Abstract
Arbuscular mycorrhizal (AM) fungi are mutualistic symbionts living in the roots of 80% of land plant species, and developing extensive, below-ground extraradical hyphae fundamental for the uptake of soil nutrients and their transfer to host plants. Since AM fungi have a wide host range, they are able to colonize and interconnect contiguous plants by means of hyphae extending from one root system to another. Such hyphae may fuse due to the widespread occurrence of anastomoses, whose formation depends on a highly regulated mechanism of self recognition. Here, we examine evidences of self recognition and non-self incompatibility in hyphal networks formed by AM fungi and discuss recent results showing that the root systems of plants belonging to different species, genera and families may be connected by means of anastomosis formation between extraradical mycorrhizal networks, which can create indefinitely large numbers of belowground fungal linkages within plant communities.
Key Words: arbuscular mycorrhizal symbiosis, extraradical mycelium, anastomosis, plant interconnectedness, self recognition, non-self incompatibility, mycorrhizal networks
Introduction
Most terrestrial plant species establish mutualistic symbioses with arbuscular mycorrhizal (AM) fungi, which develop extensive, belowground extraradical hyphae fundamental for the uptake of nutrients from soil and their transfer to the host plant.1,2 Since AM fungi have a wide host range, they are able to colonize and interconnect plants of different species, genera and families, by means of hyphae extending from one root system to another. Such mycorrhizal networks, first visualized and quantified in vivo by means of two-dimensional experimental systems, spread from colonized roots into the surrounding environment at growth rates ranging from 738 to 1067 mm per day, depending on the host plant, and reach hyphal extent of 10–40 mm per mm of root length.3 Moreover, AM extraradical networks may be interconnected by means of the widespread occurrence of anastomoses, whose formation depends on a highly regulated mechanism of self recognition between compatible hyphae. Successful anastomoses occur between hyphae belonging to the same individual and to different individuals of the same isolate, during the presymbiotic growth of AM fungi.4 By contrast, hyphae of individuals belonging to different genera and species, and even to geographic isolates of the same species, are unable to fuse, and show rejection responses, either before or after contact, revealing AM hyphal ability to discriminate against non-self.5 Extraradical mycorrhizal networks maintain the capacity of self-recognition, evidenced by the high frequency of anastomosis between hyphae originating from the same and different root systems colonized by a single AM fungal isolate.6
Here, we discuss recent advances in the study of self recognition and non-self incompatibility in hyphal networks formed by AM fungal germlings during the presymbiotic stage of their life cycle. We review evidences for the characterization of true anastomoses—i.e., complete fusions of hyphal walls, cytoplasmic flow and migration of nuclei through hyphal bridges—and for the detection of incompatibility responses—i.e., protoplasm retraction from hyphal tips and septum formation in approaching hyphae, even before physical contact—as revealed by time-lapse, video-enhanced and epifluorescence microscopy.
Finally, we discuss recent results showing that the root systems of plants belonging to different species, genera and families may become linked by means of anastomosis formation between mycorrhizal networks, which can create indefinitely large numbers of fungal linkages connecting together many plants in a community.
Evidence for the Existence of Anastomosis in Presymbiotic Mycelial Networks of AM Fungi
Although anastomoses have been extensively studied in vegetative hyphae of Ascomycota and Basidiomycota,7,8 they are believed to be lacking or rare in other fungal phyla.9,10 A few works reported sporadic observations of their occurrence in AM fungi, without giving any quantitative data on the frequency of hyphal fusions in the different isolates or on the cytological events involved.11–14
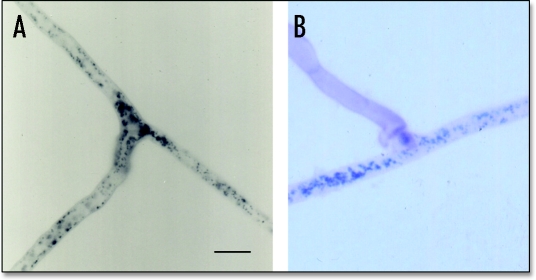
The first extensive study on anastomosis in AM fungi reported data on fusions of hyphae belonging to the same isolate in different species of the genus Glomus, by using a combination of time-lapse and video-enhanced light microscopy, image analysis, and epifluorescence microscopy.4 Protoplasmic continuity, the characteristic feature of successful hyphal fusions, was evidenced by the complete disappearance of hyphal walls and visualized by histochemical localization of formazan salts in hyphal fusions, after SDH (succinate dehydrogenase activity) staining (Fig. 1A). Time-course experiments showed that hyphal tips were able to fuse with hyphae growing nearby in about 35 min, and that a bidirectional flow of particles (vacuoles, mitochondria, nuclei, and fat droplets) moved at the speed of 1.8 ± 0.06 µm/s through hyphal bridges formed during anastomosis.4,15
Figure 1.
Light micrographs showing self recognition (A) and non-self incompatibility (B) between AM fungal hyphae. (A) Visualization of complete fusions of hyphal walls and protoplasmic continuity, evidenced by formazan salt depositions in hyphal bridges (succinate dehydrogenase activity, SDH) in two compatible hyphae of the AM fungus Glomus mosseae. (B) Incompatible interaction between hyphae of two geographically different isolates of the AM fungus Glomus mosseae, visualised after SDH and Trypan blue staining, showing protoplasm withdrawal and septum formation in the approaching hypha (isolate IN101C) after contact with a branch initial (isolate SY710). Scale bar = 10 µm.
The established protoplasmic flow was further demonstrated by the detection of nuclei in hyphal bridges, evidenced by DAPI (diamidinophenylindole) staining. Nuclear migration occurred between hyphae belonging to the same germling and to different germlings of the same AM fungal isolate, in three different Glomus species, G. caledonium, G. intraradices, G. mosseae.4 The ability of self compatible hyphae to fuse and exchange nuclei is of critical importance for the maintenance of genetic continuity within AM fungi, which are considered clonal organisms.16 Since they produce multinucleate spores, containing 1,000 to 5,000 nuclei each,17 and have been shown to be multigenomic,18,19 nuclear exchange during anastomosis within the same germling and between different germlings of the same isolate could represent a means for the maintenance of isolate genetic diversity, in the absence of sexual recombination.4,20,21
The frequency of anastomosis formation between contacting hyphae originating from the same germling or from different germlings of the same isolate ranged from 34% to 90%, in G. caledonium and G. intraradices, respectively.4 Similar results were found in other studies carried out on geographic isolates of G. mosseae originating from Europe (France and United Kingdom), USA (Arizona and Indiana) and Middle East (Syria), where anastomosis frequency ranged from 60% in the UK isolate IMA1 to 85% in the Arizona isolate AZ225C.5 Such values were obtained on total hyphal contacts ranging from 91 to 242, which are relatively high numbers, given the inability of AM fungi to grow extensively in the absence of a host plant.22–24
In the experimental data, mycelial length of each germling varied with the different isolates, from 34.5 ± 3.5 mm in the French isolate BEG69 to 119.5 ± 14.4 mm in the UK isolate IMA1. It is interesting to note that anastomosis densities detected in AM fungi, unable to grow saprophytically, ranged from 0.62 ± 0.06 to 1.3 ± 0.23 per cm of hyphal length, values comparable with those reported for the saprophytic fungi Rhizoctonia solani and Gibberella fujikuroi.25–27
Interactions between hyphae belonging to the same germling of AM fungal species of the genera Gigaspora and Scutellospora did never lead to anastomosis formation. In fact, no fusions were found over 220 hyphal contacts in G. rosea and over 460 hyphal contacts in S. castanea.4 These data were confirmed by other works, carried out in in vitro monoxenic cultures on mycelium spreading from Ri T-DNA transformed carrot roots, where no anastomoses were detected among main hyphae (runner hyphae) of Scutellospora reticulata, while only 1% of fusions was found in branching absorbing structures.28 Interestingly, the most important mechanism allowing fungal mycelium to become interconnected was represented by wound healing between broken hyphae, previously described by Gerdemann.29 Further studies, aimed at comparing the different anastomosis ability of two phylogenetically distant AM fungal families, Glomeraceae and Gigasporaceae, confirmed their fundamental diversity in mycelial developmental structure.30
Evidence for Non-Self Incompatibility in Presymbiotic Mycelial Networks of AMF
When hyphae originating from different species or genera of AM fungi come into contact, no anastomoses are formed.4,13 Different intergeneric and interspecific hyphal pairings yielded zero fusions over large numbers of contacts, ranging from 90 in the pairing G. mosseae-G. caledonium to 140 in G. mosseae-G. rosea and 232 in G. caledonium-G. rosea. Interestingly, hyphal interactions lead to different responses, ranging from no interference—i.e., hyphal intermingling—to the formation of hyphal swellings which become empty and septate after the failure of anastomosis formation. These findings, suggesting that AM fungi can recognize self entities and discriminate self from non-self, opened the way to tests of vegetative compatibility, already used for the identification of genetically different isolates of pathogenic, saprophytic and ectomycorrhizal fungi.8,31–35 Such tests, carried out on geographically different isolates of G. mosseae, showed that hyphal interactions between different isolates do never produce anastomosis, suggesting their genetic isolation. Accordingly, hyphae intermingled without any response in 49–68% of contacts, while developed incompatibility reactions in 32–51% of hyphal contacts, in the different pairings. Incompatibility responses were consistent with those detected in hyphae belonging to different genera and species after physical contact, and were characterized by hyphal swellings, vacuolization, localized wall thickenings, protoplasm withdrawal, retraction septa formation and hyphal lysis (Fig. 1B),5 and comparable to postfusion incompatibility events reported in other fungi.7,8,36–39 The strong genetic barriers to hyphal fusions exhibited by G. mosseae isolates of different geographic origins could have the function of hindering heterokaryon formation between genetically different mycelia, thus permitting the maintenance of the fittest gene combinations. Moreover, such barriers may prevent the exchange of cytoplasm and the spread of harmful genetic elements.8,40
The major evidence for the existence of a highly regulated system of self recognition and non-self discrimination in AM fungi was represented by the detection of precontact tropism and the formation of hyphal swellings and consecutive retraction septa prior to any physical contact between neighboring hyphae.5 The occurrence of hyphal tropism, previously studied also in other fungal species, Phanerochaete velutina and Stereum spp.,7,36 suggests that specific recognition signals, released by interacting hyphae, are involved in interhyphal attraction and in the regulation of hyphal fusion.32,41 Nevertheless, the nature of the specific compounds acting as signals for self recognition and non-self discrimination in AM fungi remains to be unravelled.
Visualization of Intact Mycelial Networks Spreading from Roots Colonized by AMF
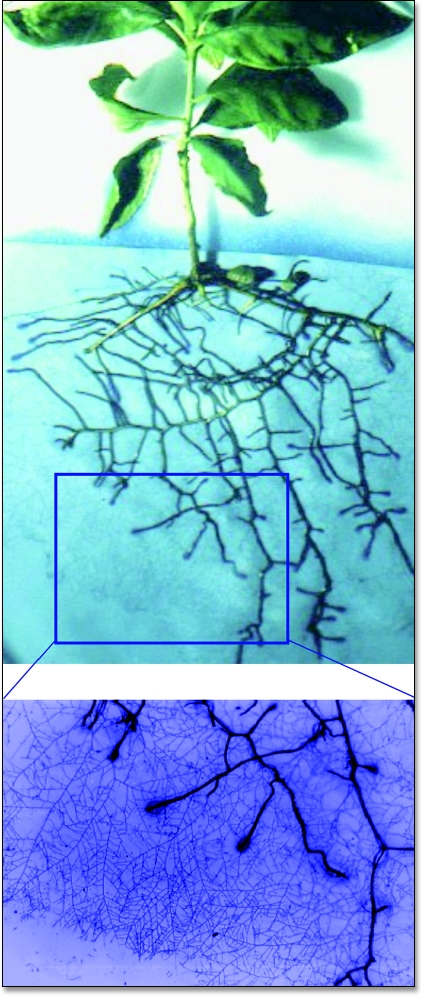
The most important AM fungal structure for plant nutrition is represented by the extraradical mycelium spreading from mycorrhizal roots into the surrounding soil, which is able to uptake mineral nutrients—N, P, S, Ca, K, Fe, Cu, Zn—and to transfer them to root cells.1,42–44 Mycorrhizal mycelium has been investigated in different experimental studies, based on either destructive extraction from soil or root observation chambers or in vitro systems, which yielded only qualitative data on its structure and growth.45–48 The first visualization of intact AM mycelium extending from mycorrhizal roots into the extraradical environment was obtained by means of a bidimensional model system which utilized two cellulose esters membranes “sandwiched” around the roots of individuals plantlets (Fig. 2). After only seven days' growth, a fine network of extramatrical hyphae growing on the membranes was visible to the naked eye, and its length extended from 5169 to 7471 mm (hyphal length), in Thymus vulgaris and Allium porrum, respectively (Fig. 3).3 In order to understand the fundamental role played by extraradical mycelium in nutrient uptake and translocation, it is interesting to calculate hyphal length per total root length, which reaches 40.2 mm mm−1 in A. porrum, and the mean growth rate, which ranges from 738 to 1067 mm per day, depending on the host plant. Such data are comparable with the higher values of hyphal densities previously detected by using destructive extraction from soil, which were much variable, ranging from 1.6 to 1420 mm of hyphal length per mm of root.49–52
Figure 2.
Visualisation of intact extraradical networks produced by the AM fungal species Glomus mosseae, spreading from mycorrhizal roots of Prunus cerasifera and uniformly colonizing the surrounding environment.
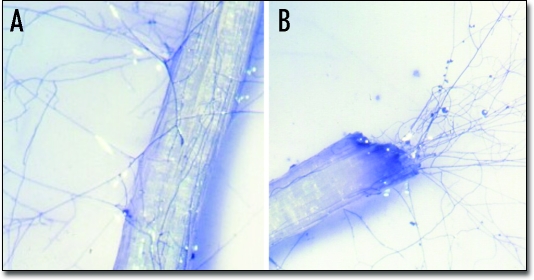
Figure 3.
Visualization under the dissecting microscope (40×) of Glomus mosseae extraradical hyphae spreading from intact (A) and cut (B) mycorrhizal roots of Allium porrum.
The experimental system deviced to visualize the mycorrhizal mycelium also evidenced that the mechanism allowing the formation of the network was self recognition and hyphal anastomosis. Since AM fungal hyphae showed many branches (8.6–9.7 cm−1) the number of anastomoses per cm of hypha was very high (4.6–5.1), as well as their frequency, 75–78% of hyphal contact (Table 1). The frequency of anastomosis was higher in extraradical mycelium (symbiotic) than in presymbiotic mycelium of AM fungi or than in self-anastomosing isolates of Rhizoctonia solani.4,5,25
Table 1.
Extension and interconnectedness of extraradical mycelial networks produced by AM fungi living in symbioses with different plant species
| Plant Species/Fungal Species | Hyphal Density (mm mm−2) | No. of Anastomoses Per Hyphal Length (cm) | Anastomosis Frequency (%) | Ref. |
| Allium porrum/Glomus mosseae | 2.7 | 4.6 | 75.0 | 3 |
| Allium porrum/Glomus mosseae | 3.5 | 3.8 | 59.3 | 6 |
| Daucus carota/Gigaspora margarita* | - | 0.0075 | 9.8 | 30 |
| Daucus carota/Gigaspora rosea* | - | 0.012 | 4.2 | 30 |
| Daucus carota/Glomus hoi* | - | 0.057 | 100 | 30 |
| Daucus carota/Glomus intraradices* | - | 0.076 | 100 | 30 |
| Daucus carota/Glomus mosseae | 3.9 | 2.5 | 45.5 | 6 |
| Daucus carota/Glomus proliferum* | - | 0.066 | 100 | 30 |
| Daucus carota/Scutellospora reticulata* | - | 0.0079 | 5.2 | 30 |
| Gossypium hirsutum/Glomus mosseae | 6.8 | 6.2 | 53.1 | 6 |
| Lactuca sativa/Glomus mosseae | 2.9 | 3.0 | 63.8 | 6 |
| Petroselinum crispum/Glomus caledonium | 3.8 | 1.5 | 18.6 | § |
| Petroselinum crispum/Glomus intraradices | 2.3 | 5.5 | 56.9 | § |
| Petroselinum crispum/Glomus mosseae | 3.5 | 4.7 | 62.3 | § |
| Prunus cerasifera/Glomus mosseae | 2.4 | 5.1 | 64.0 | 3 |
| Solanum melongena/Glomus mosseae | 4.1 | 2.1 | 47.0 | 6 |
| Thymus vulgaris/Glomus mosseae | 2.1 | 5.1 | 78.0 | 3 |
Ri T-DNA transformed carrot roots.
Unpublished results.
It is important to stress that the viability of the mycorrhizal network was 100% and that all the anastomoses showed protoplasmic continuity and nuclear occurrence in hyphal bridges, confirming the occurrence of nuclear exchange also during fusions between extraradical (symbiotic) hyphae.
Visualization of Belowground Interconnections between Plants of Different Species, Genera and Families
AM fungi have been reported to be active in mediating nutrient transfer among plants,53–58 mainly through the extensive mycelial networks, which, due to the lack of host specificity, may link the roots of contiguous plant species.57,59,60 Recent studies showed a novel mechanism by which plants may become interconnected, that is hyphal fusions between extraradical hyphae originating from different individual plant root systems of different species, genera and families.6
The bi-dimensional experimental system utilized allowed the visualization and quantification of fusions between mycorrhizal networks spreading from Allium porrum (leek) root systems—after inoculation with the AM symbiont Glomus mosseae—and those originating from Daucus carota (carrot), Gossypium hirsutum (cotton), Lactuca sativa (lettuce), Solanum melongena (eggplant). The use of plants belonging to different species allowed the detection of a host plant effect on the development of extraradical mycelium, since hyphal density in cotton was 6.8 mm mm−2, a value statistically different from those of all the other plant species, which ranged from 2.9 to 4.1 mm mm−2 in lettuce and eggplant, respectively (Table 1). Cotton was also the species which showed the highest interconnectedeness in the mycorrhizal network: the number of anastomoses per mm of hyphal length was 0.62 compared to values ranging from 0.21 to 0.38 of the other species.
The frequency of anastomoses between mycorrhizal networks originating from the different plant species was very high, ranging from 44% in the pairing leek-eggplant to 49% in the pairing leek-cotton, even though lower than that between networks spreading from the same species, leek (62%).
The occurrence of true anastomoses was verified by means of SDH and DAPI stainings: formazan salt depositions and nuclei were detected in the middle of hyphal bridges connecting different mycorrhizal networks, whereas no hyphal incompatibility reactions were found in interactions between hyphae connecting different mycorrhizal networks.
The high rate of anastomosis formation between extraradical hyphae spreading from the root systems of different plants suggests that plant interconnectedness may be greater than previously thought. Accordingly, due to the wide host range of AM fungi, mycorrhizal mycelium could give rise to an indefinitely large network of hyphae interconnecting contiguous plants, representing a major factor in the distribution of resources in plant communities.56,57,61,62 The bi-dimensional experimental system deviced for visualizing the structure of the mycorrhizal network could be further implemented, to detect and quantify nutrient and carbon transfer in the “soil food web”.63–66
Acknowledgements
This work was supported by funds from the University of Pisa (Italy) and by C.N.R. (National Research Council, Italy).
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=2227
References
- 1.Smith SE, Read DJ. Mycorrhizal symbiosis. London, UK: Academic Press; 1997. [Google Scholar]
- 2.Giovannetti M, Avio L. Biotechnology of arbuscular mycorrhizas. In: Khachatourians GG, Arora DK, editors. Appied mycology and biotechnology. Vol. 2 Agriculture and food production. Amsterdam, NL: Elsevier; 2002. pp. 275–310. [Google Scholar]
- 3.Giovannetti M, Fortuna P, Citernesi AS, Morini S, Nuti MP. The occurrence of anastomosis formation and nuclear exchange in intact arbuscular mycorrhizal networks. New Phytol. 2001;151:717–724. doi: 10.1046/j.0028-646x.2001.00216.x. [DOI] [PubMed] [Google Scholar]
- 4.Giovannetti M, Azzolini D, Citernesi AS. Anastomosis formation and nuclear and protoplasmic exchange in arbuscular mycorrhizal fungi. Appl Environ Microbiol. 1999;65:5571–5575. doi: 10.1128/aem.65.12.5571-5575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giovannetti M, Sbrana C, Strani P, Agnolucci M, Rinaudo V, Avio L. Genetic diversity of geographically different isolates of Glomus mosseae detected by vegetative compatibility and biochemical and molecular analysis. Appl Environ Microbiol. 2003;69:616–624. doi: 10.1128/AEM.69.1.616-624.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giovannetti M, Sbrana C, Avio L, Strani P. Patterns of below-ground plant interconnections established by means of arbuscular mycorrhizal networks. New Phytol. 2004;164:175–181. doi: 10.1111/j.1469-8137.2004.01145.x. [DOI] [PubMed] [Google Scholar]
- 7.Ainsworth AM, Rayner ADM. Responses of living hyphae associated with self and nonself fusions in the basidiomycete Phanerochaete velutina. J Gen Microbiol. 1986;132:191–201. [Google Scholar]
- 8.Leslie JF. Fungal vegetative compatibility. Annu Rev Phytopathol. 1993;31:127–150. doi: 10.1146/annurev.py.31.090193.001015. [DOI] [PubMed] [Google Scholar]
- 9.Gregory PH. The fungal mycelium — An historical perspective. In: Jennings DH, Rayner ADM, editors. The ecology and physiology of the fungal mycelium. Cambridge, UK: Cambridge University Press; 1984. pp. 1–22. [Google Scholar]
- 10.Carlile MJ. The success of the hypha and mycelium. In: Gow NAR, Gadd GM, editors. The Growing Fungus. London, UK: Chapman and Hall; 1995. pp. 3–19. [Google Scholar]
- 11.Godfrey RM. Studies on British species of Endogone. III. Germination of spores. Trans Br Mycol Soc. 1957;40:203–210. [Google Scholar]
- 12.Mosse B. The regular germination of resting spores and some observations on the growth requirements of an Endogone sp. causing vesicular-arbuscular mycorrhiza. Trans Br Mycol Soc. 1959;42:273–286. [Google Scholar]
- 13.Tommerup IC. The vesicular-arbuscular mycorrhizas. Adv Plant Pathol. 1988;6:81–91. [Google Scholar]
- 14.Giovannetti M, Sbrana C, Avio L, Citernesi AS, Logi C. Differential hyphal morphogenesis in arbuscular mycorrhizal fungi during preinfection stages. New Phytol. 1993;125:587–593. doi: 10.1111/j.1469-8137.1993.tb03907.x. [DOI] [PubMed] [Google Scholar]
- 15.Giovannetti M, Sbrana C. Self and nonself responses in hyphal tips of arbuscular mycorrhizal fungi. In: Geitmann A, Cresti M, editors. Cell biology of plant and fungal tip growth. Amsterdam, NL: IOS Press; 2001. pp. 221–231. (NATO Science Series, Series I: Life and Behavioural Sciences). [Google Scholar]
- 16.Rosendhal S, Taylor JW. Development of multiple genetic markers for studies of genetic variation in arbuscular mycorrhizal fungi using AFLP. Mol Ecol. 1997;6:821–829. [Google Scholar]
- 17.Viera A, Glenn MG. DNA content of vesicular-arbuscular mycorrhizal fungal spores. Mycologia. 1990;82:263–267. [Google Scholar]
- 18.Trouvelot S, van Tuinen D, Hijiri M, Gianinazzi-Pearson V. Visualization of ribosomal DNA loci in spore interphasic nuclei of glomalean fungi by fluorescence in situ hybridization. Mycorrhiza. 1999;8:201–206. [Google Scholar]
- 19.Kuhn G, Hijri M, Sanders IR. Evidence for the evolution of multiple genomes in arbuscular mycorrhizal fungi. Nature. 2001;414:745–748. doi: 10.1038/414745a. [DOI] [PubMed] [Google Scholar]
- 20.Bever JD, Morton J. Heritable variation and mechanisms of inheritance of spore shape within a population of Scutellospora pellucida, an arbuscular mycorrhizal fungus. Amer J Bot. 1999;86:1209–1216. [PubMed] [Google Scholar]
- 21.Sanders I. No sex please, we are fungi. Nature. 1999;399:737–739. doi: 10.1038/21544. [DOI] [PubMed] [Google Scholar]
- 22.Hepper CM. Limited independent growth of a vesicular-arbuscular mycorrhizal fungus in vitro. New Phytol. 1983;93:537–542. [Google Scholar]
- 23.Giovannetti M. Spore germination and presymbiotic mycelial growth. In: Kapulnik Y, Douds DD, editors. Arbuscular mycorrhizas: Physiology and function. Dordrecht, NL: Kluwer Academic Publishers; 2000. pp. 47–68. [Google Scholar]
- 24.Giovannetti M. Survival strategies in arbuscular mycorrhizal symbionts. In: Seckbach J, editor. Symbiosis mechanisms and model systems. Dordrecht, NL: Kluwer Academic Publisher; 2001. pp. 185–196. [Google Scholar]
- 25.Hyakumachi M, Ui T. Nonself-anastomosing isolates of Rhizoctonia solani obtained from fields of sugarbeet monoculture. Trans Br Mycol Soc. 1987;89:155–159. [Google Scholar]
- 26.Correll JC, Klittich CJR, Leslie JF. Heterokaryon self-incompatibility in Gibberella fujikuroi (Fusarium moniliforme) Mycol Res. 1989;93:21–27. [Google Scholar]
- 27.Leslie JF. Mating populations in Gibberella fujikuroi (Fusarium section Liseola) Phytopathology. 1991;81:1058–1060. [Google Scholar]
- 28.De Souza FA, Declerck S. Mycelium development and architecture, and spore production of Scutellospora reticulata in monoxenic culture with Ri T-DNA transformed carrot roots. Mycologia. 2003;95:1004–1012. [PubMed] [Google Scholar]
- 29.Gerdemann JW. Wound healing of hyphae in a phycomycetous mycorrhizal fungus. Mycologia. 1955;47:916–918. [Google Scholar]
- 30.De la Providencia IE, de Souza FA, Fernandez F, Séjalon Delmas N, Declerck S. Arbuscular mycorrhizal fungi reveal distinct patterns of anastomosis formation and hyphal healing mechanisms between different phylogenic groups. New Phytol. 2005;165:261–271. doi: 10.1111/j.1469-8137.2004.01236.x. [DOI] [PubMed] [Google Scholar]
- 31.Fries N. Somatic incompatibility and field distribution of the ectomycorrhizal fungus Suillus luteus (Boletaceae) New Phytol. 1987;107:735–739. [Google Scholar]
- 32.Rayner ADM. The challenge of the individualistic mycelium. Mycologia. 1991;83:48–71. [Google Scholar]
- 33.Brasier C. A champion thallus. Nature. 1992;356:382–383. [Google Scholar]
- 34.Dahlberg A, Stenlid J. Size, distribution and biomass of genets in populations of Suillus bovinus (L.: Fr.) Roussel revealed by somatic incompatibility. New Phytol. 1994;128:225–234. doi: 10.1111/j.1469-8137.1994.tb04006.x. [DOI] [PubMed] [Google Scholar]
- 35.Milgroom MG, Cortesi P. Analysis of population structure of the chestnut blight fungus based on vegetative incompatibility genotypes. Proc Natl Acad Sci USA. 1999;96:10518–10523. doi: 10.1073/pnas.96.18.10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ainsworth AM, Rayner ADM. Hyphal and mycelial responses associated with genetic exchange within and between species of the basidiomycete genus Stereum. J Gen Microbiol. 1989;135:1643–1659. [Google Scholar]
- 37.Newhouse JR, MacDonald WL. The ultrastructure of hyphal anastomoses between vegetatively compatible and incompatible virulent and hypovirulent strains of Cryphonectria parasitica. Can J Bot. 1991;69:602–614. [Google Scholar]
- 38.Jacobson DJ, Beurkens K, Klomparens KL. Microscopic and ultrastructural examination of vegetative incompatibility in partial diploids heterozygous at het loci in Neurospora crassa. Fung Genet Biol. 1998;23:45–56. doi: 10.1006/fgbi.1997.1020. [DOI] [PubMed] [Google Scholar]
- 39.Glass NL, Jacobson DJ, Shiu PKT. The genetics of hyphal fusion and vegetative incompatibility in filamentous ascomycete fungi. Annu Rev Genetics. 2000;34:165–186. doi: 10.1146/annurev.genet.34.1.165. [DOI] [PubMed] [Google Scholar]
- 40.Glass NL, Kuldau GA. Mating type and vegetative incompatibility in filamentous ascomycetes. Annu Rev Phytopathol. 1992;30:201–224. doi: 10.1146/annurev.py.30.090192.001221. [DOI] [PubMed] [Google Scholar]
- 41.Worrall JJ. Somatic incompatibility in basidiomycetes. Mycologia. 1997;89:24–36. [Google Scholar]
- 42.Cox G, Moran KJ, Sanders F, Nockolds C, Tinker PB. Translocation and transfer of nutrients in vesicular-arbuscular mycorrhizas. III. Polyphosphate granules and phosphorus translocation. New Phytol. 1980;84:649–659. [Google Scholar]
- 43.Harrison MJ, van Buuren ML. A phosphate transporter from the mycorrhizal fungus Glomus versiforme. Nature. 1995;378:626–629. doi: 10.1038/378626a0. [DOI] [PubMed] [Google Scholar]
- 44.Smith FA, Jakobsen I, Smith SE. Spatial differences in acquisition of soil phosphate between two arbuscular mycorrhizal fungi in symbiosis with Medicago truncatula. New Phytol. 2000;147:357–366. [Google Scholar]
- 45.Jakobsen I, Rosendhal L. Carbon flow into soil and external hyphae from roots of mycorrhizal cucumber plants. New Phytol. 1990;115:77–83. [Google Scholar]
- 46.Friese C, Allen MF. The spread of VA mycorrhizal fungal hyphae in the soil: Inoculum types and external hyphal architecture. Mycologia. 1991;83:409–418. [Google Scholar]
- 47.Bago B, Azcón-Aguilar C, Piché Y. Architecture and developmental dynamics of the external mycelium of the arbuscular mycorrhizal fungus Glomus intraradices grown under monoxenic conditions. Mycologia. 1998;90:52–62. [Google Scholar]
- 48.Jones MD, Durall DM, Tinker PB. Comparison of arbuscular and ectomycorrhizal Eucalyptus coccifera: Growth response, phosphorus uptake efficiency and external hyphal production. New Phytol. 1998;140:125–134. [Google Scholar]
- 49.Sanders FE, Tinker PB. Phosphate flow into mycorrhizal roots. Pesticide Science. 1973;4:385–395. [Google Scholar]
- 50.Tisdall PB, Oades JM. Stabilization of soil aggregates by the root segments of ryegrass. Australian J Soil Res. 1979;17:429–441. [Google Scholar]
- 51.Abbott LK, Robson AD. Formation of external hyphae in soil by four species of vesicular-arbuscular mycorrhizal fungi. New Phytol. 1985;99:245–255. [Google Scholar]
- 52.Sylvia DM. Activity of external hyphae of vesicular-arbuscular mycorrhizal fungi. Soil Biol Biochem. 1988;20:39–43. [Google Scholar]
- 53.Chiariello N, Hickman JC, Mooney HA. Endomycorrhizal role for interspecific transfer of phosphorus in a community of annual plants. Science. 1982;217:941–943. doi: 10.1126/science.217.4563.941. [DOI] [PubMed] [Google Scholar]
- 54.Francis R, Read DJ. Direct transfer of carbon between plants connected by vesicular-arbuscular mycorrhizal mycelium. Nature. 1984;307:53–56. [Google Scholar]
- 55.Grime JP, Mackey JML, Hillier SH, Read DJ. Floristic diversity in a model system using experimental microcosms. Nature. 1987;328:420–422. [Google Scholar]
- 56.Watkins NK, Fitter AH, Graves JD, Robinson D. Carbon transfer between C3 and C4 plants linked by a common mycorrhizal network, quantified using stable carbon isotopes. Soil Biol Biochem. 1996;28:471–477. [Google Scholar]
- 57.Graves JD, Watkins NK, Fitter AH, Robinson D, Scrimgeour C. Intraspecific transfer of carbon between plants linked by a common mycorrhizal network. Plant Soil. 1997;192:153–159. [Google Scholar]
- 58.Lerat S, Gauci R, Catford JG, Vierheilig H, Piche Y, Lapointe L. C-14 transfer between the spring ephemeral Erythronium americanum and sugar maple saplings via arbuscular mycorrhizal fungi in natural stands. Oecologia. 2002;132:181–187. doi: 10.1007/s00442-002-0958-9. [DOI] [PubMed] [Google Scholar]
- 59.Read DJ. The ties that bind. Nature. 1997;388:517–518. [Google Scholar]
- 60.Van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature. 1998;396:69–72. [Google Scholar]
- 61.Perry DA, Amaranthus MP, Borchers JG, Borchers SL, Brainerd RE. Bootstrapping in ecosystems. Bioscience. 1989;39:230–237. [Google Scholar]
- 62.Fitter AH, Graves JD, Watkins NK, Robinson D, Scrimgeour C. Carbon transfer between plants and its control in networks of arbuscular mycorrhizas. Funct Ecol. 1998;12:406–412. [Google Scholar]
- 63.Newman EI, Eason WR. Rates of phosphorus transfer within and between ryegrass (Lolium perenne) plants. Funct Ecol. 1993;7:242–248. [Google Scholar]
- 64.Pearson JN, Jakobsen I. Symbiotic exchange of carbon and phosphorus between cucumber and three arbuscular mycorrhizal fungi. New Phytol. 1993;124:481–488. [Google Scholar]
- 65.Robinson D, Fitter A. The magnitude and control of carbon transfer between plants linked by a common mycorrhizal network. J Exp Bot. 1999;50:9–13. [Google Scholar]
- 66.Simard SW, Durall DM. Mycorrhizal networks: A review of their extent, function, and importance. Can J Bot. 2004;82:1140–1165. [Google Scholar]