Abstract
Previous studies have demonstrated that co-administration of rat adrenomedullin (AM) and human AM binding protein-1 (AMBP-1) has various beneficial effects following adverse circulatory conditions. In order to reduce rat proteins to elicit possible immune responses in humans, we determined the effect of human AM combined with human AMBP-1 after intestinal ischemia and reperfusion (I/R). Intestinal ischemia was induced in the rat by occluding the superior mesenteric artery for 90 min. At 60 min after the beginning of reperfusion, human AM/AMBP-1 at 3 different dosages was administered intravenously over 30 min. At 240 min after the treatment, blood and tissue samples were harvested and measured for pro-inflammatory cytokines (i.e., TNF-α and IL-6), myeloperoxidase activities in the gut and lungs, and cleaved caspase-3 expression in the lungs, as well as serum levels of hepatic enzymes and lactate. In additional groups of animals, a 10-day survival study was conducted. Results showed that administration of human AM/AMBP-1 reduced pro-inflammatory cytokines, attenuated organ injury, and improved the survival rate in a seemingly dose-response fashion. Co-administration of the highest dose of human AM/AMBP-1 in this study had the optimal therapeutic effect in the rat model of intestinal I/R.
Keywords: Human adrenomedullin, human adrenomedullin binding protein-1, intestinal ischemia and reperfusion
1. Introduction
Acute mesenteric ischemia is a common emergency resulting from either a focal interruption of mesenteric blood supply or as a consequence of a more global hypoperfusion. Although it is increasingly recognized in patients leading to earlier diagnosis, the mortality of the condition has changed little in the last 25 years, ranging as high as 60-80% [1-3]. The disease process is usually recognized in two parts with an obstruction of blood flow (whether embolic or thrombotic, arterial or venous) causing ischemic injury to the bowel and a reperfusion injury after restoration of blood flow. The direct injury to the bowel depends both on the degree or ischemia and its duration and it ranges from minimal mucosal injury to transmural necrosis and perforation [4]. Curiously, reperfusion injury can produce even greater local damage as well as amplify the effects of the ischemia by making a focal injury a global insult [5]. Multiple explanations for this amplification have included translocation of bacteria and bacterial products [6,7], production of cytokines [8], and activation of circulation neutrophils and macrophages [3]. As a result, numerous agents and interventions have been studied to reduce gut ischemia and reperfusion (I/R)-induced organ injury and its mortality, but none has so far been entirely successful [9]. It remains important, therefore, to find an effective therapeutic agent which improves both local perfusion, ameliorates ischemic organ injury and blunts the consequent associated circulatory collapse and distant organ injury.
The polypeptide adrenomedullin (AM) has potent and long-lasting vasoactive effects which made is a plausible intervention in the management of I/R injury based upon earlier experience with vasodilatation in the clinical management of focal mesenteric ischemia [10]. AM was discovered and isolated from human pheochromocytoma extracts in 1993 [11] and is was widely distributed in the endocrine and neuroendocrine system [12,13], suggesting that AM plays an important role in the control of systemic and local circulation. AM also effects humoral secretion [14]. Elevated levels of endogenous AM have been demonstrated in various diseases such as sepsis, hypertension, renal failure, heart failure, and shock [15,16].
A 120-140 kD AM-binding protein-1 (AMBP-1) was first reported in 1999 to be identical to human complement factor H [17,18]. Previous studies in this laboratory have demonstrated that human AMBP-1 synergistically enhanced rat AM-induced vascular relaxation and that the administration of rat AM and human AMBP-1 in combination prevented the transition from the hyperdynamic to the hypodynamic state of sepsis [19-22]. We have also demonstrated that rat AM and human AMBP-1 in combination down-regulated inflammatory cytokines, attenuated tissue injury, decreased gut permeability, and prevented acute lung injury after intestinal I/R [8,23,24]. A number of obstacles to the development of AM/AMBP-1 as a therapeutic agent are obvious. One is the potential immunogenicity of rat proteins in humans. Human AM is 52-amino acids in length, while rat AM is 50-amino acids in length. Six amino acid differences are seen between the human and rat protein [10]. A second problem is that the therapeutic benefit of the AM/AMBP-1 combination may be dependent on the rat AM and not be demonstrable with the human protein. The aim of this study, therefore, was to investigate the effects of human AM combined with human AMBP-1 on intestinal I/R-induced inflammation, organ injury, and mortality in rats and, if beneficial, to determine the optimal dosage of human AM/AMBP-1.
2. Materials and methods
2.1. Experimental animals
Male Sprague-Dawley rats (275-385 g), purchased from Charles River Laboratories (Wilmington, MA), were used in this study. All procedures performed were described in protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the Feinstein Institute for Medical Research. Animal experimentation was carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources). The rats were housed in a temperature-controlled room on a 12 h light/dark cycle and fed a standard Purina rat chow diet. The rats were fasted with free access to water the night before laparotomy. Operative procedures were performed under general anesthesia with isoflurane and a steady state of sedation was maintained with subsequent intravenous injections of sodium pentobarbital (∼30 mg/kg BW). The procedures were performed using sterile surgical technique.
2.2. Experimental protocol
Experiment animals were divided into one of five groups with six animals per group: Sham (laparotomy without mesenteric occlusion), and mesenteric occlusion and treatment with either Vehicle, 1X human AM/AMBP-1, 2X human AM/AMBP-1, and 4X human AM/AMBP-1. Detailed information regarding groups is provided in Table 1. A length of PE-50 tubing was introduced into the femoral vein after carefully separating it from the femoral nerve to allow administration of drug and withdrawal of blood. The animals were not heparinized. A midline laparotomy was performed and the superior mesenteric artery (SMA) was dissected circumferentially and isolated. Ischemia was produced by complete occlusion of the SMA for 90 min after which the SMA clamp was removed. No additional fluids were administered. At 60 min post-reperfusion, human AM and human AMBP-1 in combination, or human serum albumin (Vehicle) was infused intravenously over a period of 30 min. The incision was then closed and rats were allowed to awaken and were observed for additional 240 min. At the end of experiment, blood and tissue samples (small intestines and lungs) were harvested and stored at -80°C until assayed.
Table 1. Animal groups.
| Intestinal ischemia and reperfusion (I/R) | |||||
|---|---|---|---|---|---|
| Sham | Vehicle | 1X | 2X | 4X | |
| Human Albumin (μg/kg BW) | – | 208 | – | – | – |
| Human AM (μg/kg BW) | – | – | 12 | 24 | 48 |
| Human AMBP-1 (μg/kg BW) | – | – | 40 | 80 | 160 |
2.3. Determination of tumor necrosis factor-a and interleukins-6
The lung and small bowel were excised and lysated in an ice-cold lysis buffer (1% Triton X-100 in TBS with protease inhibitors, pH, 7.5) and sonicated 30 s on ice. The tissue lysates were centrifuged at 12,000 rpm for 10 min and protein concentration was measured by using Bio-Rad DC Protein Assay Kit (Bio-Rad, Hercules, CA). Tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in gut and lung ware quantified with the use of specific enzyme linked immunosorbent assay (ELISA) kits according to the instructions provided by manufacturer (BD Biosciences Pharmingen, San Diego, CA).
2.4. Determination of serum levels of transaminases, lactate, and creatinine
Serum concentrations of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and lactate were determined by using assay kits according to the manufacturer's instructions (Pointe Scientific, Canton, MI).
2.5. Cleaved caspase-3 measurement
The lung and gut tissues were excised and lysated in an ice-cold lysis buffer (1% Triton X-100 in TBS with protease inhibitors, pH, 7.5) and sonicated 30 s on ice. The tissue lysates ware centrifuged at 12,000 rpm for 10 min and protein concentration was measured by using Bio-Rad DC Protein Assay Kit (Bio-Rad, Hercules, CA). 50 μg of total proteins were loaded onto NuPAGE Novex 10% Bis-Tris Gel (Invitrogen, Carlsbad, CA). Proteins were transferred to a 0.45 μm nitrocellulose membrane (Invitrogen, Carlsbad, CA) blocked with 5% nonfat dry milk for 2 h at 37°C, and incubated with rabbit anti-cleaved caspase-3 antibody (Cell Signaling Technology, Beverly, MA) or β-actin (Sigma, St. Louis, Mo). Bands were visualized with a horseradish peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG secondary antibody secondary antibody followed by ECL using the SuperSignal kit (GE healthcare, Buckinghamshire, UK).
2.6. Measurement of myeloperoxidase (MPO) activity
MPO activity in homogenates of whole gut and lung was determined as described by Rana et al [25] and Koike et al [26]. Briefly, samples of 100 mg were suspended in 1 ml of 0.5% hexadecyltrimethylammonium bromide in 50 mmol/L phosphate buffer (pH 6.0) and sonicated 90 s on ice. Homogenates were cleared by centrifuging at 12,000 rpm at 4°C, and the protein concentration of supernatants was determined by using Bio-Rad DC Protein Assay Kit (Bio-Rad, Hercules, CA). The reaction was carried out in a 96-well plate by adding 290 μl of 50 mmol/L phosphate buffer with 3 μl substrate solution (containing 20 mg/ml o-dianisidine hydrochloride), and 3 μl H2O2 (20 mmol/L). Sample (10 μl) was added to each well to start the reaction. Plates were read spectrophotometrically at 460 nm for 3 min on CERES UV 900C Microplate reader (Bio-Tek Inst Inc, Winooski, VT). MPO activity (1 unit defined as change in absorbance of 1 per min) was expressed as units per gram of tissue.
2.7. Survival study
Survival was assessed in an additional group of animals. The animals were divided into one of 4 groups, vehicle (n=16), 1X human AM/AMBP-1 (n=17), 2X human AM/AMBP-1 (n=16) and 3X human AM/AMBP-1 (n=18). Human albumin or human AM/AMBP-1 was administered intravenously over 30 minutes to animals who had been exposed to 90 minutes of meseneteric ischemia and 60 minutes of reperfusion as described above. After treatment, the incision was closed and the rats alowed food and water ad libitum. All surviving animals were sacrificed on day 10.
2.8. Statistical analysis
All data are expressed as means ± SE and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls method. The survival rate was estimated by Kaplan-Meier method and compared by the log-rank test. Differences in values were considered significant if p<0.05.
3. Results
3.1. Effects of human AM/AMBP-1 on serum levels of AST, ALT, and lactate after intestinal I/R
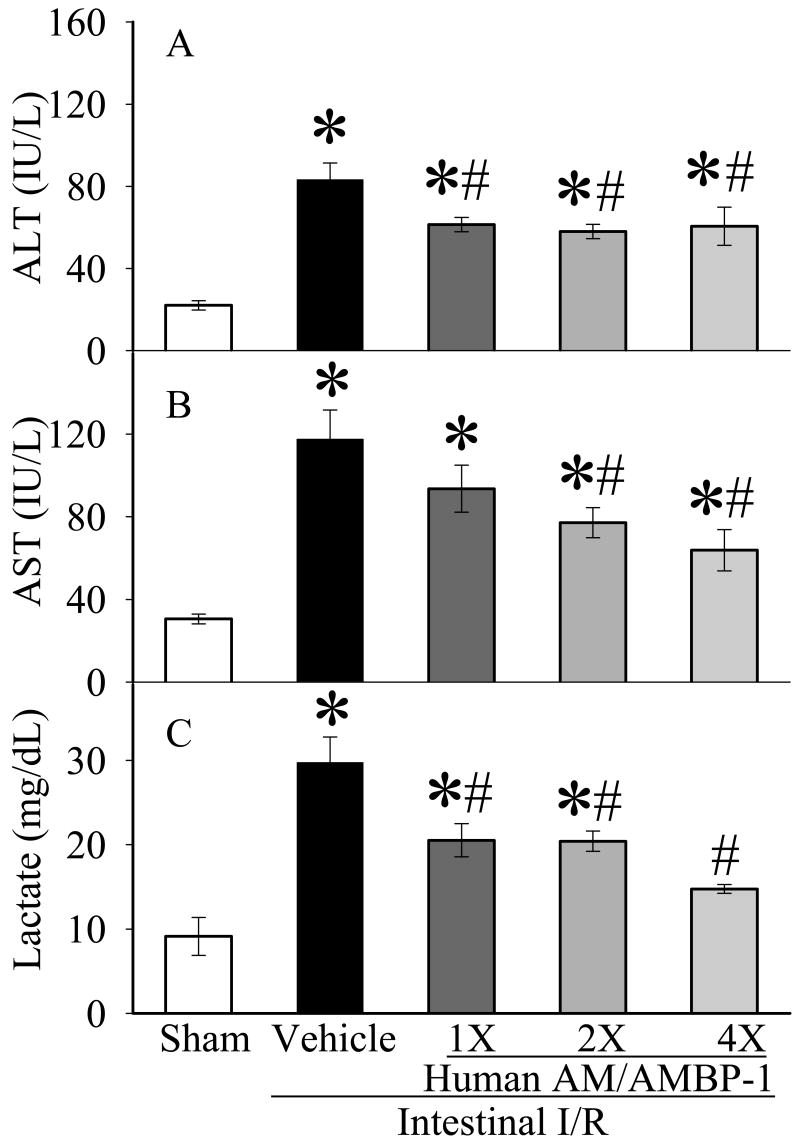
Serum levels of ALT and AST were measured as a surrogate for hepatic injury. As seen in Figures 1A-B, serum ALT and AST activity increased by 273% and 295% after intestinal I/R (p<0.05). Human AM/AMBP-1 administration significantly attenuated the ALT and AST levels. A dose-dependent effect of human AM/AMBP-1 on serum AST levels is seen in Fig. 1B. Serum lactate increased by 225% after the completion of intestinal I/R in the vehicle group (p<0.05, Fig. 1C). Administration of human AM/AMBP-1 resulted in a 30-50% decrease in serum lactate levels, again in a nearly dose-dependent (p<0.05, Fig. 3C).
Figure 1.
Alterations in serum levels of ALT (A), AST (B), and lactate (C) in sham-operated animals (Sham), and intestinal I/R animals treated with human albumin (Vehicle: 208 μg/kg BW) or different doses of human AM/AMBP-1 at 60 min post-reperfusion following 90 min ischemia (1X: 12/40 μg/kg BW; 2X: 24/80 μg/kg BW, 4X: 48/160 μg/kg BW). Data are presented as means ± SE (n=6) and compared by one-way ANOVA and student-Newman-Keuls method: *p<0.05 versus Sham group, # p<0.05 versus Vehicle group.
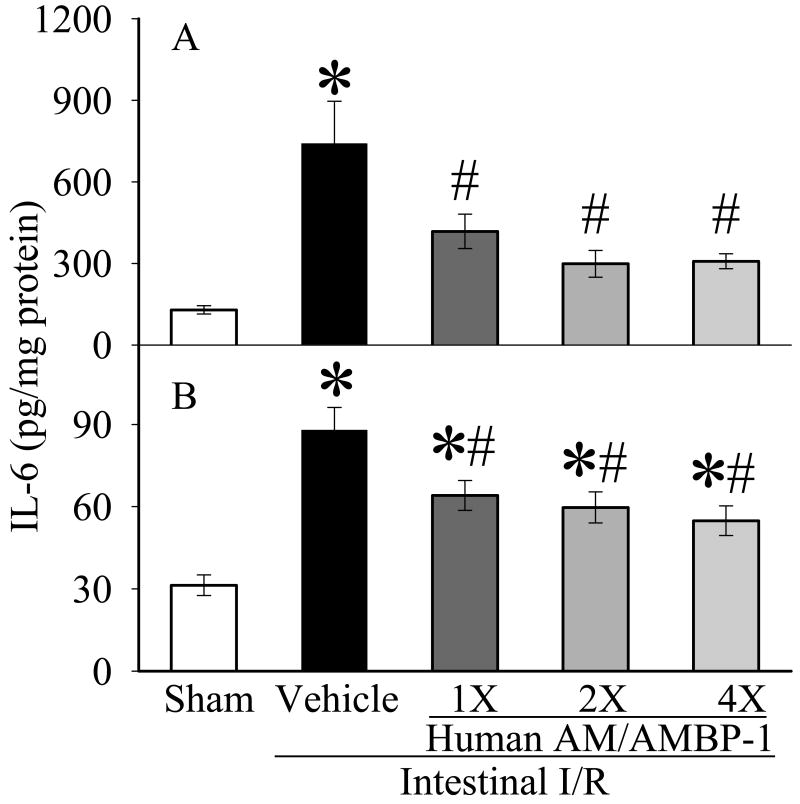
Figure 3.
Alterations of IL-6 in the small intestine (A) and lungs (B) in sham-operated animals (Sham), and intestinal I/R animals treated with human albumin (Vehicle 208 μg/kg BW) or different doses of human AM/AMBP-1 after 90 min ischemia and 60 min reperfusion (1X: 12/40 μg/kg BW; 2X: 24/80 μg/kg BW, 4×: 48/160 μg/kg BW). Data are presented as means ± SE (n=6) and compared by one-way ANOVA and student-Newman-Keuls method: *p<0.05 versus Sham group, # p<0.05 versus Vehicle group.
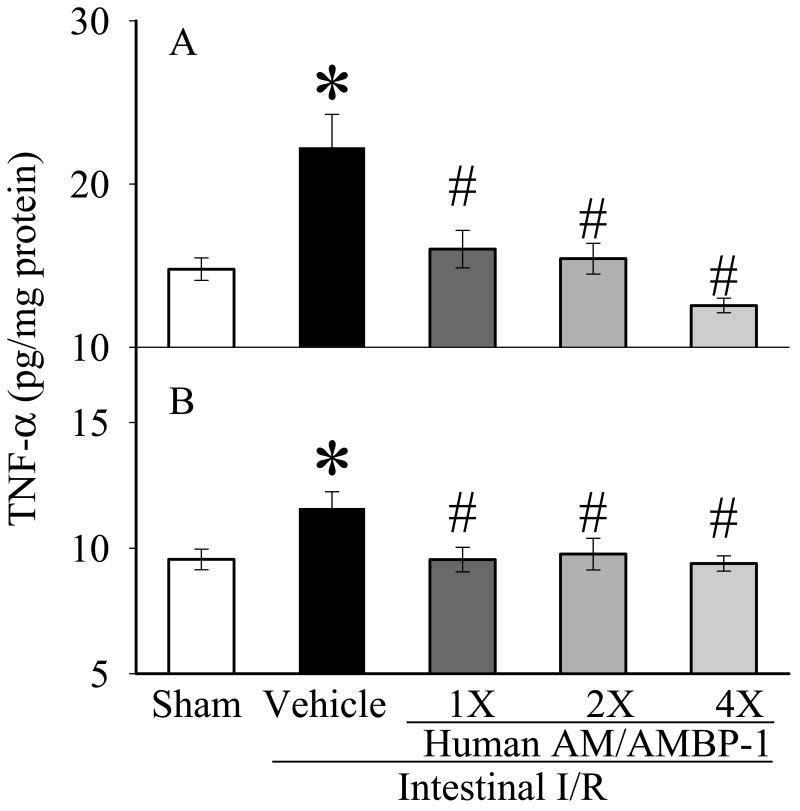
3.2. Effects of human AM/AMBP-1 on gut and lung levels of pro-inflammatory cytokines after intestinal I/R
TNF-α and IL-6 levels were increased significantly in the gut (Figs. 2A, 3A) and lungs (Figs. 2B, 3B) after intestinal I/R as compared to sham-operated animals (p<0.05). Gut and lung TNF-α and IL-6 levels decreased significantly after administration of AM/AMBP-1 across all dosages (p<0.05). The decreases in intestinal levels of TNF-α and IL-6 (Figs. 2A and 3A) appear to be dose-dependent.
Figure 2.
Alterations of TNF-α in the small intestine (A) and lungs (B) in sham-operated animals (Sham), and intestinal I/R animals treated with human albumin (Vehicle: 208 μg/kg BW) or different doses of human AM/AMBP-1 after 90 min ischemia and 60 min reperfusion (1X: 12/40 μg/kg BW; 2X: 24/80 μg/kg BW, 4X: 48/160 μg/kg BW). Data are presented as means ± SE (n=6) and compared by one-way ANOVA and student-Newman-Keuls method: *p<0.05 versus Sham group, # p<0.05 versus Vehicle group.
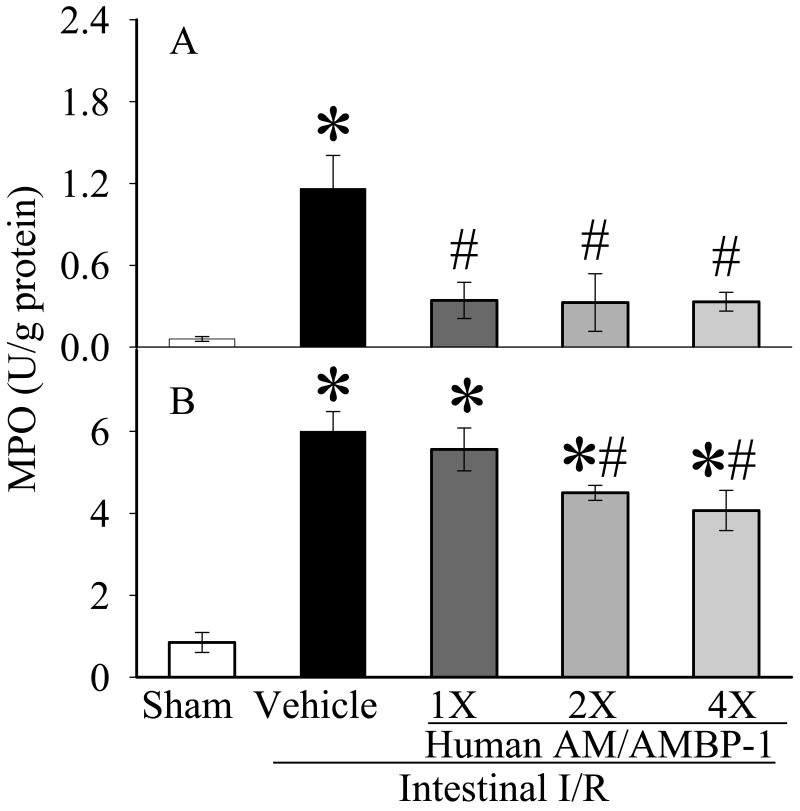
3.3. Effects of human AM/AMBP-1 on gut and lung MPO after intestinal I/R
MPO is a marker of neutrophil infiltration. MPO activities were each increased by >600% after intestinal I/R as compared to sham-operated controls in the gut (Fig. 4A) and lungs (Fig. 4B). MPO activity was decreased by >70% (p<0.05) in the gut, and reduced by 7%, 25% and 32% (p<0.05) in the lungs after 1X, 2X, and 4X human AM/AMBP-1 treatment, respectively.
Figure 4.
Alterations of MPO in the small intestine (A) and lungs (B) in sham-operated animals (Sham), and intestinal I/R animals treated with human albumin (Vehicle 208 μg/kg BW) or different doses of human AM/AMBP-1 after 90 min ischemia and 60 min reperfusion (1X: 12/40 μg/kg BW; 2X: 24/80 μg/kg BW, 4X: 48/160 μg/kg BW). Data are presented as means ± SE (n=6) and compared by one-way ANOVA and student-Newman-Keuls method: *p<0.05 versus Sham group, # p<0.05 versus Vehicle group.
3.4. Effect of human AM/AMBP-1 treatment on lung apoptosis after intestinal I/R
As shown in Figure 5, cleaved-caspase-3, a key mediator of cell apoptosis, was significantly increased in vehicle-treated I/R group in the lungs. The treatment with human AM/AMBP-1 dramatically inhibited the caspase-3 activation.
Figure 5.
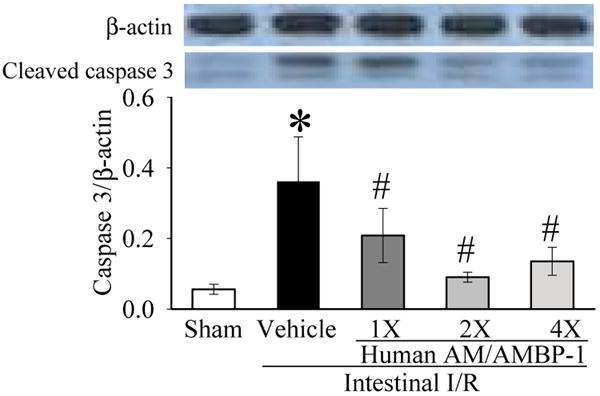
Alterations cleaved-caspase-3 protein levels in the lungs in sham-operated animals (Sham), and intestinal I/R animals treated with human albumin (Vehicle 208 μg/kg BW) or different doses of human AM/AMBP-1 after 90 min ischemia and 60 min reperfusion (1X: 12/40 μg/kg BW; 2X: 24/80 μg/kg BW, 4X: 48/160 μg/kg BW). Data are expressed as ratios of caspase-3 over β-actin and are presented as means ± SE (n=6) and compared by one-way ANOVA and student-Newman-Keuls method: *p<0.05 versus Sham group, # p<0.05 versus Vehicle group.
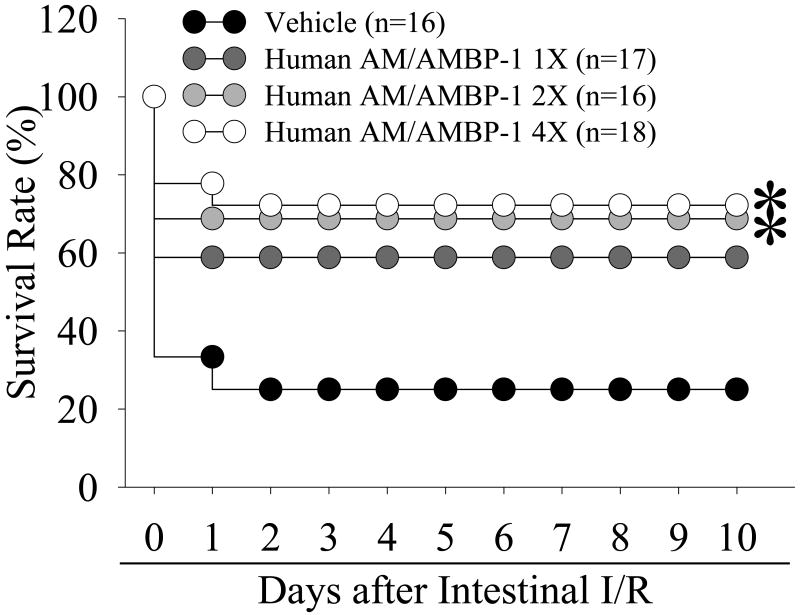
3.5. Effect of human AM/AMBP-1 on survival after intestinal I/R
Figure 6 shows that the survival rate after intestinal I/R with vehicle administration is 33% at day 1 and 25% at days 2-10. With 1X human AM/AMBP-1 treatment, the survival rate increased to 67% of day 1 and stabilized at that level thereafter. With 2X human AM/AMBP-1, the survival rate increased to 75% at days 1-10. With a 4X human AM/AMBP-1, the survival rate increased to 78% at day 1 and 72% at day 2 through 10. The survival rate is statistically significantly greater after 2X or 4X human AM/AMBP-1 as compared to the survival rate after vehicle administration (p<0.05).
Figure 6.
Alterations in the survival rate after intestinal I/R with human albumin (Vehicle: 208 μg/kg BW) or different doses (1X: 12/40 μg/kg BW; 2X: 24/80 μg/kg BW, 4X: 48/160 μg/kg BW) of human AM/AMBP-1 treatment. There are 16∼18 animals in each group. The survival rate was estimated by the Kaplan-Meier method and compared by using the log-rank test. *p<0.05 versus I/R Vehicle group.
4. Discussion
The injury of intestinal I/R is a common abdominal emergency and can result in severe tissue and organ destruction with a very high mobility and mortality rate [1,27,28]. The causes of I/R in patients are the result of occlusive disease (local) or systemic hypoperfusion (global). Focal ischemia may be the result of arterial embolization (commonly consequent to atrial fibrillation), arterial occlusion, mesenteric venous occlusion or obstruction with concomitant vascular insufficiency [29]. Occlusion and reperfusion of arteries can cause circulatory shock by releasing pro-inflammatory substances and nitrogen-derived and oxygen-derived free radicals [30,31]. I/R can also result in the loss of effective mucosal barrier, increasing intestinal permeability, allowing translocation of intestinal bacteria and bacterial products which may damage distant organs [32]. Numerous substances have been studied to reduce intestinal I/R-induced organ injury and mortality, but no successful clinical results have been reported. The problem of intestinal ischemia is further complicated by the inconsistency and unreliability of clinical signs prior to frank ischemic necrosis and bowel perforation. At this point in a patient, resection of the necrotic bowel becomes necessary, but despite this, mortality remains high presumably consequent to a septic cascade which has already been initiated and which may be amplified by the reperfusion of ischemic (but not necrotic) bowel.
Because of its potent and long-lasting vasoactive properties [11,19], AM has attracted the interest of investigators in the cardiovascular field since it was reported 15 years ago. The primary functions of AM are vasodilation, diuresis, natriuresis and inhibition of aldosterone secretion [33]. Many cells can produce AM, and endothelial cells are the main target cells of AM [34]. AM can increase the cyclic adenosine monophosphate (cAMP) of vascular smooth muscles and induce the release of nitric oxide of vascular endothelial cells [35-37]. The discovery of AM binding protein in 1999 [17] which greatly increases the vasodilatory effects of AM has expanded the role of these two substances as therapeutic agents. Direct installation of vasodilating drugs has been a part of the management of mesenteric ischemia both before and after operation for many years [38-40]. The use of heparin and other anticoagulants in general has been suggested as adjunctive therapy [41,42] to further maintain both arterial inflow and venous outflow in both focal and non-occlusive ischemia.
Our previous studies have demonstrated that rat AM combined with human AMBP-1 increased the anti-inflammatory effect of AM by down-regulating production of pro-inflammatory cytokines [43], mediating through both the cAMP-dependent pathway and proline-rich tyrosine kinase-2 (Pyk-2)-ERK1/2-dependent induction of peroxisome proliferator-activated receptor-γ (PPAR-γ) [44]. Rat AM combined with human AMBP-1, but not rat AM or human AMBP-1 alone, down-regulated inflammatory cytokines, attenuated organ injury, and improved survival after intestinal I/R in rat [8,23]. These beneficial effects of rat AM combined with human AMBP-1 lead us to explore this combination as a therapeutic agent for future clinical application. The immunogenicity, however, of therapeutic proteins or peptides is an area of concern for pharmaceutical agencies. The mRNA of human AM is 1.6 kilobases long and encodes for 185 amino acids. The precursor of the 185 amino acids is then processed to a 164-amino acid peptide, which is called proAM. The biologically active peptide is 52 amino acids in length and formed from the proAM [11]. Southern blot analyses have indicated that the human AM gene is situated in a single locus on chromosome 11 [45]. Rat AM has been cloned and differs from human AM in six positions of amino acids and is only 50 amino acids in length. Because sequence variation and the length of amino-acids are main factors of influencing immunogenicity, it is important to determine the treatment effect of human AM for decreasing the therapeutic proteins to elicit antibody responses in human. The present study proved that human AM combined with AMBP-1 down-regulated inflammatory cytokines, attenuated tissue injury, and improved the survival rate on intestinal I/R in rats. Human AM/AMBP-1 in these experiments, then, had the same therapeutic effect as rat AM.
It was also of interest to examine what dose would be optimal to reduce inflammation, prevent tissue injury and increase survival rates after intestinal I/R. To this end, we used three doses, 12/40 (1X), 24/80 (2X) and 48/160 μg/kg (4X) of human AM/AMBP-1 to treat rats after intestinal I/R. A 1X dose of human AM/AMBP-1, based on our previous studies using rat AM and human AMBP-1, already down-regulated pro-inflammatory cytokines, reduced the activity of MPO in the gut and lungs, attenuated the tissue injury, decreased lung apoptosis, and increased the survival rates significantly. A 4X dose of human AM/AMBP-1 decreased dramatically in all experiences, and the decrease was greater than that caused by 1X and 2X dosages. Most of the results showed the dose-dependent effects. It has been concluded that 12 μg/kg of human AM and 40 μg/kg of human AMBP-1 already has the therapeutic effect, but the optimal therapeutic effect is in high dose of 48 μg/kg of human AM and 160 μg/kg of human AMBP-1 on intestinal I/R in rats.
As for the injury of I/R, studies have demonstrated that reperfusion of the previously ischemic organ can result in severe tissue destruction. The problem with reconstitution of blood supply is that this may be accompanied by significant local and systemic inflammatory injury effectively limiting the potential benefits of blood flow restoration. In the present experiment, we extended the reperfusion time to 330 min to cause a great increase of pro-inflammatory cytokines of the lungs, serum lactate, ALT, AST and mortality, compared to the experimental protocol conducted in previous papers [8,23], indicating that the damage experience in our present experiment was more severe. On the other hand, after treatment with human AM/AMBP-1, percent decreases of serum ALT, AST, lactate and MPO in lung and gut was the same in the short and long perfusion time, but percent increase in survival rate after human AM/AMBP-1 application was much greater in our experiment than in previous papers. Previous study [8] showed a 54% increase only in the survival rate while the present research showed 135%, 175%, and 189% increases (in a relative term). Since human AM/AMBP-1 attenuated organ damage as demonstrated by decreased organ injury markers in the circulation and improved survival, it is ascertained that organ function was improved. Nevertheless, we will determine whether human AM/AMBP-1 will improve gut and lung function in our future studies.
I/R injury can diminish the barrier function of the gut, and promote an increase in the leakage of molecules or bacterial translocation to result in harm distant tissues and organs. Intestinal I/R can induce multiple organ injuries, but the lungs seem particularly susceptible to insult [27,46]. Besides increased pro-inflammatory cytokines-TNF-α in the lungs, we found that the expression of cleaved caspase-3 up-regulated significantly. Studies have demonstrated that TNF-α is a strong inducer of apoptosis and may act through apoptosis signal transduction pathways to influence pulmonary cellular activity to lead to lung cells apoptosis [47]. It is important that human AM/AMBP-1 inhibits significantly caspase-3 activation and prevents lung apoptosis when compared to vehicle controls. We did not observed significant upregulation of cleaved caspase-3 in the gut (data not shown). The lack of an increase in intestinal cleaved caspase-3 may be due to the possibility that sever I/R injury results in necrosis, but not apoptosis.
It is important, however, to consider the limitations of this animal model in contrast to the human clinical situation. These caveats are applicable to other agents such as erythropoietin [48], which have found effective in the management of gut I/R injury in rats. Standard human therapy today includes fluid resuscitation to increase filling pressure and therefore cardiac output, correction of acidosis and electrolyte disturbances consequent to changes in pH, aggressive support of pulmonary function with increased oxygen concentrations to improve tissue oxygen availability even as oxygen diffusion is decreased consequent to pulmonary inflammation, and the use of broad spectrum antibiotics to decrease circulating viable bacteria (whether demonstrated in blood culture or not). Additionally, as described above, some clinical centers will add aggressive vasodilatation, pressor therapy to maintain perfusion, and glucocorticoids. Any clinical intervention based upon the data presented here would have to be in addition to these “standard” therapies.
In conclusion, intestinal and pulmonary levels of pro-inflammatory cytokines (TNF-α and IL-6) and MPO activities, apoptotic cells in lung and serum levels of ALT, AST and lactate were significantly elevated after vehicle administration. The different doses of human AM/AMBP-1 for treatment to intestinal I/R down-regulated pro-inflammatory cytokines TNF-α and IL-6, attenuated organ injury, decreased lung cell apoptosis, and improved survival rate. It appears that the highest dose of human AM/AMBP-1 used in this study is the optimal therapeutic dose for intestinal I/R injury.
Acknowledgments
This study was supported in part by National Institutes of Health grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koike K, Moore FA, Moore EE, Read RA, Carl VS, Banerjee A. Gut ischemia mediates lung injury by a xanthine oxidase-dependent neutrophil mechanism. J Surg Res. 1993;54:469–73. doi: 10.1006/jsre.1993.1072. [DOI] [PubMed] [Google Scholar]
- 2.Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology. 2001;94:1133–8. doi: 10.1097/00000542-200106000-00030. [DOI] [PubMed] [Google Scholar]
- 3.Moore EE, Moore FA, Franciose RJ, Kim FJ, Biffl WL, Banerjee A. The postischemic gut serves as a priming bed for circulating neutrophils that provoke multiple organ failure. J Trauma. 1994;37:881–7. doi: 10.1097/00005373-199412000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Oldenburg WA, Lau LL, Rodenberg TJ, Edmonds HJ, Burger CD. Acute mesenteric ischemia: a clinical review. Arch Intern Med. 2004;164:1054–62. doi: 10.1001/archinte.164.10.1054. [DOI] [PubMed] [Google Scholar]
- 5.Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol. 1986;250:G749–G753. doi: 10.1152/ajpgi.1986.250.6.G749. [DOI] [PubMed] [Google Scholar]
- 6.Raujo-Filho I, Rego AC, Pinheiro LA, Azevedo IM, Medeiros VB, Brandao-Neto J, Medeiros AC. Prevention of bacterial translocation using beta-(1-3)-D-glucan in small bowel ischemia and reperfusion in rats. Acta Cir Bras. 2006;21 4:18–22. doi: 10.1590/s0102-86502006001000005. [DOI] [PubMed] [Google Scholar]
- 7.Medeiros AC, Chacon DA, Sales VS, Egito ES, Brandao-Neto J, Pinheiro LA, Carvalho MR. Glucan and glutamine reduce bacterial translocation in rats subjected to intestinal ischemia-reperfusion. J Invest Surg. 2006;19:39–46. doi: 10.1080/08941930500444453. [DOI] [PubMed] [Google Scholar]
- 8.Carrizo GJ, Wu R, Cui X, Dwivedi AJ, Simms HH, Wang P. Adrenomedullin and adrenomedullin-binding protein-1 downregulate inflammatory cytokines and attenuate tissue injury after gut ischemia-reperfusion. Surgery. 2007;141:245–53. doi: 10.1016/j.surg.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359–77. doi: 10.1023/b:ddas.0000042232.98927.91. [DOI] [PubMed] [Google Scholar]
- 10.Sakata J, Shimokubo T, Kitamura K, Nakamura S, Kangawa K, Matsuo H, Eto T. Molecular cloning and biological activities of rat adrenomedullin, a hypotensive peptide. Biochem Biophys Res Commun. 1993;195:921–7. doi: 10.1006/bbrc.1993.2132. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–60. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 12.Pearson LJ, Rait C, Nicholls MG, Yandle TG, Evans JJ. Regulation of adrenomedullin release from human endothelial cells by sex steroids and angiotensin-II. J Endocrinol. 2006;191:171–7. doi: 10.1677/joe.1.06815. [DOI] [PubMed] [Google Scholar]
- 13.Minamino N. Adrenomedullin, its distribution and regulation of production. Nippon Rinsho. 2004;62 9:193–7. [PubMed] [Google Scholar]
- 14.Burley DS, Hamid SA, Baxter GF. Cardioprotective actions of peptide hormones in myocardial ischemia. Heart Fail Rev. 2007;12:279–91. doi: 10.1007/s10741-007-9029-y. [DOI] [PubMed] [Google Scholar]
- 15.Hirata Y, Mitaka C, Sato K, Nagura T, Tsunoda Y, Amaha K, Marumo F. Increased circulating adrenomedullin, a novel vasodilatory peptide, in sepsis. J Clin Endocrinol Metab. 1996;81:1449–53. doi: 10.1210/jcem.81.4.8636349. [DOI] [PubMed] [Google Scholar]
- 16.Nishikimi T. Adrenomedullin in the kidney-renal physiological and pathophysiological roles. Curr Med Chem. 2007;14:1689–99. doi: 10.2174/092986707780830943. [DOI] [PubMed] [Google Scholar]
- 17.Elsasser TH, Kahl S, Martinez A, Montuenga LM, Pio R, Cuttitta F. Adrenomedullin binding protein in the plasma of multiple species: characterization by radioligand blotting. Endocrinology. 1999;140:4908–11. doi: 10.1210/endo.140.10.7157. [DOI] [PubMed] [Google Scholar]
- 18.Pio R, Martinez A, Unsworth EJ, Kowalak JA, Bengoechea JA, Zipfel PF, Elsasser TH, Cuttitta F. Complement factor H is a serum-binding protein for adrenomedullin, and the resulting complex modulates the bioactivities of both partners. J Biol Chem. 2001;276:12292–300. doi: 10.1074/jbc.M007822200. [DOI] [PubMed] [Google Scholar]
- 19.Wang P. Andrenomedullin and cardiovascular responses in sepsis. Peptides. 2001;22:1835–40. doi: 10.1016/s0196-9781(01)00534-4. [DOI] [PubMed] [Google Scholar]
- 20.Fowler DE, Yang S, Zhou M, Chaudry IH, Simms HH, Wang P. Adrenomedullin and adrenomedullin binding protein-1: their role in the septic response. J Surg Res. 2003;109:175–81. doi: 10.1016/s0022-4804(02)00086-0. [DOI] [PubMed] [Google Scholar]
- 21.Zhou M, Ba ZF, Chaudry IH, Wang P. Adrenomedullin binding protein-1 modulates vascular responsiveness to adrenomedullin in late sepsis. Am J Physiol Regul Integr Comp Physiol. 2002;283:R553–R560. doi: 10.1152/ajpregu.00544.2001. [DOI] [PubMed] [Google Scholar]
- 22.Yang S, Zhou M, Chaudry IH, Wang P. Novel approach to prevent the transition from the hyperdynamic phase to the hypodynamic phase of sepsis: role of adrenomedullin and adrenomedullin binding protein-1. Ann Surg. 2002;236:625–33. doi: 10.1097/00000658-200211000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dwivedi AJ, Wu R, Nguyen E, Higuchi S, Wang H, Krishnasastry K, Marini CP, Ravikumar TS, Wang P. Adrenomedullin and adrenomedullin binding protein-1 prevent acute lung injury after gut ischemia-reperfusion. J Am Coll Surg. 2007;205:284–93. doi: 10.1016/j.jamcollsurg.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Higuchi S, Wu R, Zhou M, Marini CP, Ravikumar TS, Wang P. Gut hyperpermiability after ischemia and reperfusion: Attenuation with adrenomedullin and its binding protein treatment. Int J Clin Exp Pathol. 2008;1:409–18. [PMC free article] [PubMed] [Google Scholar]
- 25.Rana SN, Li X, Chaudry IH, Bland KI, Choudhry MA. Inhibition of IL-18 reduces myeloperoxidase activity and prevents edema in intestine following alcohol and burn injury. J Leukoc Biol. 2005;77:719–28. doi: 10.1189/jlb.0704396. [DOI] [PubMed] [Google Scholar]
- 26.Koike K, Moore EE, Moore FA, Read RA, Carl VS, Banerjee A. Gut ischemia/reperfusion produces lung injury independent of endotoxin. Crit Care Med. 1994;22:1438–44. doi: 10.1097/00003246-199409000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Collange O, Fabienne T, Nathalie R, Christian T, Vincent R, Bertrand D, Didier P. Pulmonary apoptosis after supraceliac aorta clamping in a rat model. J Surg Res. 2005;129:190–5. doi: 10.1016/j.jss.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Cerqueira NF, Hussni CA, Yoshida WB. Pathophysiology of mesenteric ischemia/reperfusion: a review. Acta Cir Bras. 2005;20:336–43. doi: 10.1590/s0102-86502005000400013. [DOI] [PubMed] [Google Scholar]
- 29.Stefanutti G, Vejchapipat P, Williams SR, Pierro A, Eaton S. Heart energy metabolism after intestinal ischaemia and reperfusion. J Pediatr Surg. 2004;39:179–83. doi: 10.1016/j.jpedsurg.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Yao YM, Sheng ZY, Yu Y, Tian HM, Wang YP, Lu LR, Xu SH. The potential etiologic role of tumor necrosis factor in mediating multiple organ dysfunction in rats following intestinal ischemia-reperfusion injury. Resuscitation. 1995;29:157–68. doi: 10.1016/0300-9572(95)00831-d. [DOI] [PubMed] [Google Scholar]
- 31.Cuzzocrea S, Chatterjee PK, Mazzon E, Dugo L, De SA, Van de Loo FA, Caputi AP, Thiemermann C. Role of induced nitric oxide in the initiation of the inflammatory response after postischemic injury. Shock. 2002;18:169–76. doi: 10.1097/00024382-200208000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Turnage RH, Guice KS, Oldham KT. Endotoxemia and remote organ injury following intestinal reperfusion. J Surg Res. 1994;56:571–8. doi: 10.1006/jsre.1994.1091. [DOI] [PubMed] [Google Scholar]
- 33.Samson WK. Adrenomedullin and the control of fluid and electrolyte homeostasis. Annu Rev Physiol. 1999;61:363–89. doi: 10.1146/annurev.physiol.61.1.363. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi K. Adrenomedullin from a pheochromocytoma to the eye: implications of the adrenomedullin research for endocrinology in the 21st century. Tohoku J Exp Med. 2001;193:79–114. doi: 10.1620/tjem.193.79. [DOI] [PubMed] [Google Scholar]
- 35.Koo DJ, Zhou M, Chaudry IH, Wang P. The role of adrenomedullin in producing differential hemodynamic responses during sepsis. J Surg Res. 2001;95:207–18. doi: 10.1006/jsre.2000.6013. [DOI] [PubMed] [Google Scholar]
- 36.Eguchi S, Hirata Y, Iwasaki H, Sato K, Watanabe TX, Inui T, Nakajima K, Sakakibara S, Marumo F. Structure-activity relationship of adrenomedullin, a novel vasodilatory peptide, in cultured rat vascular smooth muscle cells. Endocrinology. 1994;135:2454–8. doi: 10.1210/endo.135.6.7988431. [DOI] [PubMed] [Google Scholar]
- 37.Eguchi S, Hirata Y, Kano H, Sato K, Watanabe Y, Watanabe TX, Nakajima K, Sakakibara S, Marumo F. Specific receptors for adrenomedullin in cultured rat vascular smooth muscle cells. FEBS Lett. 1994;340:226–30. doi: 10.1016/0014-5793(94)80143-6. [DOI] [PubMed] [Google Scholar]
- 38.Takagi T, Yoshida N, Isozaki Y, Shimozawa M, Katada K, Manabe H, Hanada O, Kokura S, Ichikawa H, Naito Y, Okanoue T, Yoshikawa T. CV-11974, angiotensin II type I receptor antagonist, protects against ischemia-reperfusion injury of the small intestine in rats. Eur J Pharmacol. 2006;535:283–90. doi: 10.1016/j.ejphar.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)--summary of recommendations. J Vasc Interv Radiol. 2006;17:1383–97. doi: 10.1097/01.RVI.0000240426.53079.46. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan N, Yagmurdur H, Kilinc K, Baltaci B, Tezel S. The protective effects of intravenous anesthetics and verapamil in gut ischemia/reperfusion-induced liver injury. Anesth Analg. 2007;105:1371–8. doi: 10.1213/01.ane.0000284696.99629.3a. table. [DOI] [PubMed] [Google Scholar]
- 41.Sternbergh WC, III, Sobel M, Makhoul RG. Heparinoids with low anticoagulant potency attenuate postischemic endothelial cell dysfunction. J Vasc Surg. 1995;21:477–83. doi: 10.1016/s0741-5214(95)70290-3. [DOI] [PubMed] [Google Scholar]
- 42.Okajima K, Uchiba M. The anti-inflammatory properties of antithrombin III: new therapeutic implications. Semin Thromb Hemost. 1998;24:27–32. doi: 10.1055/s-2007-995820. [DOI] [PubMed] [Google Scholar]
- 43.Wu R, Zhou M, Wang P. Adrenomedullin and adrenomedullin binding protein-1 downregulate TNF-alpha in macrophage cell line and rat Kupffer cells. Regul Pept. 2003;112:19–26. doi: 10.1016/s0167-0115(03)00018-1. [DOI] [PubMed] [Google Scholar]
- 44.Miksa M, Wu R, Cui X, Dong W, Das P, Simms HH, Ravikumar TS, Wang P. Vasoactive hormone adrenomedullin and its binding protein: anti-inflammatory effects by up-regulating peroxisome proliferator-activated receptor-gamma. J Immunol. 2007;179:6263–72. doi: 10.4049/jimmunol.179.9.6263. [DOI] [PubMed] [Google Scholar]
- 45.Ishimitsu T, Kojima M, Kangawa K, Hino J, Matsuoka H, Kitamura K, Eto T, Matsuo H. Genomic structure of human adrenomedullin gene. Biochem Biophys Res Commun. 1994;203:631–9. doi: 10.1006/bbrc.1994.2229. [DOI] [PubMed] [Google Scholar]
- 46.Steinberg J, Halter J, Schiller H, Gatto L, Nieman G. The development of acute respiratory distress syndrome after gut ischemia/reperfusion injury followed by fecal peritonitis in pigs: a clinically relevant model. Shock. 2005;23:129–37. doi: 10.1097/01.shk.0000148053.66645.2e. [DOI] [PubMed] [Google Scholar]
- 47.Berman KS, Verma UN, Harburg G, Minna JD, Cobb MH, Gaynor RB. Sulindac enhances tumor necrosis factor-alpha-mediated apoptosis of lung cancer cell lines by inhibition of nuclear factor-kappaB. Clin Cancer Res. 2002;8:354–60. [PubMed] [Google Scholar]
- 48.Guneli E, Cavdar Z, Islekel H, Sarioglu S, Erbayraktar S, Kiray M, Sokmen S, Yilmaz O, Gokmen N. Erythropoietin protects the intestine against ischemia/reperfusion injury in rats. Mol Med. 2007;13:509–17. doi: 10.2119/2007-00032.Guneli. [DOI] [PMC free article] [PubMed] [Google Scholar]