Abstract
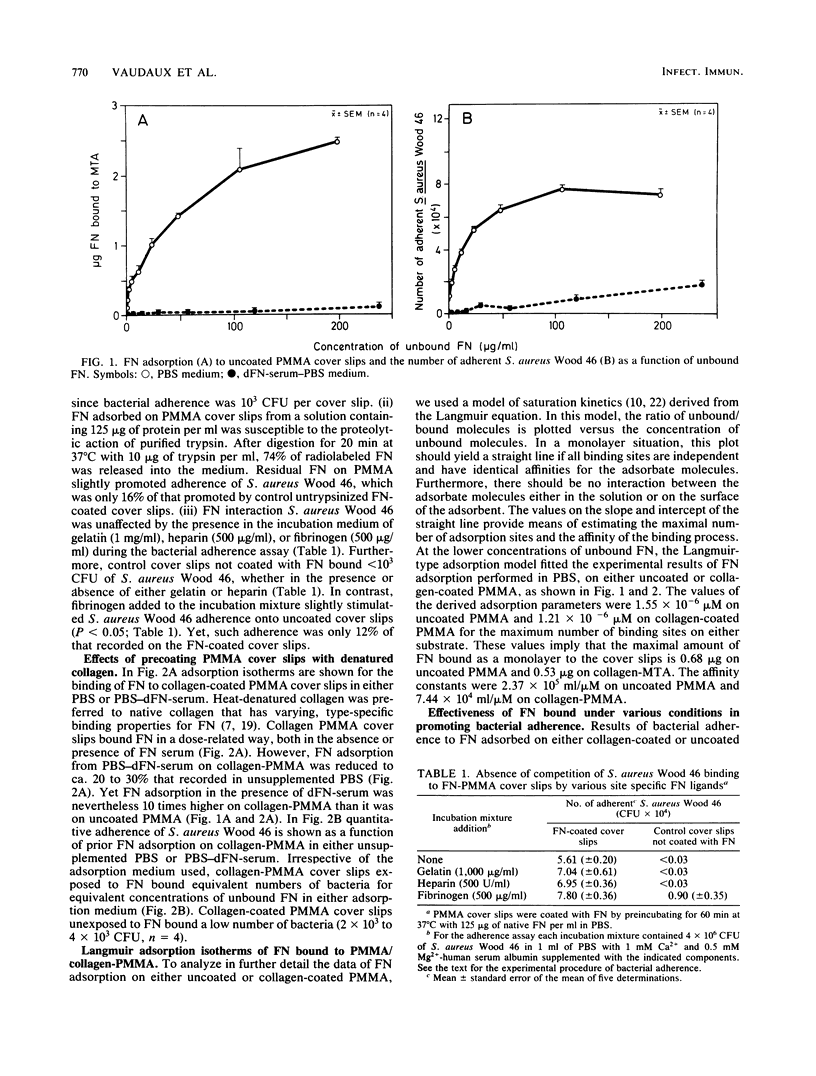
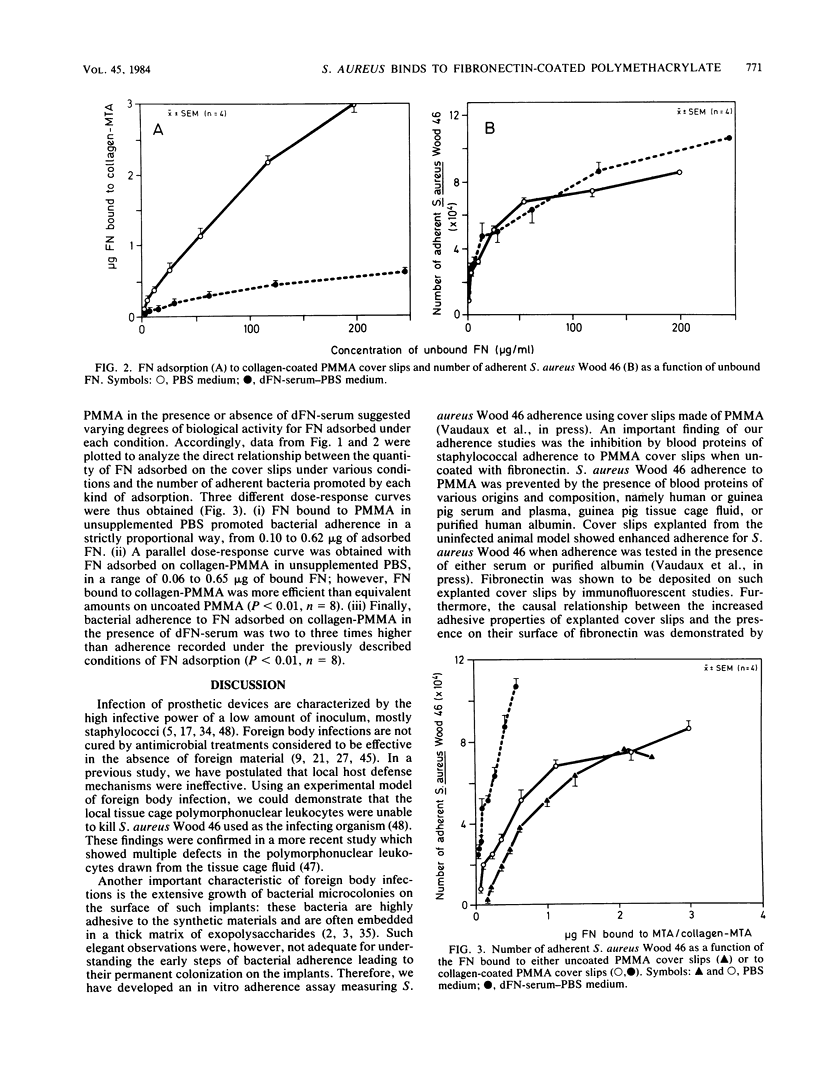
Recent data suggest that fibronectin may favor Staphylococcus aureus infection by promoting attachment to either injured tissues or implanted foreign bodies. We studied the quantitative adsorption of fibronectin onto polymethylmethacrylate (PMMA) cover slips by using a 125I-labeled preparation of the purified plasma glycoprotein. Fibronectin in buffer solutions showed a high affinity to PMMA coverslips. Adherence of S. aureus Wood 46 was studied on PMMA pre-exposed to fibronectin, using an assay specifically adapted to the cover slip model. Whereas S. aureus adherence in an albumin-containing buffer was less than or equal to 10(3) CFU on control uncoated cover slips, adherence in the same medium increased up to maximum of 7.7 X 10(4) CFU on cover slips preincubated in a solution of fibronectin (125-micrograms/ml). At intermediate fibronectin concentrations, bacterial adherence was a linear function of both the quantity in solution and of the quantity adsorbed on the PMMA cover slips. The presence of human serum proteins, as represented by a fibronectin-depleted pool, essentially prevented adsorption of radiolabeled fibronectin on PMMA and subsequent bacterial adherence on the cover slips. Precoating of PMMA with denatured collagen resulted in increased fibronectin adsorption on PMMA, even in the presence of serum proteins, and S. aureus adherence was optimal on such surfaces. Collagen may therefore play a role as a cofactor contributing to S. aureus adherence onto fibronectin-coated substrata or foreign bodies.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton B. T., Hynes R. O. A difference between plasma and cellular fibronectins located with monoclonal antibodies. Cell. 1981 Jul;25(1):133–141. doi: 10.1016/0092-8674(81)90237-3. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982 Jul;37(1):318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Geesey G. G., Cheng K. J. How bacteria stick. Sci Am. 1978 Jan;238(1):86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- Doran J. E., Raynor R. H. Fibronectin binding to protein A-containing staphylococci. Infect Immun. 1981 Sep;33(3):683–689. doi: 10.1128/iai.33.3.683-689.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELEK S. D., CONEN P. E. The virulence of Staphylococcus pyogenes for man; a study of the problems of wound infection. Br J Exp Pathol. 1957 Dec;38(6):573–586. [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E., Miller E. J. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978 Jun 1;147(6):1584–1595. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espersen F., Clemmensen I. Isolation of a fibronectin-binding protein from Staphylococcus aureus. Infect Immun. 1982 Aug;37(2):526–531. doi: 10.1128/iai.37.2.526-531.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald R. H., Jr, Peterson L. F., Washington J. A., 2nd, Van Scoy R. E., Coventry M. B. Bacterial colonization of wounds and sepsis in total hip arthroplasty. J Bone Joint Surg Am. 1973 Sep;55(6):1242–1250. [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Spinell D. M. Model delineating the effects of a salivary pellicle on the adsorption of Streptococcus miteor onto hydroxyapatite. Infect Immun. 1976 Oct;14(4):1109–1112. doi: 10.1128/iai.14.4.1109-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Cellular adhesiveness and extracellular substrata. Int Rev Cytol. 1978;53:65–144. doi: 10.1016/s0074-7696(08)62241-x. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Feld M. K. Adsorption characteristics of plasma fibronectin in relationship to biological activity. J Biomed Mater Res. 1981 May;15(3):363–381. doi: 10.1002/jbm.820150308. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Feld M. K. Fibronectin adsorption on hydrophilic and hydrophobic surfaces detected by antibody binding and analyzed during cell adhesion in serum-containing medium. J Biol Chem. 1982 May 10;257(9):4888–4893. [PubMed] [Google Scholar]
- Hahn L. H., Yamada K. M. Isolation and biological characterization of active fragments of the adhesive glycoprotein fibronectin. Cell. 1979 Dec;18(4):1043–1051. doi: 10.1016/0092-8674(79)90217-4. [DOI] [PubMed] [Google Scholar]
- Hedman K., Johansson S., Vartio T., Kjellén L., Vaheri A., Hök M. Structure of the pericellular matrix: association of heparan and chondroitin sulfates with fibronectin-procollagen fibers. Cell. 1982 Mar;28(3):663–671. doi: 10.1016/0092-8674(82)90221-5. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMES R. C., MACLEOD C. J. Induction of staphylococcal infections in mice with small inocula introduced on sutures. Br J Exp Pathol. 1961 Jun;42:266–277. [PMC free article] [PubMed] [Google Scholar]
- Klebe R. J., Bentley K. L., Schoen R. C. Adhesive substrates for fibronectin. J Cell Physiol. 1981 Dec;109(3):481–488. doi: 10.1002/jcp.1041090314. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., McGoodwin E. B., Martin G. R., Klebe R. J., Fietzek P. P., Woolley D. E. Localization of the binding site for cell attachment in the alpha1(I) chain of collagen. J Biol Chem. 1978 Aug 25;253(16):5642–5646. [PubMed] [Google Scholar]
- Kloster F. E. Complications of artificial heart valves. JAMA. 1979 May 18;241(20):2201–2203. [PubMed] [Google Scholar]
- Kronvall G., Quie P. G., Williams R. C., Jr Quantitation of staphylococcal protein A: Determination of equilibrium constant and number of protein A residues on bacteria. J Immunol. 1970 Feb;104(2):273–278. [PubMed] [Google Scholar]
- Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978 Dec 14;276(5689):718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanser M. E., Saba T. M. Fibronectin as a co-factor necessary for optimal granulocyte phagocytosis of Staphylococcus aureus. J Reticuloendothel Soc. 1981 Nov;30(5):415–424. [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. A., Broekelmann T. J., Kelley D. G., Villiger B. Gelatin-binding domain-specific anti-human plasma fibronectin Fab' inhibits fibronectin-mediated gelatin binding but not cell spreading. J Biol Chem. 1981 Jun 10;256(11):5583–5587. [PubMed] [Google Scholar]
- Mosesson M. W., Amrani D. L. The structure and biologic activities of plasma fibronectin. Blood. 1980 Aug;56(2):145–158. [PubMed] [Google Scholar]
- Mosher D. F. Fibronectin. Prog Hemost Thromb. 1980;5:111–151. [PubMed] [Google Scholar]
- Mosher D. F., Proctor R. A. Binding and factor XIIIa-mediated cross-linking of a 27-kilodalton fragment of fibronectin to Staphylococcus aureus. Science. 1980 Aug 22;209(4459):927–929. doi: 10.1126/science.7403857. [DOI] [PubMed] [Google Scholar]
- Noble W. C. The production of subcutaneous staphylococcal skin lesions in mice. Br J Exp Pathol. 1965 Jun;46(3):254–262. [PMC free article] [PubMed] [Google Scholar]
- Peters G., Locci R., Pulverer G. Microbial colonization of prosthetic devices. II. Scanning electron microscopy of naturally infected intravenous catheters. Zentralbl Bakteriol Mikrobiol Hyg B. 1981;173(5):293–299. [PubMed] [Google Scholar]
- Pierschbacher M. D., Hayman E. G., Ruoslahti E. Location of the cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell. 1981 Oct;26(2 Pt 2):259–267. doi: 10.1016/0092-8674(81)90308-1. [DOI] [PubMed] [Google Scholar]
- Proctor R. A., Hamill R. J., Mosher D. F., Textor J. A., Olbrantz P. J. Effects of subinhibitory concentrations of antibiotics on Staphylococcus aureus interactions with fibronectin. J Antimicrob Chemother. 1983 Oct;12 (Suppl 100):85–95. doi: 10.1093/jac/12.suppl_c.85. [DOI] [PubMed] [Google Scholar]
- Proctor R. A., Prendergast E., Mosher D. F. Fibronectin mediates attachment of Staphylococcus aureus to human neutrophils. Blood. 1982 Apr;59(4):681–687. [PubMed] [Google Scholar]
- Rydén C., Rubin K., Speziale P., Hök M., Lindberg M., Wadström T. Fibronectin receptors from Staphylococcus aureus. J Biol Chem. 1983 Mar 10;258(5):3396–3401. [PubMed] [Google Scholar]
- Vaheri A., Mosher D. F. High molecular weight, cell surface-associated glycoprotein (fibronectin) lost in malignant transformation. Biochim Biophys Acta. 1978 Sep 18;516(1):1–25. doi: 10.1016/0304-419x(78)90002-1. [DOI] [PubMed] [Google Scholar]
- Van de Water L., Destree A. T., Hynes R. O. Fibronectin binds to some bacteria but does not promote their uptake by phagocytic cells. Science. 1983 Apr 8;220(4593):201–204. doi: 10.1126/science.6338594. [DOI] [PubMed] [Google Scholar]
- Verbrugh H. A., Peterson P. K., Smith D. E., Nguyen B. Y., Hoidal J. R., Wilkinson B. J., Verhoef J., Furcht L. T. Human fibronectin binding to staphylococcal surface protein and its relative inefficiency in promoting phagocytosis by human polymorphonuclear leukocytes, monocytes, and alveolar macrophages. Infect Immun. 1981 Sep;33(3):811–819. doi: 10.1128/iai.33.3.811-819.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti G. M., Lussenhop D., Peterson P. K., Furcht L. T., McCarthy J. B., Jacob H. S., Moldow C. F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984 Jan;103(1):34–43. [PubMed] [Google Scholar]
- Wilson P. D., Jr, Salvati E. A., Aglietti P., Kutner L. J. The problem of infection in endoprosthetic surgery of the hip joint. Clin Orthop Relat Res. 1973 Oct;(96):213–221. [PubMed] [Google Scholar]
- Zardi L., Siri A., Carnemolla B., Cosulich E., Viale G., Santi L. A simplified procedure for the preparation of antibodies to serum fibronectin. J Immunol Methods. 1980;34(2):155–165. doi: 10.1016/0022-1759(80)90169-6. [DOI] [PubMed] [Google Scholar]
- Zimmerli W., Lew P. D., Waldvogel F. A. Pathogenesis of foreign body infection. Evidence for a local granulocyte defect. J Clin Invest. 1984 Apr;73(4):1191–1200. doi: 10.1172/JCI111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli W., Waldvogel F. A., Vaudaux P., Nydegger U. E. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982 Oct;146(4):487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]


