Differ. 2006;13(9):1506–14. doi: 10.1038/sj.cdd.4401831. [DOI] [PubMed] [Google Scholar]
- Shapiro L, Scherer PE. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr Biol. 1998;8(6):335–8. doi: 10.1016/s0960-9822(98)70133-2. [DOI] [PubMed] [Google Scholar]
- Shelmet JJ, Reichard GA, Skutches CL, Hoeldtke RD, Owen OE, Boden G. Ethanol causes acute inhibition of carbohydrate, fat, and protein oxidation and insulin resistance. J Clin Invest. 1988;81(4):1137–45. doi: 10.1172/JCI113428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheron N, Bird G, Goka J, Alexander G, Williams R. Elevated plasma interleukin-6 and increased severity and mortality in alcoholic hepatitis. Clin Exp Immunol. 1991;84(3):449–53. [PMC free article] [PubMed] [Google Scholar]
- Shimano H, Horton JD, Hammer RE, Shimomura I, Brown MS, Goldstein JL. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J Clin Invest. 1996;98(7):1575–84. doi: 10.1172/JCI118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimano H, Horton JD, Shimomura I, Hammer RE, Brown MS, Goldstein JL. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Invest. 1997;99(5):846–54. doi: 10.1172/JCI119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JS, Lee JJ, Yang JW, Kim CW. Ethanol decreases basal insulin secretion from HIT-T15 cells. Life Sci. 2002;70(17):1989–97. doi: 10.1016/s0024-3205(02)01484-4. [DOI] [PubMed] [Google Scholar]
- Shklyaev S, Aslanidi G, Tennant M, Prima V, Kohlbrenner E, Kroutov V, Campbell-Thompson M, Crawford J, Shek EW, Scarpace PJ, Zolotukhin S. Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc Natl Acad Sci U S A. 2003;100(24):14217–22. doi: 10.1073/pnas.2333912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla SD, Velazquez J, French SW, Lu SC, Ticku MK, Zakhari S. Emerging role of epigenetics in the actions of alcohol. Alcohol Clin Exp Res. 2008;32(9):1525–34. doi: 10.1111/j.1530-0277.2008.00729.x. [DOI] [PubMed] [Google Scholar]
- Sims HF, Brackett JC, Powell CK, Treem WR, Hale DE, Bennett MJ, Gibson B, Shapiro S, Strauss AW. The molecular basis of pediatric long chain 3-hydroxyacyl-CoA dehydrogenase deficiency associated with maternal acute fatty liver of pregnancy. Proc Natl Acad Sci U S A. 1995;92(3):841–5. doi: 10.1073/pnas.92.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Patel DG, Snyder AK, Pullen GL. Ethanol influence on insulin secretion from isolated rat islets. Experientia. 1986;42(1):58–60. doi: 10.1007/BF01975895. [DOI] [PubMed] [Google Scholar]
- Solinas G, Naugler W, Galimi F, Lee MS, Karin M. Saturated fatty acids inhibit induction of insulin gene transcription by JNK-mediated phosphorylation of insulin-receptor substrates. Proc Natl Acad Sci U S A. 2006;103(44):16454–9. doi: 10.1073/pnas.0607626103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh Y, Jeong KS, Lee IJ, Bae MA, Kim YC, Song BJ. Selective activation of the c-Jun N-terminal protein kinase pathway during 4-hydroxynonenal-induced apoptosis of PC12 cells. Mol Pharmacol. 2000;58(3):535–41. doi: 10.1124/mol.58.3.535. [DOI] [PubMed] [Google Scholar]
- Sozio M, Crabb DW. Alcohol and lipid metabolism. Am J Physiol Endocrinol Metab. 2008;295(1):E10–6. doi: 10.1152/ajpendo.00011.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BJ, Koop DR, Ingelman-Sundberg M, Nanji A, Cederbaum AI. Ethanol-inducible cytochrome P450 (CYP2E1): biochemistry, molecular biology and clinical relevance: 1996 update. Alcohol Clin Exp Res. 1996;20(Suppl):138A–146A. doi: 10.1111/j.1530-0277.1996.tb01764.x. [DOI] [PubMed] [Google Scholar]
- Song BJ, Moon KH, Olsson N, Salem N., Jr Prevention of alcoholic fatty liver and mitochondrial dysfunction in the rat by long-chain polyunsaturated fatty acids. J Hepatol. 2008;49:262–73. doi: 10.1016/j.jhep.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BJ, Soh Y, Bae M, Pie J, Wan J, Jeong K. Apoptosis of PC12 cells by 4-hydroxy-2-nonenal is mediated through selective activation of the c-Jun N-terminal protein kinase pathway. Chem Biol Interact. 2001;130-132(1-3):943–54. doi: 10.1016/s0009-2797(00)00247-7. [DOI] [PubMed] [Google Scholar]
- Sotaniemi EA, Keinänen K, Lahtela JT, Arranto AJ, Kairaluoma M. Carbohydrate intolerance associated with reduced hepatic glucose phosphorylating and releasing enzyme activities and peripheral insulin resistance in alcoholics with liver cirrhosis. J Hepatol. 1985;1(3):277–90. doi: 10.1016/s0168-8278(85)80055-6. [DOI] [PubMed] [Google Scholar]
- Stewart SF, Vidali M, Day CP, Albano E, Jones DE. Oxidative stress as a trigger for cellular immune responses in patients with alcoholic liver disease. Hepatology. 2004;39(1):197–203. doi: 10.1002/hep.20021. [DOI] [PubMed] [Google Scholar]
- Suh SK, Hood BL, Kim BJ, Conrads TP, Veenstra TD, Song BJ. Identification of oxidized mitochondrial proteins in alcohol-exposed human hepatoma cells and mouse liver. Proteomics. 2004;4(11):3401–12. doi: 10.1002/pmic.200400971. [DOI] [PubMed] [Google Scholar]
- Sun Z, Klein AS, Radaeva S, Hong F, El-Assal O, Pan HN, Jaruga B, Batkai S, Hoshino S, Tian Z, Kunos G, Diehl AM, Gao B. In vitro interleukin-6 treatment prevents mortality associated with fatty liver transplants in rats. Gastroenterology. 2003;125(1):202–15. doi: 10.1016/s0016-5085(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Szabo G, Aloman C, Polyak SJ, Weinman SA, Wands J, Zakhari S. Hepatitis C infection and alcohol use: A dangerous mix for the liver and antiviral immunity. Alcohol Clin Exp Res. 2006;30(4):709–19. doi: 10.1111/j.1530-0277.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- Tomita K, Azuma T, Kitamura N, Nishida J, Tamiya G, Oka A, Inokuchi S, Nishimura T, Suematsu M, Ishii H. Pioglitazone prevents alcohol-induced fatty liver in rats through up-regulation of c-Met. Gastroenterology. 2004;126(3):873–85. doi: 10.1053/j.gastro.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Yu S, Rao S, Reddy JK. Peroxisome proliferator-activated receptors, fatty acid oxidation, steatohepatitis and hepatocarcinogenesis. Curr Mol Med. 2003;3(6):561–72. doi: 10.2174/1566524033479537. [DOI] [PubMed] [Google Scholar]
- Valerio LG, Jr, Parks T, Petersen DR. Alcohol mediates increases in hepatic and serum nonheme iron stores in a rat model for alcohol-induced liver injury. Alcohol Clin Exp Res. 1996;20(8):1352–61. doi: 10.1111/j.1530-0277.1996.tb01134.x. [DOI] [PubMed] [Google Scholar]
- Vankoningsloo S, Piens M, Lecocq C, Gilson A, De Pauw A, Renard P, Demazy C, Houbion A, Raes M, Arnould T. Mitochondrial dysfunction induces triglyceride accumulation in 3T3-L1 cells: role of fatty acid beta-oxidation and glucose. J Lipid Res. 2005;46(6):1133–49. doi: 10.1194/jlr.M400464-JLR200. [DOI] [PubMed] [Google Scholar]
- Venkatraman A, Landar A, Davis AJ, Chamlee L, Sanderson T, Kim H, Page G, Pompilius M, Ballinger S, Darley-Usmar V, Bailey SM. Modification of the mitochondrial proteome in response to the stress of ethanol-dependent hepatotoxicity. J Biol Chem. 2004a;279(21):22092–101. doi: 10.1074/jbc.M402245200. [DOI] [PubMed] [Google Scholar]
- Venkatraman A, Landar A, Davis AJ, Ulasova E, Page G, Murphy MP, Darley-Usmar V, Bailey SM. Oxidative modification of hepatic mitochondria protein thiols: effect of chronic alcohol consumption. Am J Physiol Gastrointest Liver Physiol. 2004b;286(4):G521–7. doi: 10.1152/ajpgi.00399.2003. [DOI] [PubMed] [Google Scholar]
- Vidali M, Stewart SF, Rolla R, Daly AK, Chen Y, Mottaran E, Jones DE, Leathart JB, Day CP, Albano E. Genetic and epigenetic factors in autoimmune reactions toward cytochrome P4502E1 in alcoholic liver disease. Hepatology. 2003;37(2):410–9. doi: 10.1053/jhep.2003.50049. [DOI] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfred de Alwis NM, Day CP. Genetics of alcoholic liver disease and nonalcoholic fatty liver disease. Semin Liver Dis. 2007;27(1):44–54. doi: 10.1055/s-2006-960170. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999;277(1 Pt 1):E1–10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- Woodcroft KJ, Hafner MS, Novak RF. Insulin signaling in the transcriptional and posttranscriptional regulation of CYP2E1 expression. Hepatology. 2002;35(2):263–73. doi: 10.1053/jhep.2002.30691. [DOI] [PubMed] [Google Scholar]
- Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112(1):91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahagi N, Shimano H, Hasty AH, Matsuzaka T, Ide T, Yoshikawa T, Amemiya-Kudo M, Tomita S, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, Osuga J, Harada K, Gotoda T, Nagai R, Ishibashi S, Yamada N. Absence of sterol regulatory element-binding protein-1 (SREBP-1) ameliorates fatty livers but not obesity or insulin resistance in Lep(ob)/Lep(ob) mice. J Biol Chem. 2002;277(22):19353–7. doi: 10.1074/jbc.M201584200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, Monia BP, Li YX, Diehl AM. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45(6):1366–74. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278(4):2461–8. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117(4):942–52. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- You M, Cao Q, Liang X, Ajmo JM, Ness GC. Mammalian sirtuin 1 is involved in the protective action of dietary saturated fat against alcoholic fatty liver in mice. J Nutr. 2008a;138(3):497–501. doi: 10.1093/jn/138.3.497. [DOI] [PubMed] [Google Scholar]
- You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42:568–577. doi: 10.1002/hep.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1–G6. doi: 10.1152/ajpgi.00056.2004. [DOI] [PubMed] [Google Scholar]
- You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP) J Biol Chem. 2002;277(32):29342–7. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- You M, Liang X, Ajmo JM, Ness GC. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol. 2008b;294(4):G892–8. doi: 10.1152/ajpgi.00575.2007. [DOI] [PubMed] [Google Scholar]
- You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127(6):1798–808. doi: 10.1053/j.gastro.2004.09.049. [DOI] [PubMed] [Google Scholar]
- Zakhari S, Li TK. Determinants of alcohol use and abuse: Impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46(6):2032–9. doi: 10.1002/hep.22010. [DOI] [PubMed] [Google Scholar]
- Zhao P, Kalhorn TF, Slattery JT. Selective mitochondrial glutathione depletion by ethanol enhances acetaminophen toxicity in rat liver. Hepatology. 2002;36(2):326–35. doi: 10.1053/jhep.2002.34943. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]


