Abstract
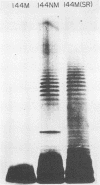
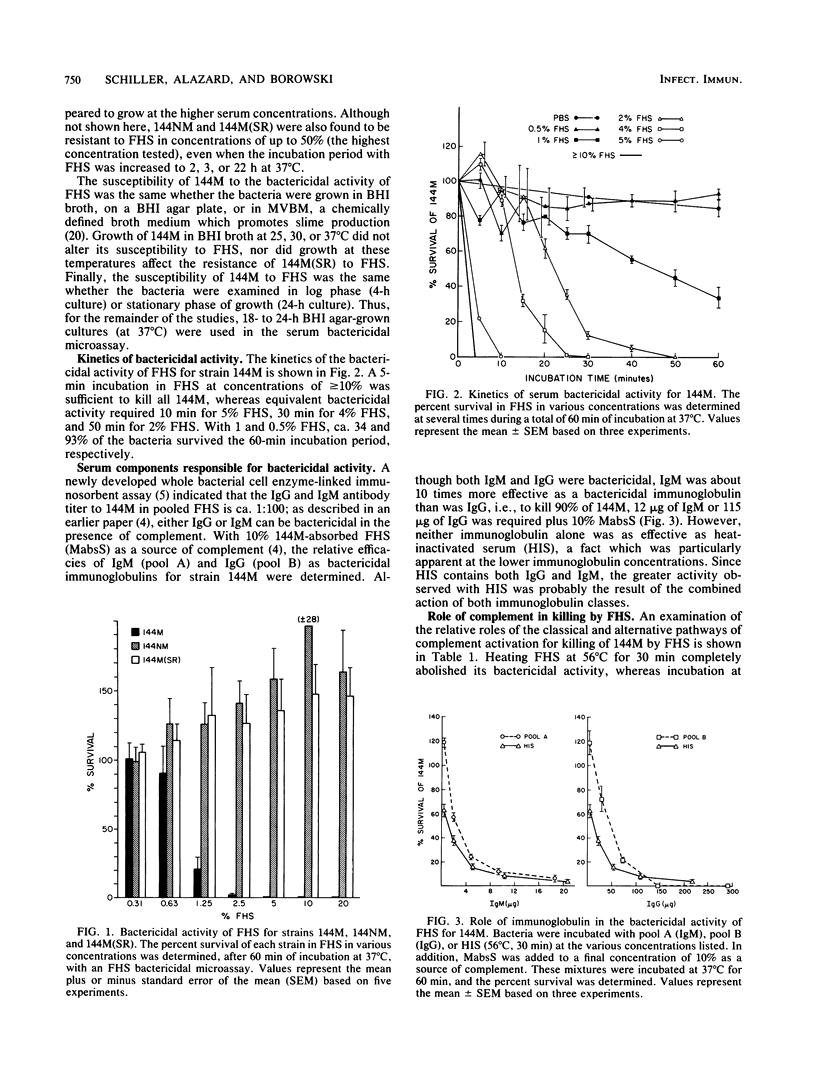
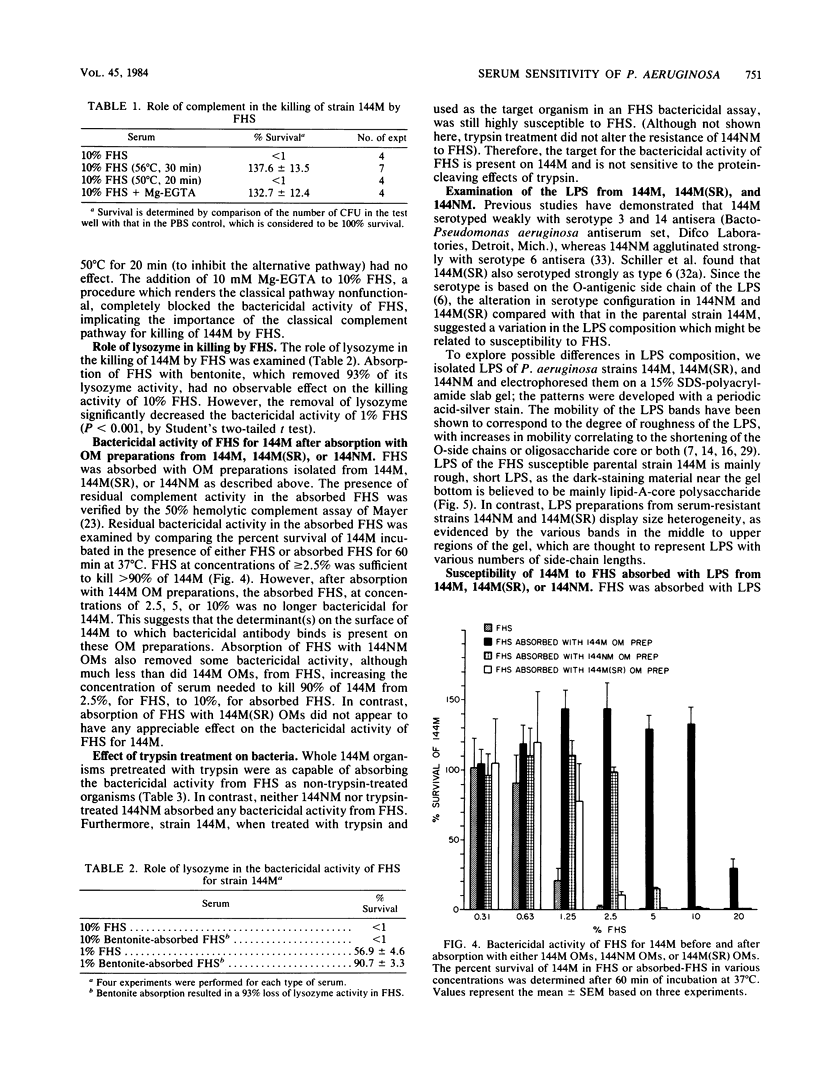
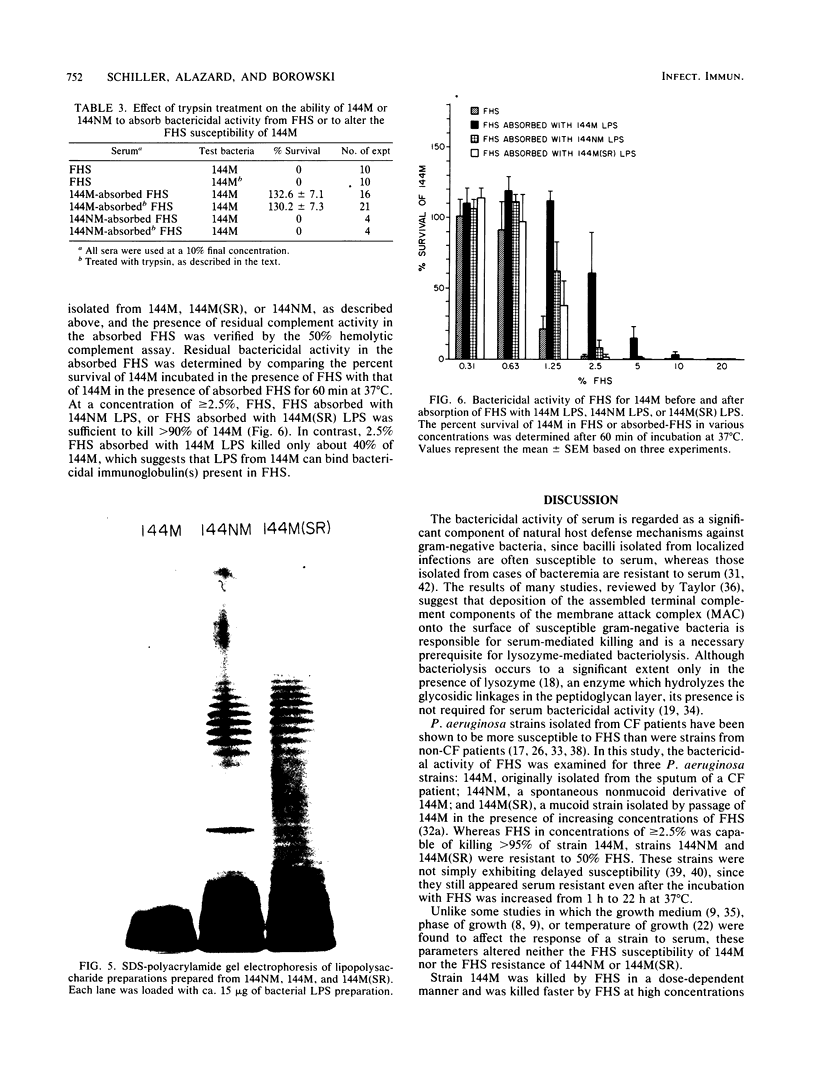
The susceptibility of Pseudomonas aeruginosa 144M (a mucoid strain isolated from the sputum of a cystic fibrosis patient) to the bactericidal activity of pooled fresh normal human serum (FHS) was examined. FHS at concentrations of greater than or equal to 2.5% was capable of killing greater than 95% of strain 144M. Strain 144M was killed by FHS in a dose-dependent manner. Although either immunoglobulin M (IgM) or IgG was bactericidal in the presence of complement, IgM was about 10 times as effective as IgG. However, optimal killing activity required both IgM and IgG and complement, activated by the classical pathway. A role for lysozyme in the killing of 144M was demonstrated only when low concentrations of FHS were used. In contrast to 144M, P. aeruginosa strains 144NM and 144M(SR) were totally resistant to FHS at all of the concentrations tested (up to 50%). Neither the FHS susceptibility of 144M nor the FHS resistance of 144NM or 144M(SR) was altered by choice of growth medium, growth phase, or temperature of growth. Results of absorption studies with whole organisms, isolated outer membrane preparations, or lipopolysaccharide (LPS) from each strain suggest that the antigen(s) which binds the bactericidal immunoglobulins is accessible on the surface of 144M but not on the surface of 144NM or 144M(SR), is insensitive to trypsin treatment, and is believed to be LPS. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the three LPS preparations demonstrated that 144M LPS contained primarily lipid-A-core polysaccharide components, whereas the LPS from 144NM and 144M(SR) were heterogeneous, with various degrees of O-side-chain substitution. These results suggest that at least one target for bactericidal antibody on the surface of 144M is contained in the rough LPS of this strain.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjornson A. B., Michael J. G. Biological Activities of Rabbit Immunoglobulin M and Immunoglobulin G Antibodies to Pseudomonas aeruginosa. Infect Immun. 1970 Oct;2(4):453–461. doi: 10.1128/iai.2.4.453-461.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P., Bolivar R., Fainstein V., Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- Borowski R. S., Stock L. M., Schiller N. L. Development of an enzyme-linked immunosorbent assay for studying Pseudomonas aeruginosa cell surface antigens. J Clin Microbiol. 1984 Jun;19(6):736–741. doi: 10.1128/jcm.19.6.736-741.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester I. R., Meadow P. M., Pitt T. L. The relationship between the O-antigenic lipopolysaccharides and serological specificity in strains of Pseudomonas aeruginosa of different O-serotypes. J Gen Microbiol. 1973 Oct;78(2):305–318. doi: 10.1099/00221287-78-2-305. [DOI] [PubMed] [Google Scholar]
- DAVIS S. D., WEDGWOOD R. J. KINETICS OF THE BACTERICIDAL ACTION OF NORMAL SERUM ON GRAM-NEGATIVE BACTERIA. J Immunol. 1965 Jul;95:75–79. [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMatteo C. S., Hammer M. C., Baltch A. L., Smith R. P., Sutphen N. T., Michelsen P. B. Susceptibility of Pseudomonas aeruginosa to serum bactericidal activity. A comparison of three methods with clinical correlations. J Lab Clin Med. 1981 Oct;98(4):511–518. [PubMed] [Google Scholar]
- Eidinger D., Bello E., Mates A. The heterocytotoxicity of human serum. I. Activation of the alternative complement pathway by heterologous target cells. Cell Immunol. 1977 Mar 1;29(1):174–186. doi: 10.1016/0008-8749(77)90286-6. [DOI] [PubMed] [Google Scholar]
- Fine D. P. Comparison of ethyleneglycoltetraacetic acid and its magnesium salt as reagents for studying alternative complement pathway function. Infect Immun. 1977 Apr;16(1):124–128. doi: 10.1128/iai.16.1.124-128.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAINES S., LANDY M. Prevalence of antibody to Pseudomonas in normal human sera. J Bacteriol. 1955 Jun;69(6):628–633. doi: 10.1128/jb.69.6.628-633.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A. A., Milne C. M. A kinetic study of the bacteriolytic and bactericidal action of human serum. Immunology. 1967 Jun;12(6):639–653. [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Chan L., Darveau R. P., Speert D. P., Pier G. B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983 Oct;42(1):170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holby N., Olling S. Pseudomonas aeruginosa infection in cystic fibrosis. Bactericidal effect of serum from normal individuals and patients with cystic fibrosis on P. aeruginosa strains from patients with cystic fibrosis or other diseases. Acta Pathol Microbiol Scand C. 1977 Apr;85(2):107–114. [PubMed] [Google Scholar]
- Inoue K., Yonemasu K., Takamizawa A., Amano T. [Studies on the immune bacteriolysis. XIV. Requirement of all nine components of complement for immune bacteriolysis]. Biken J. 1968 Sep;11(3):203–206. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lam J., Chan R., Lam K., Costerton J. W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 1980 May;28(2):546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHAEL J. G., ROSEN F. S. ASSOCIATION OF "NATURAL" ANTIBODIES TO GRAM-NEGATIVE BACTERIA WITH THE GAMMA-1-MACROGLOBULINS. J Exp Med. 1963 Oct 1;118:619–626. doi: 10.1084/jem.118.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R. J. Plasmid-mediated and temperature-regulated surface properties of Yersinia enterocolitica. Infect Immun. 1983 Sep;41(3):921–930. doi: 10.1128/iai.41.3.921-930.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshulam T., Verbrugh H., Verhoef J. Serum-induced lysis of Pseudomonas aeruginosa. Eur J Clin Microbiol. 1982 Feb;1(1):1–6. doi: 10.1007/BF02014132. [DOI] [PubMed] [Google Scholar]
- Muschel L. H., Ahl L. A., Fisher M. W. Sensitivity of Pseudomonas aeruginosa to normal serum and to polymyxin. J Bacteriol. 1969 May;98(2):453–457. doi: 10.1128/jb.98.2.453-457.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offredo-Hemmer C., Berche P., Véron M. A complement-sensitive mutant of Pseudomonas aeruginosa. Ann Microbiol (Paris) 1983 May-Jun;134A(3):281–294. [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Roantree R. J., Rantz L. A. A STUDY OF THE RELATIONSHIP OF THE NORMAL BACTERICIDAL ACTIVITY OF HUMAN SERUM TO BACTERIAL INFECTION. J Clin Invest. 1960 Jan;39(1):72–81. doi: 10.1172/JCI104029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller N. L., Hatch R. A. The serum sensitivity, colonial morphology, serogroup specificity, and outer membrane protein of Pseudomonas aeruginosa strains isolated from several clinical sites. Diagn Microbiol Infect Dis. 1983 Jun;1(2):145–157. doi: 10.1016/0732-8893(83)90044-5. [DOI] [PubMed] [Google Scholar]
- Schreiber R. D., Morrison D. C., Podack E. R., Müller-Eberhard H. J. Bactericidal activity of the alternative complement pathway generated from 11 isolated plasma proteins. J Exp Med. 1979 Apr 1;149(4):870–882. doi: 10.1084/jem.149.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W., Kroll H. P. Killing of an encapsulated strain of Escherichia coli by human serum. Infect Immun. 1983 Jan;39(1):122–131. doi: 10.1128/iai.39.1.122-131.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen M. J., Demko C. A. Serum bactericidal effect on Pseudomonas aeruginosa isolates from cystic fibrosis patients. Infect Immun. 1981 Aug;33(2):512–518. doi: 10.1128/iai.33.2.512-518.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub W. H., Acker G., Kleber I. Ultrastructural surface alterations of serratia marcescens after exposure to polymyxin B and/or fresh human serum. Chemotherapy. 1976;22(2):104–113. doi: 10.1159/000221919. [DOI] [PubMed] [Google Scholar]
- Traub W. H., Kleber I. Studies on the additive effect of polymyxin B and the bactericidal activity of human serum against Serratia marcescens. Chemotherapy. 1975;21(3-4):189–204. doi: 10.1159/000221860. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Vosti K. L., Randall E. Sensitivity of serologically classified strains of escherichia coli of human origin to the serum bactericidal system. Am J Med Sci. 1970 Feb;259(2):114–119. doi: 10.1097/00000441-197002000-00005. [DOI] [PubMed] [Google Scholar]
- WARDLAW A. C. The complement-dependent bacteriolytic activity of normal human serum. I. The effect of pH and ionic strength and the role of lysozyme. J Exp Med. 1962 Jun 1;115:1231–1249. doi: 10.1084/jem.115.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Levine R. P. How complement kills E. coli. I. Location of the lethal lesion. J Immunol. 1981 Sep;127(3):1146–1151. [PubMed] [Google Scholar]
- Wright S. D., Levine R. P. How complement kills E. coli. II. The apparent two-hit nature of the lethal event. J Immunol. 1981 Sep;127(3):1152–1156. [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Human immunity to Pseudomonas aeruginosa. I. In-vitro interaction of bacteria, polymorphonuclear leukocytes, and serum factors. J Infect Dis. 1972 Sep;126(3):257–276. doi: 10.1093/infdis/126.3.257. [DOI] [PubMed] [Google Scholar]
- Young L. S. Human immunity to Pseudomonas aeruginosa. II. Relationship between heat-stable opsonins and type-specific lipopolysaccharides. J Infect Dis. 1972 Sep;126(3):277–287. doi: 10.1093/infdis/126.3.277. [DOI] [PubMed] [Google Scholar]