Abstract
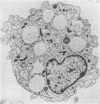
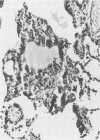
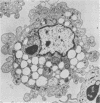
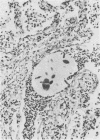
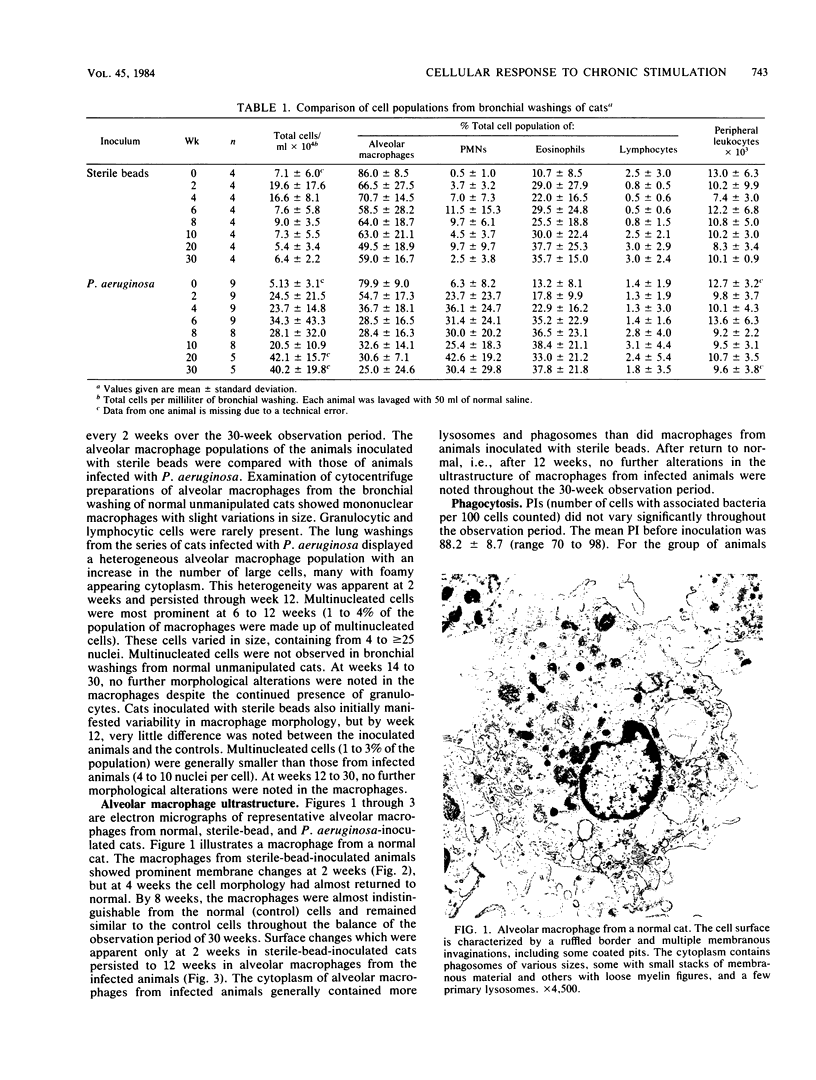
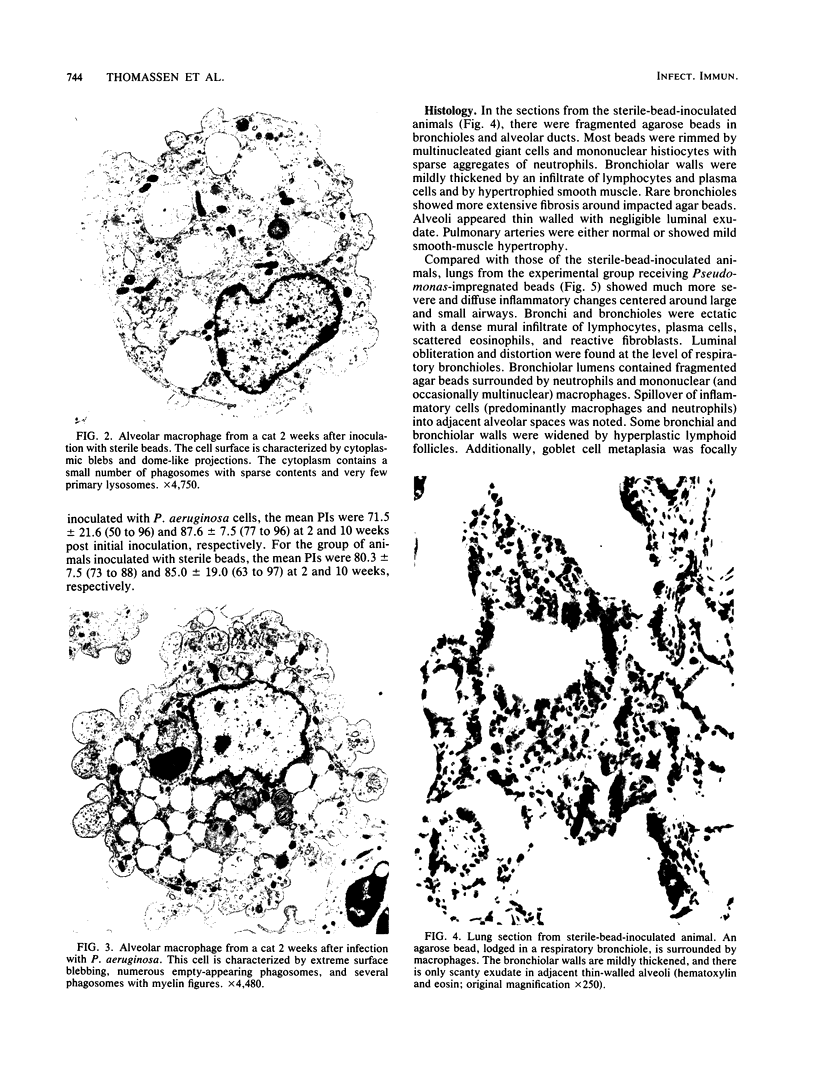
A model of chronic pulmonary infection was used for studying cellular events in a sequential manner. In this model, agarose beads containing Pseudomonas aeruginosa were instilled endotracheally into cats. Nine cats were inoculated with agarose beads containing P. aeruginosa, and four others were inoculated with sterile beads. With a fiberoptic bronchoscope, bronchial washings were obtained biweekly for up to 30 weeks. The quantitative pulmonary inflammatory cell response and alveolar macrophage morphology of the animals exposed to P. aeruginosa were compared with those for the animals exposed to a chronic irritant (agarose beads). Bronchial washings of all animals before inoculation showed that 70 to 90% of the cells were macrophages. After inoculation with P. aeruginosa, a persistent inflammatory response was observed (60 to 70% granulocytes). In the sterile-bead-inoculated group, the response was less prominent (30 to 40% granulocytes). As early as 2 weeks after inoculation, alveolar macrophages from infected animals were larger and had cytoplasmic features that differed from those of controls. Electron microscope examination showed prominent surface alterations in alveolar macrophages from the infected cats. These alterations persisted from 2 to 12 weeks after infection. In animals inoculated with sterile beads, alveolar macrophages exhibited less extensive surface changes that had resolved by week 8. Histologically, chronic bronchiolitis and pneumonia were more severe in the infected animals than in controls. This model of chronic inflammation and macrophage stimulation, which is similar to the chronic pneumonia of cystic fibrosis, may be a useful approach to answer questions on the role of macrophage activation in chronic lung disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwood L. L., Stone R. M., Iglewski B. H., Pennington J. E. Evaluation of Pseudomonas aeruginosa exotoxin A and elastase as virulence factors in acute lung infection. Infect Immun. 1983 Jan;39(1):198–201. doi: 10.1128/iai.39.1.198-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash H. A., Woods D. E., McCullough B., Johanson W. G., Jr, Bass J. A. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am Rev Respir Dis. 1979 Mar;119(3):453–459. doi: 10.1164/arrd.1979.119.3.453. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Matthay R. A., Reynolds H. Y. Cystic fibrosis pseudomonas opsonins. Inhibitory nature in an in vitro phagocytic assay. J Clin Invest. 1981 Oct;68(4):899–914. doi: 10.1172/JCI110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holby N., Olling S. Pseudomonas aeruginosa infection in cystic fibrosis. Bactericidal effect of serum from normal individuals and patients with cystic fibrosis on P. aeruginosa strains from patients with cystic fibrosis or other diseases. Acta Pathol Microbiol Scand C. 1977 Apr;85(2):107–114. [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. L., Lazdins J. K. Biochemical criteria for activated macrophages. J Immunol. 1978 Sep;121(3):809–813. [PubMed] [Google Scholar]
- Lam J., Chan R., Lam K., Costerton J. W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 1980 May;28(2):546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The concept of the activated macrophage. J Immunol. 1978 Sep;121(3):806–809. [PMC free article] [PubMed] [Google Scholar]
- Tandler B., Walter R. J. Epon-maraglas embedment for electron microscopy. Stain Technol. 1977 Jul;52(4):238–239. doi: 10.3109/10520297709116783. [DOI] [PubMed] [Google Scholar]
- Thomassen M. J., Boxerbaum B., Demko C. A., Kuchenbrod P. J., Dearborn D. G., Wood R. E. Inhibitory effect of cystic fibrosis serum on pseudomonas phagocytosis by rabbit and human alveolar macrophages. Pediatr Res. 1979 Sep;13(9):1085–1088. doi: 10.1203/00006450-197909000-00030. [DOI] [PubMed] [Google Scholar]
- Thomassen M. J., Demko C. A. Serum bactericidal effect on Pseudomonas aeruginosa isolates from cystic fibrosis patients. Infect Immun. 1981 Aug;33(2):512–518. doi: 10.1128/iai.33.2.512-518.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen M. J., Demko C. A., Wood R. E., Tandler B., Dearborn D. G., Boxerbaum B., Kuchenbrod P. J. Ultrastructure and function of alveolar macrophages from cystic fibrosis patients. Pediatr Res. 1980 May;14(5):715–721. doi: 10.1203/00006450-198005000-00003. [DOI] [PubMed] [Google Scholar]
- Winnie G. B., Klinger J. D., Sherman J. M., Thomassen M. J. Induction of phagocytic inhibitory activity in cats with chronic Pseudomonas aeruginosa pulmonary infection. Infect Immun. 1982 Dec;38(3):1088–1093. doi: 10.1128/iai.38.3.1088-1093.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]