Abstract
Language acquisition and processing are governed by genetic constraints. A crucial unresolved question is how far these genetic constraints have coevolved with language, perhaps resulting in a highly specialized and species-specific language “module,” and how much language acquisition and processing redeploy preexisting cognitive machinery. In the present work, we explored the circumstances under which genes encoding language-specific properties could have coevolved with language itself. We present a theoretical model, implemented in computer simulations, of key aspects of the interaction of genes and language. Our results show that genes for language could have coevolved only with highly stable aspects of the linguistic environment; a rapidly changing linguistic environment does not provide a stable target for natural selection. Thus, a biological endowment could not coevolve with properties of language that began as learned cultural conventions, because cultural conventions change much more rapidly than genes. We argue that this rules out the possibility that arbitrary properties of language, including abstract syntactic principles governing phrase structure, case marking, and agreement, have been built into a “language module” by natural selection. The genetic basis of human language acquisition and processing did not coevolve with language, but primarily predates the emergence of language. As suggested by Darwin, the fit between language and its underlying mechanisms arose because language has evolved to fit the human brain, rather than the reverse.
Keywords: Baldwin effect, coevolution, cultural evolution, language acquisition
The mechanisms involved in the acquisition and processing of language are closely intertwined with the structure of language itself. Children routinely acquire language with little intentional tutoring by their parents and as adults use language with minimal effort. Indeed, our unique and nearly universal capacity to acquire and use language has even been cited as one of eight key transitions in the evolution of life (1). These features of species specificity and species universality, combined with the intimate fit between language structure and the mechanisms by which language is acquired and used, point to substantial genetic constraints. The nature and origin of the genetic basis for language remain the focus of much debate, however (2–4).
An influential line of thinking in the cognitive sciences suggests that the genes involved in language predetermine a highly specialized and species-specific language “module” (5), “instinct” (6), or “organ” (7). This module has been assumed to specify a number of domain-specific linguistic properties, including case marking, agreement, and conformity to highly abstract syntactic constraints, such as X-bar theory (8). Although some have argued that the genes encoding a language module arose through a sudden “catastrophic” genetic change (9), and others have remained agnostic on this point (10), “the default prediction from a Darwinian perspective on human psychological abilities” (ref. 11; p. 16) is the adaptationist view, that genes for language coevolved with human language itself for the purpose of communication (1, 8, 12–18).
A challenge for the adaptationists is to pinpoint an evolutionary mechanism by which a language module could become genetically encoded (19). The problem is that many of the linguistic properties purported to be included in the language module are highly abstract and have no obvious functional basis—they cannot be explained in terms of communicative effectiveness or cognitive constraints—and have even been suggested to hinder communication (20). By analogy with the conventions of communication protocols between computers, it has been suggested that even completely arbitrary linguistic properties can have an adaptive value, if the same conventions are adopted by all members of a speech community (12). That is, although any number of equally effective communicative “protocols” may serve equally well for communication, what matters is that everyone adopts the same set of culturally mediated conventions.
The subsequent shift from initially learned linguistic conventions to genetically encoded principles necessary to evolve a language module may appear to require Lamarckian inheritance. The Baldwin effect (21, 22) provides a possible Darwinian solution to this challenge, however. Baldwin proposed that characteristics that are initially learned or developed over the lifespan can become gradually encoded in the genome over many generations, because organisms with a stronger predisposition to acquire a trait have a selective advantage. Over generations, the amount of environmental exposure required to develop the trait decreases, and eventually no environmental exposure may be needed—the trait is genetically encoded. A frequently cited example of the Baldwin effect is the development of calluses on the keels and sterna of ostriches (22). The proposal is that calluses were initially developed in response to abrasion where the keel and sterna touch the ground during sitting. Natural selection then favored individuals that could develop calluses more rapidly, until callus development became triggered within the embryo and could occur without environmental stimulation. We investigated the circumstances under which a similar evolutionary mechanism could genetically assimilate properties of language in a domain-specific module (1, 12, 13).
Simulation 1: Establishing the Baldwin Effect.
We first specified a model of the mutual influence of language and language genes. To provide the best chance for the Baldwin effect to operate, we chose the simplest possible relationship between language and genes (23). We considered a language governed by n principles, P1, … ,Pi … ,Pn, which potentially may be encoded in n genes. Each principle has two variants, +L and −L. The corresponding genes, G1, … ,Gi … ,Gn, have three alleles, +G, −G, and ?G, two of which encode a bias toward learning, +L or −L, with the third being neutral.
In each generation, a population of N agents attempts to learn the principles using trial-and-error learning. Fitness is based on the number of trials required to learn the principles. Only the f fittest agents reproduce, by sexual recombination and mutation. The genes of each “child” in the new generation are derived by randomly combining the genes of two “parents” chosen at random. Each gene has a probability of mutation, m—that is, a locus is randomly reassigned to one of the alleles +G, −G, or ?G. Thus, the population is genetically selected to be good at learning the principles of the language.
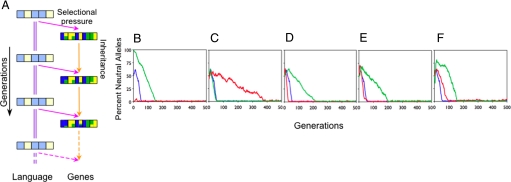
To see how the Baldwin effect might operate, suppose that the ith principle in the language is +L. Learners with the appropriately biased allele, +G, in the ith position in the genome will tend to learn more rapidly and thus have a higher probability of reproducing. Thus, over generations, correctly biased alleles will predominate, and learning time will approach 0; the principles of the language will have become genetically fixed. Fig. 1 shows that this process occurs robustly in Simulation 1. The Baldwin effect is stable across wide variations in the initial percentage of neutral alleles, group size, genome size, percentage of learners allowed to reproduce, and strength of genetic bias. The Baldwin effect emerges reliably; neutral alleles are eliminated, and “correctly” biased alleles are established. Hence, aspects of language that are stable over evolutionary time can become genetically assimilated (13, 24), particularly if there is a cost associated with language learning (25, 26) and no conflicting functional pressures (27).
Fig. 1.
Arbitrary linguistic principles can become genetically encoded via the Baldwin effect. (A) Influences across generations for language and genes. The principles, Pi, of the language, L, are indicated by light-yellow (+L) and light-blue (-L) squares. The corresponding biasing alleles, +G and -G, are indicated by dark-yellow and dark-blue squares. Neutral alleles, ?G, are shown in green. For illustration, just five linguistic principles and the mean population values for genes are shown. Here L is fixed across generations (purple double lines); L exerts selectional pressure on the genes, G, at each generation (pink arrows); and the genes of the fastest language learners are transmitted across generations (orange arrows), subject to sexual recombination and mutation. (B–F) The percentage of neutral alleles, plotted against the number of generations, across variations in (B) percentage of neutral alleles in the first generation: 0 (red), 50 (blue), and 100 (green); (C) group size: 24 (red), 100 (blue), and 250 (green); (D) genome size: 10 (red), 20 (blue), and 50 (green); (E) percentage of learners allowed to reproduce: 26 (red), 50 (blue), and 74 (green); and (F) bias of +G/-G alleles for sampling the corresponding +L/-L principles during learning: 0.8 (red), 0.95 (blue), and 1 (green). The remaining parameters in these simulations take the default values indicated in blue.
Simulation 2: Language Change Eliminates the Baldwin Effect.
As with ostrich calluses, linguistic principles that initially arise after substantial interaction with the environment can, after a period of natural selection, develop with minimal environmental input. Yet the Baldwin effect typically applies in fixed environments (28), whereas simulations of biological coevolution suggest that the Baldwin effect is eliminated in a changing environment (29). So, whereas the stable environment of the ostrich provides a reliable adaptive advantage for calluses, the linguistic environment appears highly unstable. Arbitrary linguistic conventions before genetic encoding are, like other cultural phenomena, subject to rapid change, which is generally considered to be much faster than genetic evolution (30–32). Indeed, in the modern era, many properties of language continue to change rapidly; for example, the languages in the Indo-European language group (including Breton, Danish, Faroese, Gujarati, Hittite, Tadzik, and Waziri), which have huge variations in case systems, word order, and phonology, have diverged in just 10,000 years (33). Thus, the “environment” of linguistic conventions changes far more rapidly, and yields far greater diversity (34), than the typical properties of physical and biological environments to which organisms must adapt, and which typically jump between a relatively small numer of states [depending on climatic variation, or the population size of key prey/predators (35)].
Can the Baldwin effect lead to the genetic assimilation of linguistic properties (e.g., agreement, case marking, and X-bar theory) that putatively began as cultural conventions? In particular, is this possible given that cultural conventions provide a rapidly “moving target” for any biological adaptation?
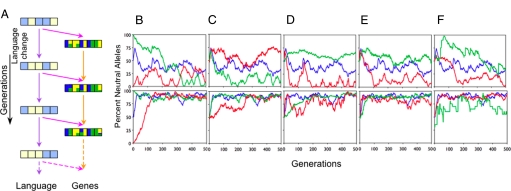
In a second set of simulations (Fig. 2A), we introduced language change [which might arise, e.g., via grammaticalization or language contact (36)] by modifying a randomly chosen principle, Pi, with probability l. Fig. 2 B–F (Upper) illustrates that the Baldwin effect is substantially reduced even when linguistic principles change at the same rate as the mutation rate, m, of the genes (l = m); Fig. 2 B–F (Lower) shows that when linguistic change is more rapid (l = 10m), the Baldwin effect is eliminated. Thus, genetic commitment to specific principles is maladaptive when the language is a “moving target.” The most successful learners end up with neutral alleles.
Fig. 2.
The effect of language change on the genetic encoding of arbitrary linguistic principles. (A) Influence across generations is as in Fig. 1, except that language also varies across generations (purple arrows). (B–F) As in Fig. 1, except that language changes at the same rate as genetic mutation, l = m (Upper), or 10 times faster, l = 10m (Lower). The Baldwin effect is substantially reduced in the first case and minimal in the second case.
Simulation 3: Language–Gene Coevolution.
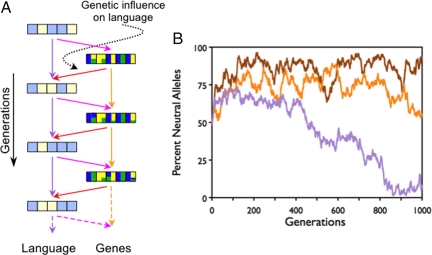
In Simulation 2, language change is governed by cultural forces and uninfluenced by genetic factors. But linguistic properties also may be influenced by genetic biases. If linguistic principles coevolve with genetic factors, this might potentially stabilize the linguistic principles and provide a fixed target on which natural selection over language genes might operate [cf. Baldwinian niche construction (37)]. In a third set of simulations, we explored this by allowing the language at generation t + 1 to be determined by a combination of genes and language at the previous generation t. Specifically, for the ith principle at t + 1, there is a probability, g, that this principle is determined by the genetic allele that is most prevalent at the ith location at t. Otherwise, with probability 1 - g, the principle is not influenced by the genes, but is subject to linguistic change with probability l, as before. If g is 0, then the results are as in Simulation 2. If g is 1, then the language is “reinvented” afresh at each generation to fit the genetic biases in the population. In the latter case, the Baldwin effect is not required to explain a putative language module, because the linguistic properties are already determined by preexisting genes. The critical question is whether low values of g (i.e., moderate genetic influence) can lead to a “runaway” tendency for a certain set of principles to be adopted and thus provide stable selection pressure, so that the Baldwin effect can operate. Fig. 3 shows that only when g is high (≈50%) does the Baldwin effect reemerge. But this case seems implausible for most, if not all, arbitrary linguistic principles; if selection pressure on the relevant locus is influenced mainly by the genes, then this indicates that the principle has a strong preexisting genetic basis.
Fig. 3.
Coevolution of language and language genes. (A) Influence across generations as in Fig. 2, except that the genome, G, also influences language, L, in the next generation (red arrows). Thus, L and G at generation t + 1 are influenced by both L and G at generation t. (B) The percentage of neutral alleles plotted across generations. Default parameters are as in Figs. 1 and 2. Genetic influence, g, varies from 10% (brown) to 25% (orange) to 50% (purple). With high genetic influence, the Baldwin effect reemerges.
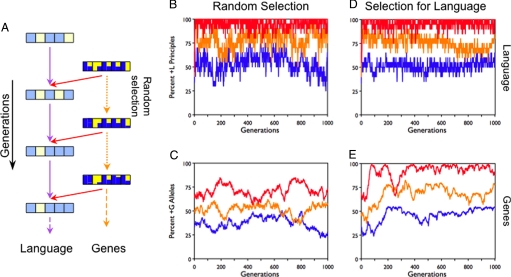
Note, however, that if the genetic influence g is sufficiently high to lead to the Baldwin effect (i.e., high enough to outweigh the influence of language change), g also is sufficiently high to determine language structure even in the absence of language-driven genetic selection (Fig. 4). Specifically, the degree and direction of language change remain almost unaffected when language-specific genetic selection (implementing the Baldwin effect) is replaced by genetic drift. Thus, language is determined by the genetic biases of the population at the outset, independent of selection pressure from the language to the genome (cf. ref. 38). The Baldwin effect serves merely to entrench existing genetic biases in the population.
Fig. 4.
The shaping of language by genetic influence. (A) Influence across generations is as in Fig. 3, except that the genes are no longer influenced by language or any other selective pressure. The genes of randomly chosen agents are sexually recombined and mutated (orange dotted arrows), leading to genetic drift. (B) The percentage of +L principles in the language, as a function of number of generations. The interaction between high population influence (g = 50%) and previous genetic biases is determined by varying the probability of allele reassignment during mutation. The neutral ?G allele always has a probability of 1/3. The +G allele has a mutation reassignment probability of 2/3, 1/2, or 1/3. The initial proportion of +G alleles is set to be the same as the mutation reassignment probability, with the remaining parameters taking the default values from Figs. 1–3. There are three degrees of +G bias: strong (2/3, red line), medium (1/2, orange), and none (1/3, blue). The previous genetic bias toward +G is reflected in the proportion of +L principles; language becomes aligned with the genes even when there is no selective feedback from language to genes. (C) The percentage of +G alleles in the population, plotted across generations. The genes follow a random-walk distribution, based on the reassignment probabilities for +G. (D and E) For comparison, the simulations in (B) and (C) were rerun, but with selection for language reintroduced (as in Fig. 3). Comparing (B) and (C) with (D) and (E) shows that with strong population influence, language simply converges on the initial genetic bias of the population (+G) regardless of whether or not genes are selected for language (as in the Baldwin effect).
Conclusion
Although our results demonstrate that the Baldwin effect may apply to functional properties of language (Simulation 1), the Baldwin effect is unlikely to be the mechanism for genetic assimilation of arbitrary linguistic properties that began as learned cultural conventions (Simulations 2 and 3). Thus, a highly intricate and abstract language “module” (5), “instinct” (6), or “organ” (7) postulated to explain language acquisition (7, 39), language universals (7), and the species-specificity of human language (8) could not have arisen through biological adaptation. Indeed, this conclusion is reinforced by the observation that had such adaptation occurred in the human lineage, these processes would have operated independently on modern human populations as they spread throughout Africa and the rest of the world over the last 100,000 years. If this were so, then genetic populations should have coevolved with their own language groups, leading to divergent and mutually incompatible language modules (40). Linguists have found no evidence of this, however (6); for example, native Australasian populations have been largely isolated for 50,000 years (31) but learn European languages readily.
To make the conditions for Baldwinian coadaptation as favorable as possible, our model embodies the simplest possible relationship between linguistic principles and language genes: one-to-one correspondence. The complexity of the actual many-to-many relationship between genes and behavior makes Baldwinian coevolution even less plausible, because selectional pressures from the linguistic environment are substantially diluted (41). Perhaps the best candidate for a “language gene” is FOXP2 (42), damage to which has been associated with morphosyntactic deficits (43). But FOXP2 damage leads to a broader developmental impairment in the form of orofacial apraxia (44), of which linguistic difficulties may be a consequence. This gene also is associated with gut, lung, and heart function (45). Thus, any selectional pressures on FOXP2 due to language processing would not be directly associated with a putative language module and would compete with selection pressures from essential biological processes. Indeed, current knowledge suggests that FOXP2 is not tied to arbitrary properties of language but instead appears to influence domain-general procedural learning systems (46) and a down-stream gene, CNTNAP2, which in turn affects phonological short-term memory (47).
Although we have shown that arbitrary linguistic properties cannot be genetically encoded through adaptation, this does not preclude genetic adaptation to aspects of language held stable by functional pressures. For example, changes in the vocal apparatus may have arisen from functional pressures to produce more intelligible vocalization, although this point is controversial (48–50). Possible functional properties include duality of patterning (51) [i.e., the presence of two levels of symbolic structure: a pool of phonetic resources from which to compose word forms (52) and an inventory of constructions from which to compose sentences (53)]; syntactic devices to express propositional attitudes (12); and, more constroversially, recursion (8, 48, 54). It is possible, however, that culturally-mediated linguistic change may shield the relevant learning and processing mechanisms from adapting to selective pressures even from functional properties of language (55).
If a biologically specialized language module incorporating arbitrary constraints is ruled out, what explains the close fit between language and its underlying mechanisms? One possibility is that the properties of natural languages, although apparently capricious, arise from underlying functional considerations [i.e., optimizing communication (56)] or from noncommunication factors concerned with optimizing the relationship between sound and meaning (20, 57). More generally, this suggests that the biology of language results primarily from exaptation, not adaptation (58–60). If this is so, language may have been shaped to a large extent by evolutionary processes of cultural transmission across generations of language learners (61–63). These processes include grammaticalization, the continual routinization, generalization, and erosion that underlie historical patterns of language change (36). Importantly, such cultural evolution is constrained by properties of the human neural and perceptuo-motor systems, which themselves have a genetic basis largely predating the emergence of language (40).
Although our simulations indicate that some biological adaptations for functional aspects of language could have taken place, we suggest that the close fit between the structure of language and the mechanisms employed to acquire and use it primarily arose because language has been shaped by the brain through cultural evolution. Indeed, the astonishing subtlety and diversity of patterns in human language (34) may for the most part result from the complex interaction of multiple constraints on cultural evolution, deriving from the nature of thought, the perceptuo-motor system, cognitive limitations on learning and processing, and pragmatic/communicative factors (40). Thus, as suggested by Darwin (64), the evolution of human language may be best understood in terms of cultural evolution, not biological adaptation.
Methods
Setup of Simulations.
The simulations investigated the coevolution of genes and language in a small population of hominids. At each generation, a population of N learners attempts to learn by trial and error a language governed by n principles, P1, … ,Pi … ,Pn, each of which has two variations, +L and −L. The corresponding genes, G1, … ,Gi … ,Gn, have three alleles, +G, −G, and ?G, two of which encode a bias toward learning, +L or −L, and the third of which is neutral. The simulations begin with x neutral alleles, where x varies between 0 and 100%; the remaining biased alleles are set randomly to +G or −G. Group size, N, was varied between 24 and 250 (65); language/genome size, n, was varied between 10 and 50; percentage of learners allowed to reproduce, f, was varied between 26 and 74; and strength of the bias, p, of +G/−G alleles for sampling the corresponding +L/−L principles during learning was varied between 0.8 and 1. The mutation rate, m, was fixed at 0.01 throughout the simulations. (This is rather high from a biological standpoint, but it did not qualitatively affect the results.) In Simulations 2 and 3, the rate of linguistic change, l, was either the same as the mutation rate (0.01) or a factor of 10 larger (0.1). In Simulation 3, the genetic influence, g, on the language was varied between 10% and 50%. Here and throughout, multiple runs with the same parameter values yielded qualitatively similar results. Figs. 1–4 show typical, randomly chosen runs.
Learning by Trial and Error.
To produce the initial hypothesis concerning the target language, the learner first stochastically samples a set of candidate principles, according to its genetic biases. At locus i, a learner with a genetic bias +G will sample the +L variant of the ith principle with probability p > 0.5 and will sample the −L variant with probability 1-p; the converse holds for a learner with genetic bias −G. The neutral ?G allele samples +L and −L with equal probability. Initially, and throughout learning, as soon as a principle is guessed correctly, it is fixed. Learning involves sequentially resampling any incorrect candidate principles according to the initial genetic bias, in a random order, until all principles match the target language. The fitness of the agent, and thus whether it reproduces, is determined by the total number of resamplings required for the agent to learn the entire language.
Speed of Learning.
Learners with an allelic bias +G on the ith locus will rapidly fix the correct variation for this principle (with expected number of steps 1/p); those with bias −G typically will require more samples (expectation, 1/1 - p), and those with variant ?G will be intermediate [expectation, 1/(1/2) = 2]. Note that unless p is either 0 or 1 and incorrectly set for some allele, all learners acquire the language eventually.
Reproduction by Sexual Recombination.
For all simulations presented here, sexual recombination involves randomly pairing “parent” agents and then creating a “child” agent for each genetic locus, i, taking the allele at that locus randomly from either parent. Mutation then occurs randomly for each gene at each generation with probability m. The reassignment takes the value +G, −G, or ?G with equal probability.
Acknowledgments.
This work was supported by the Human Frontiers Science Program Grant RGP0177/2001-B. N.C. was supported by a Major Research Fellowship from the Leverhulme Trust and by ESRC Grant RES-000–22-2768 and RES-000-22-2488. M.H.C. was supported by a Charles A. Ryskamp Fellowship from the American Council of Learned Societies.
Footnotes
The authors declare no conflicts of interest.
This article is a PNAS Direct Submission.
References
- 1.Maynard Smith J, Szathmáry E. Major Transitions in Evolution. New York: Oxford Univ Press; 1997. [Google Scholar]
- 2.Bickerton D, Szathmáry E, editors. Biological Foundations and Origin of Syntax. Cambridge MA: MIT Press; 2009. [Google Scholar]
- 3.Christiansen MH, Kirby S. Language evolution: Consensus and controversies. Trends Cogn Sci. 2003;7:300–307. doi: 10.1016/s1364-6613(03)00136-0. [DOI] [PubMed] [Google Scholar]
- 4.Smith ADM, Smith K, Ferrer i Cancho R, editors. Proceedings of the Sixth International Conference on the Evolution of Language. London: World Scientific Publishing; 2008. [Google Scholar]
- 5.Fodor J. The Modularity of Mind. Cambridge, MA: MIT Press; 1983. [Google Scholar]
- 6.Pinker S. The Language Instinct. New York: Harper Collins; 1994. [Google Scholar]
- 7.Chomsky N. Rules and Representations. Oxford, UK: Blackwell; 1980. [Google Scholar]
- 8.Pinker S, Jackendoff R. The faculty of language: What's special about it? Cognition. 2005;95:201–236. doi: 10.1016/j.cognition.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Bickerton D. Language and Human Behavior. London: UCL Press; 1995. [Google Scholar]
- 10.Chomsky N. Language and Problems of Knowledge: The Managua Lectures. Cambridge, MA: MIT Press; 1988. [Google Scholar]
- 11.Pinker S. In: Language Evolution. Christiansen MH, Kirby S, editors. New York: Oxford Univ Press; 2003. pp. 16–37. [Google Scholar]
- 12.Pinker S, Bloom P. Natural language and natural selection. Behav Brain Sci. 1990;13:707–784. [Google Scholar]
- 13.Briscoe E. Grammatical acquisition: Inductive bias and coevolution of language and the language acquisition device. Language. 2000;76:245–296. [Google Scholar]
- 14.Corballis MC. In: Language Evolution. Christiansen MH, Kirby S, editors. New York: Oxford Univ Press; 2003. pp. 201–218. [Google Scholar]
- 15.Culicover PW, Jackendoff R. Simpler Syntax. New York: Oxford Univ Press; 2005. [Google Scholar]
- 16.Dunbar RIM. In: Language Evolution. Christiansen MH, Kirby S, editors. New York: Oxford Univ Press; 2003. pp. 219–234. [Google Scholar]
- 17.Számadó S, Szathmáry E. Competing selective scenarios for the emergence of natural language. Trends Ecol Evol. 2006;21:555–561. doi: 10.1016/j.tree.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Tooby J, Cosmides L. In: The Handbook of Evolutionary Psychology. Buss DM, editor. Hoboken NJ: Wiley; 2005. pp. 5–67. [Google Scholar]
- 19.Nowak MA, Komarova NL, Niyogi P. Evolution of universal grammar. Science. 2001;291:114–118. doi: 10.1126/science.291.5501.114. [DOI] [PubMed] [Google Scholar]
- 20.Chomsky N. Three factors in language design. Ling Inq. 2005;36:1–22. [Google Scholar]
- 21.Baldwin JM. A new factor in evolution. Am Nat. 1896;30:441–451. [Google Scholar]
- 22.Waddington CH. Canalization of development and the inheritance of acquired characters. Nature. 1942;150:563–565. doi: 10.1038/1831654a0. [DOI] [PubMed] [Google Scholar]
- 23.Hinton GE, Nowlan SJ. How learning can guide evolution. Complex Systems. 1987;1:495–502. [Google Scholar]
- 24.Batali J. In: Artificial Life 4: Proceedings of the Fourth International Workshop on the Synthesis and Simulations of Living Systems. Brooks R, Maes P, editors. Redwood City CA: Addison-Wesley; 1994. pp. 160–171. [Google Scholar]
- 25.Kirby S, Hurford J. In: Fourth European Conference on Artificial Life. Husbands P, Harvey I, editors. Cambridge, MA: MIT Press; 1997. pp. 493–502. [Google Scholar]
- 26.Munroe S, Cangelosi A. Learning and the evolution of language: The role of cultural variation and learning cost in the Baldwin effect. Artif Life. 2002;8:311–339. doi: 10.1162/106454602321202408. [DOI] [PubMed] [Google Scholar]
- 27.Reali F, Christiansen MH. The relative role of biological and linguistic adaptation in language evolution: A computational approach. Interact Stud. 2009 in press. [Google Scholar]
- 28.Maynard Smith J. When learning guides evolution. Nature. 1987;329:761–762. doi: 10.1038/329761a0. [DOI] [PubMed] [Google Scholar]
- 29.Ancel L. A quantitative model of the Simpson-Baldwin effect. J Theor Biol. 1999;196:197–209. doi: 10.1006/jtbi.1998.0833. [DOI] [PubMed] [Google Scholar]
- 30.Boyd R, Richerson PJ. The Origin and Evolution of Cultures. Oxford UK: Oxford Univ Press; 2005. [Google Scholar]
- 31.Cavalli-Sforza LL, Feldman MW. The application of molecular genetic approaches to the study of human evolution. Nat Genet. 2003;33:266–275. doi: 10.1038/ng1113. [DOI] [PubMed] [Google Scholar]
- 32.Dawkins R. The Selfish Gene. Oxford, UK: Oxford Univ Press; 1976. [Google Scholar]
- 33.Gray RD, Atkinson QD. Language-tree divergence times support the Anatolian theory of Indo-European origin. Nature. 2003;426:435–439. doi: 10.1038/nature02029. [DOI] [PubMed] [Google Scholar]
- 34.Evans N. Context, culture, and structuration in the languages of Australia. Annu Rev Anthropol. 2003;32:13–40. [Google Scholar]
- 35.Levins R. Evolution in Changing Environments. Princeton, NJ: Princeton Univ Press; 1967. [Google Scholar]
- 36.Heine B, Kuteva T. Language Contact and Grammatical Change. Cambridge, UK: Cambridge Univ Press; 2005. [Google Scholar]
- 37.Odling-Smee FJ, Laland KN, Feldman MW. Niche Construction: The Neglected Process in Evolution. Princeton, NJ: Princeton Univ Press; 2003. [Google Scholar]
- 38.Kirby S, Dowman M, Griffiths TL. Innateness and culture in the evolution of language. Proc Natl Acad Sci U S A. 2007;104:5241–5245. doi: 10.1073/pnas.0608222104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crain S, Pietroski P. Nature, nurture and universal grammar. Ling Philos. 2001;24:139–185. [Google Scholar]
- 40.Christiansen MH, Chater N. Language as shaped by the brain. Behav Brain Sci. 2008;31:489–558. doi: 10.1017/S0140525X08004998. [DOI] [PubMed] [Google Scholar]
- 41.Newman SA. Developmental mechanisms: Putting genes in their place. J Biosci. 2002;27:97–104. doi: 10.1007/BF02703765. [DOI] [PubMed] [Google Scholar]
- 42.Lai CSL, Fisher SE, Hurst JA, Vargha-Khadem F, Monoca AP. A fork-head domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- 43.Gopnik M. Feature-blind grammar and dysphasia. Nature. 1990;344:715. doi: 10.1038/344715a0. [DOI] [PubMed] [Google Scholar]
- 44.Vargha-Khadem F, Watkins K, Alcock K, Fletcher P, Passingham R. Praxic and nonverbal cognitive deficits in a large family with a genetically transmitted speech and language disorder. Proc Natl Acad Sci U S A. 1995;92:930–933. doi: 10.1073/pnas.92.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shu W, Yang H, Zhang L, Lu MM, Morrisey EE. Characterization of a new subfamily of winged-helix/forkhead (Fox) genes that are expressed in the lung and act as transcriptional repressors. J Biol Chem. 2001;276:27488–27497. doi: 10.1074/jbc.M100636200. [DOI] [PubMed] [Google Scholar]
- 46.Fisher SE. Tangled webs: Tracing the connections between genes and cognition. Cognition. 2006;101:270–297. doi: 10.1016/j.cognition.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Vernes, et al. A functional genetic link between distinct developmental language disorders. N Engl J Med. 2008;359:2337–2345. doi: 10.1056/NEJMoa0802828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hauser MD, Chomsky N, Fitch WT. The faculty of language: What is it, who has it and how did it evolve? Science. 2002;298:1569–1579. doi: 10.1126/science.298.5598.1569. [DOI] [PubMed] [Google Scholar]
- 49.Fitch WT. The evolution of speech: a comparative review. Trends Cogn Sci. 2000;4:258–267. doi: 10.1016/s1364-6613(00)01494-7. [DOI] [PubMed] [Google Scholar]
- 50.Lieberman P. The evolution of human speech: Its anatomical and neural bases. Curr Anthropol. 2007;48:39–53. [Google Scholar]
- 51.Hockett CF. The origin of speech. Sci Am. 1960;203:88–111. [PubMed] [Google Scholar]
- 52.Pierrehumbert J. What people know about the sounds of language. Linguistic Sci. 2000;29:111–120. [Google Scholar]
- 53.Goldberg AE. Constructions at Work: The Nature of Generalization in Language. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- 54.Everett D. Cultural constraints on grammar and cognition in Pirahã: Another look at the design features of human language. Curr Anthropol. 2005;46:621–646. [Google Scholar]
- 55.Smith K, Kirby S. Cultural evolution: Implications for understanding the human language faculty and its evolution. Phil Trans R Soc B. 2008;363:3591–3603. doi: 10.1098/rstb.2008.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bybee J. A functionalist approach to grammar and its evolution. Evol Comm. 1998;2:249–278. [Google Scholar]
- 57.Chomsky N. In: Interfaces + Recursion = Language? Sauerland U, Gärtner H, editors. Berlin: Mouton de Gruyter; 2007. pp. 1–29. [Google Scholar]
- 58.Fodor J. In Critical Condition. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- 59.Gould SJ. Exaptation: A crucial tool for evolutionary psychology. J Soc Issues. 1991;47:43–65. [Google Scholar]
- 60.Tattersall I. Commentary on Lieberman “The evolution of human speech: Its anatomical and neural bases”. Curr Anthrop. 2007;48:57–58. [Google Scholar]
- 61.Deacon TW. The Symbolic Species: The Coevolution of Language and the Brain. New York: Norton; 1997. [Google Scholar]
- 62.Kirby S, Hurford JR. In: Simulating the Evolution of Language. Cangelosi A, Parisi D, editors. Berlin: Springer-Verlag; 2002. pp. 121–148. [Google Scholar]
- 63.Tomasello M. In: Language Evolution. Christiansen MH, Kirby S, editors. New York: Oxford Univ Press; 2003. pp. 94–110. [Google Scholar]
- 64.Darwin C. The Descent of Man and Selection in Relation to Sex. 2nd Ed. London: John Murray; 1882. [Google Scholar]
- 65.Dunbar RIM, Shultz S. Evolution in the social brain. Science. 2007;317:1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]