Abstract
Background
During the time of lowest white blood cell count (nadir) of allogeneic hematopoietic stem cell transplantation (allo-HSCT), cancer patients suffer from tremendous symptom burden related to therapy that requires intensive patient care. However, the mechanism underlying the development of multiple symptoms has not been established.
Methods
To explore the role of inflammatory cytokines in the development of treatment-related symptoms, we studied dynamic changes in serum concentrations of inflammatory cytokines (interleukin [IL]-6, IL-8, soluble tumor necrosis factor receptor 1 [sTNF-R1], IL-1 receptor antagonist [IL-1RA], and IL-12p40p70) and symptoms from pretherapy throughout the first 30 days of allo-HSCT in 30 patients with acute myelogenous leukemia or myelodysplastic syndrome. We measured multiple symptoms repeatedly using the M. D. Anderson Symptom Inventory (MDASI). Mixed-effects modeling was used to analyze longitudinal data.
Results
In response to conditioning and stem-cell infusion, IL-6 and the severity of multiple symptoms increased rapidly and peaked at nadir, the time of lowest white blood cell count. From baseline to nadir (approximately day 8 posttransplantation), increase in IL-6 was significantly associated with worsening of the most severe symptoms (fatigue, poor appetite, pain, drowsiness, dry mouth, and disturbed sleep; P < .01). During the first 30 days after transplantation, increases in IL-6 (P < .001) and sTNF-R1 (P < .05) significantly predicted the increasing severity of these symptoms.
Conclusion
These results suggest that release of systemic inflammatory cytokines, mainly IL-6, corresponds to an increase in treatment-related multiple-symptom burden during the nadir period of allo-HSCT.
Keywords: treatment-related symptoms, inflammatory cytokines, allogeneic HSCT, MDASI, nadir, longitudinal study, mixed-effects modeling
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an aggressive, curative treatment for acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS).1 Although most of the literature on allo-HSCT–related complications focuses on the development of acute graft-versus-host disease (aGVHD), clinicians have observed that a cluster of distressing non-aGVHD–related symptoms, such as fatigue, pain, poor appetite, sleep disturbance, and psychological distress, create considerable sickness during the initial 30 days after allo-HSCT (the acute phase), affecting patients’ daily functioning and necessitating the use of intensive care facilities.2–10 Increased attention is thus being given to the impact of allo-HSCT on patients’ self-reported symptoms during the acute phase.2–9 Even so, the mechanism underlying the development of multiple moderate to severe symptoms during this initial phase of allo-HSCT has not been well studied.
The collective effect of this cluster of moderate to severe symptoms constitutes a “symptom burden”11 and suggests that a shared mechanism may underlie the development of treatment-related symptoms in cancer patients undergoing aggressive therapy. Dysregulation of inflammatory cytokines (eg, interleukin [IL]-1, IL-6, and tumor necrosis factor [TNF]-α) has been proposed as a predominant mechanism underlying sickness symptoms in patients with cancer.12,13 IL-6 is well recognized to be at the core of the inflammatory response related to symptom severity in patients with cancer.14–22 TNF-α is produced in association with the insult from cancer treatments, including chemotherapy,23 and has been recognized as a crucial factor in a cascading proinflammatory process related to host tissue damage in organs targeted by aGVHD during allo-HSCT.24
Mounting evidence that cytokines play a role in sickness (for example, neuropathic pain, cachexia, chronic fatigue syndrome, disturbed sleep, and depression) has been found in studies of both animal models and humans in cancer research11,12,23 and in neuropsychoimmunology.25,26 Patients in the acute phase of allo-HSCT experience a symptom cluster (eg, fatigue, pain, sleep disturbance, poor appetite, and distress)10,27 that is quite similar to cytokine-induced sickness behavior (hyperalgesia, disturbed sleep, reduced water and food intake, and lack of activity) seen in animal models.12,23,28–30
Patients with AML/MDS experience 3 levels of insult in the initial weeks of allo-HSCT that potentially stimulate a massive release of internal inflammatory cytokines. First, before patients commence the allo-HSCT process, they may have elevated baseline circulating inflammatory cytokines as a result of their disease, induction therapy, comorbidities, or ongoing infections. Second, the initial transplantation-related insult to the immune system is triggered by a conditioning regimen of high-dose cytotoxic chemotherapy, which produces tissue injury and a corresponding release of inflammatory cytokines that may be the primary cause of biologic and behavioral changes from baseline to white blood cell (WBC) nadir. Third, after the transplantation of allogeneic stem cells several days later, activated mature donor T cells can set off a cytokine response that in turn stimulates host T-cell production of cytokines related to the onset of aGVHD.28,31 Although symptoms can be caused by mechanisms other than release of inflammatory cytokines, we hypothesize that the multiple symptoms of sickness behavior that develop after transplantation are rooted in dysregulation of inflammatory cytokines as a common denominator or initiator.17 The association between inflammatory cytokines and the development of these symptoms has not been quantitatively studied.
To establish the relationship over time between levels of systemic cytokines and the development of multiple distressing symptoms during the acute phase of allo-HSCT, we conducted a longitudinal study that simultaneously measured dynamic changes in inflammatory cytokines and multiple symptoms during the first 30 days after allo-HSCT and examined the strength of their association. We designed the study to assess the most common proinflammatory and anti-inflammatory cytokines identified in animal sickness-behavior studies and in human studies, and used validated methods for studying human circulating cytokines and patient self-reported outcomes. We hypothesized that circulating inflammatory cytokines, particularly IL-6 and TNF-α, would be crucial contributors to the development of a cluster of severe symptoms triggered by high-dose conditioning chemotherapy and stem cell transplantation, specifically around nadir (the time of lowest WBC count32) of allo-HSCT.12,14–16,23 Confirmation of this hypothesis would be a critical step toward establishing a mechanism-driven strategy for managing the severe symptom burden caused by aggressive cancer treatments, such as allo-HSCT.
PATIENTS AND METHODS
Subjects
Thirty patients with AML/MDS who were receiving an allo-HSCT participated in the study. Patients were consecutively recruited from the outpatient clinic in the Department of Stem Cell Transplantation at The University of Texas M. D. Anderson Cancer Center in Houston, Texas. All enrolled patients had a confirmed pathological diagnosis of AML/MDS, were at least 18 years old, and were scheduled for allo-HSCT. Of the 34 eligible patients approached, 4 (11.8%) declined to participate. The study was approved by M. D. Anderson Cancer Center’s Institutional Review Board. All participants gave informed consent.
Patient characteristics and clinical parameters (age, sex, race, disease type and status, source of infusion cell product, conditioning regimen, type of donor [sibling or unrelated], infusion dose of allogeneic product, and blood counts) were recorded before and during the study.
Multiple-Symptom Assessment
The M. D. Anderson Symptom Inventory (MDASI) was designed and validated for use in cancer populations regardless of disease diagnosis or type of therapy.33 The MDASI includes 13 symptom items that are rated on a 0 to 10 scale, with 0 being “not present” and 10 being “as bad as you can imagine.” The recall period for MDASI items is the previous 24 hours.
MDASI assessments were conducted at baseline (hospital admission), during pretransplantation chemotherapy conditioning (2–5 days after admission), day of allo-HSCT (day 0), and twice a week until 30 days post–allo-HSCT.
Cytokine Assay
Blood samples were obtained from each patient, most often in the early morning, at baseline, during conditioning, and days 0, +3, +8, +15, +22 and +29 of transplantation. The blood samples were centrifuged to separate the serum from the clot, after which the serum was harvested and stored at −20°C for batch analyses to measure cytokines. The cytokines to be measured included IL-1β, IL-6, IL-8, IL-10, IL-12p40p70, interferon (IFN)-γ, IL-1 receptor antagonist (IL-1RA), TNF-α, and soluble tumor necrosis factor receptor 1 (sTNF-R1). Cytokine levels were measured in 50 µL of serum by Multiplex cytometric bead array (Multiplex) assay on a Luminex 100 Analyzer (Luminex Corp., Austin, Texas).34 Cytometric bead array reagents for the detection of IL-1RA, IL-6, IL-12p40p70, IFN-γ, TNF-α̣, and sTNF-R1 were obtained from BioSources International (Camarillo, California); reagents for IL-1β, IL-8, and IL-10 were obtained from R&D Systems (Minneapolis, Minnesota).34 The intervariability for all inflammatory cytokines tested was less than 10%, indicating the highly satisfactory reliability of the Multiplex-Luminex method of cytokine assay.
Statistical Analysis
To visually demonstrate the development of study outcomes, we constructed Lowess curves depicting the average levels of serum IL-6, symptom severity, and WBC count from baseline to day +30 after allo-HSCT. Descriptive analysis was used to present patient characteristics and the mean level of symptoms and serum cytokines over time. The Wilcoxon rank sum test was used to compare baseline and peak serum cytokine levels.
Mixed-effects modeling was used for longitudinal analysis of symptom severity and cytokine data,18 (a) to investigate the potential impact of patient-related and allo-HSCT–related factors on symptom development from baseline to symptom peak at WBC nadir, (b) to identify the critical serum inflammatory cytokine(s) that temporally correlate with dynamic changes in symptoms over time, and (c) to examine the strength of the association between serum cytokine levels and the severity of the symptoms from baseline to peak at nadir, as well as throughout the entire 30 days of the acute posttransplantation period. Using patient-reported symptom outcomes from the MDASI, we identified the 6 most severe symptoms at their peak levels during the first 30 days: pain, fatigue, sleep disturbance, drowsiness, poor appetite, and dry mouth. The analyses in this study used a symptom component score, which was the mean of the 6 symptom scores for each patient.
All mixed-effects models were achieved using SAS PROC MIXED (SAS Institute Inc, NC). Symptom severity scores were treated as continuous variables and cytokine levels as time-dependent variables.18,35 The spatial power law (SP(POW)) covariance structure was used for unequally spaced data and adjustment of time-independent variables (age, gender, race, pre-HSCT disease status, conditioning regimen, source of allogeneic stem cell product [bone marrow or peripheral blood], and infusion dose of CD34+ cells). All cytokine concentrations were transformed to log base 10 (log10) in the mixed-effects models. The best-fitting models were selected on the basis of Bayesian information criterion (BIC).36 P values lower than .05 were considered statistically significant; all tests were two-sided.
RESULTS
Patient and Treatment Characteristics
Table 1 presents patient demographics and clinical features. Eighteen of 30 patients developed aGVHD between day +9 and +30; 11 of the 18 patients developed grade 1 aGVHD and 7 patients developed grade 2–3 aGVHD. No relapse or death occurred during the first 30 days.
TABLE 1.
Patient Characteristics and Symptom Severity*
| n | % | Baseline† mean (SD) | Symptom peak (day +11) mean (SD) | ||
|---|---|---|---|---|---|
| Age | Younger than 50 | 13 | 43.3 | 2.04 (2.36) | 4.08 (2.29) |
| 50 to 59 | 9 | 30.0 | 1.78 (1.60) | 5.24 (2.18) | |
| 60 and older | 8 | 26.7 | 1.35 (0.90) | 4.78 (2.21) | |
| Sex | Male | 17 | 56.7 | 1.81 (1.64) | 4.22 (2.13) |
| Female | 13 | 43.3 | 1.73 (2.09) | 5.13 (2.32) | |
| Race | White | 22 | 73.3 | 2.19 (1.94) ‡ | 5.09 (1.96) |
| Asian, Black, and Hispanic | 8 | 26.7 | 0.65 (0.61) ‡ | 3.31 (1.96) | |
| Education | College or higher | 27 | 90.0 | 1.88 (1.88) | 4.68 (2.17) |
| High school | 3 | 10.0 | 0.89 (0.67) | 4.06 (3.08) | |
| Pretransplantation disease status | Complete remission | 18 | 60.0 | 1.73 (1.79) | 4.71 (2.08) |
| Relapse | 9 | 30.0 | 1.67 (1.71) | 4.15 (2.64) | |
| Other | 3 | 10.0 | 2.39 (2.84) | 5.44 (2.14) | |
| Reduced-intensity conditioning regimen | No | 17 | 56.7 | 1.82 (1.88) | 4.76 (2.34) |
| Yes | 13 | 43.3 | 1.75 (1.82) | 4.51 (2.19) | |
| Degree of matching | Sibling | 13 | 43.3 | 1.92 (2.26) | 4.42 (2.75) |
| Unrelated donor | 17 | 56.7 | 1.67 (1.45) | 4.76 (1.79) | |
| Source of cell product | Bone marrow | 13 | 43.3 | 1.23 (0.97) | 4.37 (1.63) |
| Peripheral blood stem cells | 17 | 56.7 | 2.20 (2.20) | 4.80 (2.62) | |
| Type of disease | AML | 14 | 46.7 | 1.63 (2.05) | 4.60 (2.48) |
| MDS | 5 | 16.7 | 2.80 (2.70) | 4.83 (1.76) | |
| MDS to AML | 11 | 36.6 | 1.50 (0.73) | 4.54 (2.25) |
SD indicates standard deviation; AML, acute myelogenous leukemia; MDS, myelodysplastic syndrome.
6 symptoms (pain, fatigue, sleep disturbance, dry mouth, lack of appetite, and drowsiness) had the highest mean severity at symptom peak (day +11 after allo-HSCT).
Baseline data were collected on the day of hospital admission, an average of 5.5 days before conditioning.
P < .01 by Wilcoxon rank sum test comparing the component symptom score at baseline between groups.
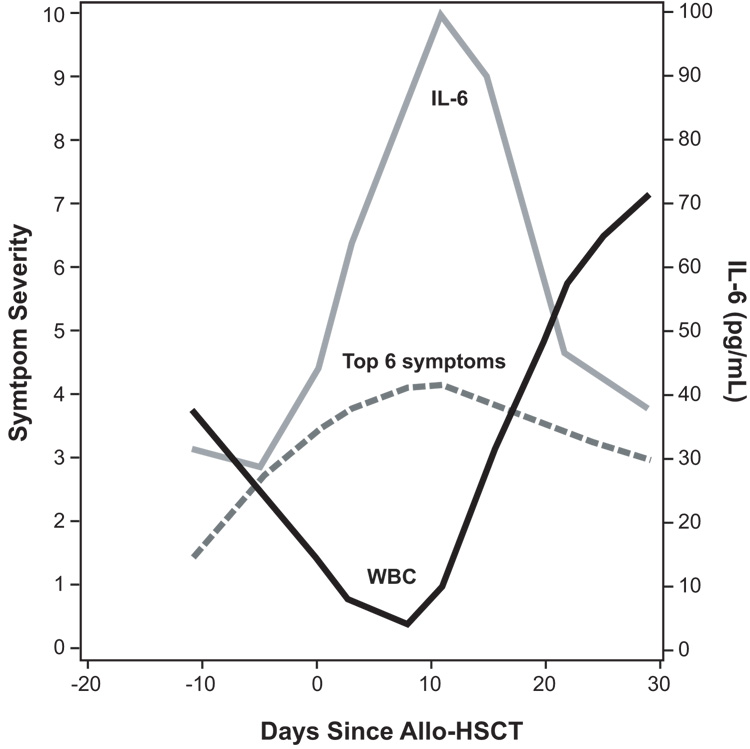
Nadir, defined as the first day that a patient has the lowest WBC count,32 was observed on day +8 for most patients. See Fig. 1 for a Loess curve of the average WBC count during the 30-day study period.
FIGURE 1.
Lowess curves of the estimated severity levels of multiple symptoms and white blood cell (WBC) count during 30 days of allo-HSCT.
Symptom Patterns and Predictors of Symptom Severity
The Loess curves in Fig. 1 present the average symptom component scores from baseline to 30 days posttransplantation. The overall random missing data rate for MDASI items was 1.7%, stemming primarily from either patient fatigue or administrative error. Mixed-effects modeling demonstrated that the 6 most severe patient-reported symptoms from the MDASI increased significantly in severity from baseline (day −10) to their highest levels (day +11, 3 days after nadir), gradually decreasing in severity thereafter (all P < .001).
Mixed-effects modeling also demonstrated that, among the potential patient-related and allo-HSCT–related factors, (age, gender, race/ethnicity, pretransplantation disease status, source of infused allogeneic stem cell product, dose of infused stem cells, degree of donor matching [sibling or unrelated], and conditioning regimen), being white non-Hispanic was the only significant predictor that had an impact on the change in severity of the symptom component score from baseline to day +30 (all P < .05). Race was the only factor that made a significant difference in baseline symptom severity, with white non-Hispanic patients reporting higher symptom levels (P < .01, Table 1).
Serum Cytokine Patterns Over Time During Allo-HSCT
Although we had hypothesized that TNF-α would play a critical role in the development of severe symptoms triggered by high-dose conditioning chemotherapy and stem cell transplantation, serum TNF-α was not detectable in all samples, possibly due to the sampling process or to storage conditions or duration.37,38 We found that its soluble receptor, sTNF-R1, which highly reflects serum TNF-α activity, was measurable in all samples, and we used it as a proxy for TNF-α.39 Serum levels of IFN-γ also were not detectable in all samples and therefore were not included in the final analyses.
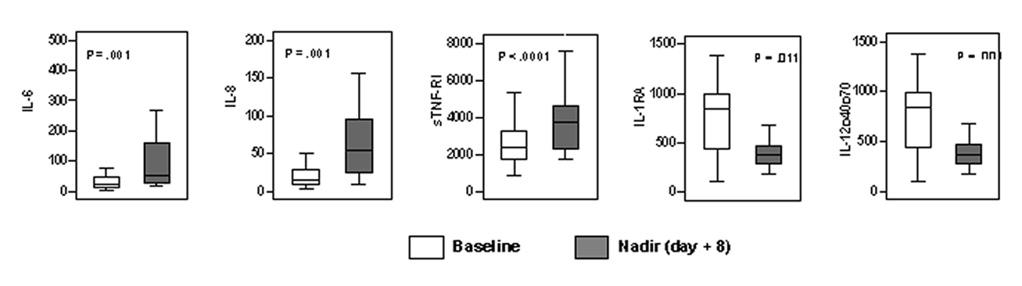
We observed fluctuations in serum concentrations of cytokines from baseline to nadir (day −10 to day +8). The levels of IL-6, IL-8, and sTNF-R1 increased significantly, whereas the levels of IL-1RA and IL-12p40p70 decreased significantly (all P < .01 by Wilcoxon rank sum test Fig. 2). During the study, the highest levels of IL-6 and the lowest levels of IL-1RA and IL-12p40p70 occurred at nadir; in contrast, sTNF-R1 rapidly increased from baseline to day of transplantation (day 0) and reached its highest levels by end of the 30-day posttransplantation period (Table 2). Fig. 1 also depicts average levels of serum IL-6, which peaked at nadir.
FIGURE 2.
Changes in serum cytokine levels over time. The figure illustrates changes in serum cytokine levels (pg/mL) from baseline to white blood cell count nadir (approximately day +8 after allo-HSCT) and from nadir to day +30 after allo-HSCT (P values derived from Wilcoxon rank sum test).
TABLE 2.
Mean (Standard Deviation) of Serum Cytokine Levels by each Time Point During First 30 Days of Allo-HSCT
| Time point | Number of days since allo-HSCT | sTNF-R1 | IL-6, pg/mL | IL-1RA, pg/mL | IL-12p40+p70, pg/mL |
|---|---|---|---|---|---|
| Baseline | −10.80 (4.09) | 3113.77 (2601.92) | 37.47 (44.19) | 1269.71 (1753.88) | 91.13 (65.15) |
| Conditioning regimen | −5.03 (1.54) | 3064.54 (2594.08) | 55.31 (106.13) | 1610.08 (3195.46) | 104.74 (77.59) |
| Allo-HSCT day 0 | 0.00 (0.00) | 4951.11 (5050.07) | 100.85 (158.81) | 912.56 (894.12) | 59.93 (43.45) |
| Allo-HSCT day +3 | 3.07 (0.25) | 4576.18 (4735.58) | 85.08 (106.85) | 876.95 (1130.00) | 43.30 (23.69) |
| Allo-HSCT + 8 (nadir) | 7.93 (0.37) | 4110.26 (2234.13) | 118.30 (135.69) | 675.15 (885.50) | 34.57 (15.28) |
| Allo-HSCT day +15 | 14.72 (0.59) | 4977.94 (3906.98) | 85.81 (93.99) | 1376.50 (987.61) | 43.48 (25.69) |
| Allo-HSCT day +22 | 21.73 (0.78) | 5338.91 (4913.75) | 52.04 (60.59) | 1679.47 (1133.26) | 88.82 (81.84) |
| Allo-HSCT day +29 | 29.00 (0.83) | 5487.96 (4532.02) | 56.42 (67.29) | 1659.72 (1568.59) | 145.16 (168.59) |
Allo-HSCT indicates allogeneic hematopoietic stem cell transplantation; sTNF-R1, soluble tumor necrosis factor receptor 1; IL, interleukin; RA, receptor antagonist
In a general linear model, baseline levels of IL-6, IL-8, IL-10, and sTNF-R1 predicted each cytokine’s level at nadir (all P <.01). There was no significant difference in baseline cytokine levels by race or by allo-HSCT-associated factors (reduced-intensity conditioning regimen, cell source, dose of infused cells).
Association of Cytokine(s) with Symptom Severity
Table 3, Mixed-Effects Model A presents an analysis of 150 concurrent measurements of symptoms and cytokines to identify whether increased serum cytokines were temporally associated with a rapid increase in symptom severity from baseline to peak after patients received conditioning therapy and underwent allo-HSCT. Age, gender, race/ethnicity, disease status, infusion cell source, conditioning regimen, and dose of infused cells were adjusted in the model. Among the examined inflammatory cytokines, increased IL-6 from baseline to nadir was the only significant predictor of increased symptom scores from baseline to symptom peak (P = .006).
TABLE 3.
Dynamic Changes in Serum Cytokines Associated with Changes in Component Score of Symptom Severity, by Mixed-Effects Modeling
| Mixed-Effects Model A. Increase in symptom severity (baseline to peak) | |||||||
|---|---|---|---|---|---|---|---|
| Cytokines changes (baseline to day +8 after allo-HSCT) | |||||||
| IL-6 | IL-8 | IL-10 | IL-1RA | IL-1β | sTNF-R1 | IL-12p40p70 | |
| Estimate* | 1.112 | 0.294 | 0.390 | 0.147 | 1.613 | 1.133 | −0.245 |
| Standard error | 0.505 | 0.393 | 0.357 | 0.500 | 0.517 | 0.915 | 1.842 |
| P † | 0.006 | 0.411 | 0.437 | 0.776 | 0.383 | 0.218 | 0.710 |
| Mixed-Effects Model B. Change in symptom severity (baseline to +30 days) | |||||||
|---|---|---|---|---|---|---|---|
| Cytokines changes (baseline to day +30 after allo-HSCT) | |||||||
| IL-6 | IL-8 | IL-10 | IL-1RA | IL-1β | sTNF-R1 | IL-12p40p70 | |
| Estimate* | 1.050 | 0.169 | 0.610 | 0.037 | −0.135 | 1.367 | −0.454 |
| Standard error | 0.245 | 0.194 | 0.369 | 0.304 | 0.564 | 0.650 | 0.294 |
| P † | < .0001 | 0.383 | 0.100 | 0.903 | 0.812 | 0.036 | 0.124 |
Age, sex, race, disease status, infusion cell service, conditioning regimen, and infusion dose of allogeneic product were adjusted in all models. Selection of symptoms (pain, fatigue, sleep disturbance, drowsiness, poor appetite, and dry mouth) is based on the highest mean levels at symptom peak (day +11 of allo-HSCT).
Allo-HSCT indicates allogeneic hematopoietic stem cell transplantation; IL, interleukin; RA, receptor antagonist; sTNF-R1, soluble tumor necrosis factor receptor 1.
Estimate: The parameter estimated for a predictor. For log10 transformed independent variables, it shows how the outcome changes when multiplying the predictor by 10. For example, the estimate of baseline log10 IL-6 levels for the mean severity of the symptom cluster was 1.88 on a 0 to 10 scale, which meant that when the level of IL-6 increases by 10 times, there was a corresponding 1.88-unit increase in the mean symptom score.
Boldface indicates a statistically significant result.
Table 3, Mixed-Effects Model B presents an analysis of 274 concurrent measurements of symptoms and cytokines from baseline to day +30, indicating that changes in IL-6 (P < .0001) and sTNF-R1 (P < .05) significantly predicted the change in severity of the symptom component score over the entire period. The sample correlation between the predicted symptom severity and the observed value was 0.78, which yields a pseudo-R2 statistic of 0.61, suggesting that 61% of the total variability can be explained by this model. The power to detect such an association in 30 patients with 13 predictors is 95%.
DISCUSSION
The escalated patient–reported symptom burden in this study fits clinicians’ impressions that patients develop significant sickness around the time of WBC count nadir after allo-HSCT. Our results demonstrated that the increased release of the serum inflammatory cytokines IL-6 and sTNF-R1 in immediate response to the allo-HSCT conditioning regimen and the infusion of stem cells was temporally associated with the worsening in severity of a cluster of the most severe symptoms during the 30 days after allo-HSCT. The positive relationship between symptom severity and both IL-6 and sTNF-R1 remained consistent throughout the 30 days posttransplantation, regardless of whether individual patient symptoms were thought to be the result of the preparative regimen, infection, aGVHD, mucositis or some other clinical condition. The results suggests a true “side effect” of inflammation, in which overexpressed circulating cytokines exert symptomatic influences beyond their role in mediating immune function.17
Our longitudinal results provide an accurate profile of the dynamic development of symptom burden and demonstrate that the MDASI is an effective tool for measuring multiple subjective symptoms. The 6 most severe MDASI symptom items (pain, fatigue, sleep disturbance, dry mouth, lack of appetite, and drowsiness) remained consistent during this period, reflecting an acute symptom burden related to allo-HSCT. There were no observed significant differences in symptom outcomes by cell product, conditioning regimen, or degree of donor matching at symptom peak during 30 days after allo-HSCT. Whereas toxicity measures are often not based on patient report, may not include all symptoms related to the treatment, and may not be repeated frequently over time,40 the MDASI used in this longitudinal study is sensitive for detecting the most disturbing nonspecific symptoms over the treatment period.11 The low missing-data rate (less than 2%) reflects the excellent feasibility of repeated symptom assessment conducted on a reasonable time schedule in very ill patients undergoing aggressive cancer therapy.
This pattern of parallel cytokine and symptom development at nadir (approximately 8 days after allo-HSCT), before any onset of aGVHD, supports our hypothesis that human sickness behavior occurs with aggressive cancer treatment, due at least in part to inflammatory processes.12,23,41 Our observation that dynamic change in serum IL-6 was the only significant inflammation factor positively promoting the changes in symptom outcomes from baseline to peak is consistent with another study in humans that identified inflammatory cytokines as the cause of chemotherapy-related symptoms in cancer patients,16 and is supported by evidence that IL-6, as an activator or an inhibitor of T-cell response, is at the core of the inflammatory response related to symptom severity in patients with cancer.14–22 Previous research suggesting that an IL-6 antibody could reduce mucositis and fever without hematological toxicity implies that interventions targeted at the mechanisms of symptom production may be a promising way of addressing treatment-related symptom burden during the transplantation period.22,42
From a methodological viewpoint, the homogenous sample, longitudinal study design and careful data collection, use of a validated patient-reported outcome measure, and use of mixed-effects modeling for longitudinal data analysis are essential elements for understanding the role of cytokines in dynamic symptom development during the acute phase of allo-HSCT.18 The study was sufficiently powered to detect the association between symptom scores and cytokine expression while controlling multiple critical covariances. Given the complexity of the role of inflammatory cytokines in both disease outcomes and symptom outcomes in patients with cancer,12,16,17,23,43 we believe that mixed-effects modeling provides a number of advantages over other traditional analysis methods (such as correlation coefficients, regression models, and multivariate analysis of variance). Mixed-effects modeling has improved ability to account for correlations between repeated observations in the same subject, handle unequally spaced or missing observations, and express linear correlations while controlling for confounding variables. 18,35,38,44
The current study had several limitations. Symptoms peaked unexpectedly on day +11 rather than on day +8 (nadir), when cytokines peaked. Because we did not collect blood samples on day +11, we cannot confirm whether cytokine levels did or did not peak along with symptoms on day +11. Also, future studies could benefit from measurements of cognitive function beyond self report, because confirmation of a shared inflammation mechanism could provide a more complete picture of symptom burden in this cohort.26 In the future, the development of salivary cytokine assays would greatly simplify patient sampling over time and would allow for a more complete picture of the temporal relationship between symptoms and fluctuations in cytokines. Although we measured pain and mouth sores and assumed that fluctuations in reported pain and mouth sores would adequately characterize mucositis, we could have included additional items that specifically address this distressing side effect. Future studies might include examiner ratings of mucositis, also not done in this study. Additionally, examination of cytokine production by different types of cells could provide a better understanding of this detailed mechanism. Finally, we did not measure a standard marker of inflammation, C reactive protein (CRP). If CRP reflects what is found with more specific cytokine fluctuations, it might be used to guide symptom management in the clinical setting.
In summary, changes in the levels of serum IL-6 were associated in this study with changes in symptom severity during the initial weeks of aggressive allo-HSCT. Our findings warrant further study with a randomized clinical trial using IL-6 antibody to confirm whether IL-6 plays a role in symptom production and worsening symptom severity in response to high-dose chemotherapy and transplantation20,22,45 Confirming a causal role for IL-6 in producing symptom burden would provide a mechanism-driven symptom intervention or prevention option for patients during the acute phase of allo-HSCT.
ACKNOWLEDGEMENTS
The authors acknowledge the editorial assistance of Jeanie F. Woodruff, ELS; cytokine assay assistance from Evan N. Cohen, BS; and protocol management support from Marilyn Morrissey, MPH.
Acknowledgement of Research Support: This study was supported by a grant from the National Institutes of Health (R01 CA026582) and by a Multidisciplinary Research Project award from The University of Texas M. D. Anderson Cancer Center.
Footnotes
Statement of prior presentation: Presented in part at the American Society of Hematology 47th Annual Meeting and Exposition, December 2005, Atlanta, Georgia, and at the 48th Annual Meeting and Exposition, December 2006, Orlando, Florida.
REFERENCES
- 1.Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324:667–674. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 2.Andrykowski MA, Greiner CB, Altmaier EM, et al. Quality of life following bone marrow transplantation: findings from a multicentre study. Br J Cancer. 1995;71:1322–1329. doi: 10.1038/bjc.1995.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hjermstad MJ, Kaasa S. Quality of life in adult cancer patients treated with bone marrow transplantation--a review of the literature. Eur J Cancer. 1995;31A:163–173. doi: 10.1016/0959-8049(94)00464-g. [DOI] [PubMed] [Google Scholar]
- 4.Whedon M, Ferrell BR. Quality of life in adult bone marrow transplant patients: beyond the first year. Semin Oncol Nurs. 1994;10:42–57. doi: 10.1016/s0749-2081(05)80044-0. [DOI] [PubMed] [Google Scholar]
- 5.Gaston-Johansson F, Foxall M. Psychological correlates of quality of life across the autologous bone marrow transplant experience. Cancer Nurs. 1996;19:170–176. doi: 10.1097/00002820-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Pederson C, Parran L. Pain and distress in adults and children undergoing peripheral blood stem cell or bone marrow transplant. Oncol Nurs Forum. 1999;26:575–582. [PubMed] [Google Scholar]
- 7.Syrjala KL, Chapko ME. Evidence for a biopsychosocial model of cancer treatment-related pain. Pain. 1995;61:69–79. doi: 10.1016/0304-3959(94)00153-6. [DOI] [PubMed] [Google Scholar]
- 8.Syrjala KL, Langer SL, Abrams JR, et al. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA. 2004;291:2335–2343. doi: 10.1001/jama.291.19.2335. [DOI] [PubMed] [Google Scholar]
- 9.Edman L, Larsen J, Hagglund H, Gardulf A. Health-related quality of life, symptom distress and sense of coherence in adult survivors of allogeneic stem-cell transplantation. Eur J Cancer Care (Engl) 2001;10:124–130. doi: 10.1046/j.1365-2354.2001.00251.x. [DOI] [PubMed] [Google Scholar]
- 10.Prieto JM, Saez R, Carreras E, et al. Physical and psychosocial functioning of 117 survivors of bone marrow transplantation. Bone Marrow Transplant. 1996;17:1133–1142. [PubMed] [Google Scholar]
- 11.Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007;37:16–21. doi: 10.1093/jncimonographs/lgm005. [DOI] [PubMed] [Google Scholar]
- 12.Cleeland CS, Bennett GJ, Dantzer R, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? Cancer. 2003;97:2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 13.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurzrock R. The role of cytokines in cancer-related fatigue. Cancer. 2001;92:1684–1688. doi: 10.1002/1097-0142(20010915)92:6+<1684::aid-cncr1497>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Pusztai L, Mendoza TR, Reuben JM, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25:94–102. doi: 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Wood LJ, Nail LM, Gilster A, Winters KA, Elsea CR. Cancer chemotherapy-related symptoms: evidence to suggest a role for proinflammatory cytokines. Oncol Nurs Forum. 2006;33:535–542. doi: 10.1188/06.ONF.535-542. [DOI] [PubMed] [Google Scholar]
- 17.Esch T, Stefano G. Proinflammation: a common denominator or initiator of different pathophysiological disease processes. Med Sci Monit. 2002;8:HY1–HY9. [PubMed] [Google Scholar]
- 18.Fairclough DL, Wang XS. Understanding the correlations between biologic and symptom measures over time. In: Lenderking WR, Revicki DA, editors. Advancing Health Outcomes Research Methods and Clinical Applications. McLean, VA: Degnon Associates; 2005. pp. 177–190. [Google Scholar]
- 19.Kelley KW, Bluthe RM, Dantzer R, et al. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17 Suppl 1:S112–S118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 20.Monk JP, Phillips G, Waite R, et al. Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J Clin Oncol. 2006;24:1852–1859. doi: 10.1200/JCO.2005.04.2838. [DOI] [PubMed] [Google Scholar]
- 21.Pihusch M, Pihusch R, Fraunberger P, et al. Evaluation of C-reactive protein, interleukin-6, and procalcitonin levels in allogeneic hematopoietic stem cell recipients. Eur J Haematol. 2006;76:93–101. doi: 10.1111/j.0902-4441.2005.00568.x. [DOI] [PubMed] [Google Scholar]
- 22.Trikha M, Corringham R, Klein B, Rossi JF. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res. 2003;9:4653–4665. [PMC free article] [PubMed] [Google Scholar]
- 23.Lee BN, Dantzer R, Langley KE, et al. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11:279–292. doi: 10.1159/000079408. [DOI] [PubMed] [Google Scholar]
- 24.Goker H, Haznedaroglu IC, Chao NJ. Acute graft-vs-host disease: pathobiology and management. Exp Hematol. 2001;29:259–277. doi: 10.1016/s0301-472x(00)00677-9. [DOI] [PubMed] [Google Scholar]
- 25.Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist. 2007;12 Suppl 1:22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- 26.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altmaier EM, Gingrich RD, Fyfe MA. Two-year adjustment of bone marrow transplant survivors. Bone Marrow Transplant. 1991;7:311–316. [PubMed] [Google Scholar]
- 28.Holler E, Kolb HJ, Moller A, et al. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation. Blood. 1990;75:1011–1016. [PubMed] [Google Scholar]
- 29.Remberger M, Ringden O, Markling L. TNF alpha levels are increased during bone marrow transplantation conditioning in patients who develop acute GVHD. Bone Marrow Transplant. 1995;15:99–104. [PubMed] [Google Scholar]
- 30.Xun CQ, Thompson JS, Jennings CD, Brown SA, Widmer MB. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood. 1994;83:2360–2367. [PubMed] [Google Scholar]
- 31.Ferrara JL. Cytokine dysregulation as a mechanism of graft versus host disease. Curr Opin Immunol. 1993;5:794–799. doi: 10.1016/0952-7915(93)90139-j. [DOI] [PubMed] [Google Scholar]
- 32.International Bone Marrow Transplant Registry, Autologous Bone Marrow Transplant Registry. Manual for Clinical Research Professionals. Milwaukee WI: Statistical Center, Medical College of Wisconsin; 2003. Granulopoiesis, items 362–371: instructions for completing the 2002 CORE insert, 2002 COREFU insert, 2002 DCI insert; pp. 35–36. [Google Scholar]
- 33.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M. D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 34.Reuben JM, Lee BN, Li C, et al. Biologic and immunomodulatory events after CTLA-4 blockade with ticilimumab in patients with advanced malignant melanoma. Cancer. 2006;106:2437–2444. doi: 10.1002/cncr.21854. [DOI] [PubMed] [Google Scholar]
- 35.Laird NM, Donnelly C, Ware JH. Longitudinal studies with continuous responses. Stat Methods Med Res. 1992;1:225–247. doi: 10.1177/096228029200100302. [DOI] [PubMed] [Google Scholar]
- 36.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, editors. SAS System for Mixed Models. Cary NC: SAS Institute, Inc; 1996. Analysis of repeated measures data; pp. 87–134. [Google Scholar]
- 37.Thavasu PW, Longhurst S, Joel SP, Slevin ML, Balkwill FR. Measuring cytokine levels in blood: importance of anticoagulants, processing, and storage conditions. J Immunol Methods. 1992;153:115–124. doi: 10.1016/0022-1759(92)90313-i. [DOI] [PubMed] [Google Scholar]
- 38.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Ritchie D, Seconi J, Wood C, Walton J, Watt V. Prospective monitoring of tumor necrosis factor alpha and interferon gamma to predict the onset of acute and chronic graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:706–712. doi: 10.1016/j.bbmt.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Lee SJ, Joffe S, Kim HT, et al. Physicians' attitudes about quality-of-life issues in hematopoietic stem cell transplantation. Blood. 2004;104:2194–2200. doi: 10.1182/blood-2003-07-2430. [DOI] [PubMed] [Google Scholar]
- 41.Ferrara JL. Cytokine inhibitors and graft-versus-host disease. Ann N Y Acad Sci. 1995;770:227–236. doi: 10.1111/j.1749-6632.1995.tb31058.x. [DOI] [PubMed] [Google Scholar]
- 42.Rossi JF, Fegueux N, Lu ZY, et al. Optimizing the use of anti-interleukin-6 monoclonal antibody with dexamethasone and 140 mg/m2 of melphalan in multiple myeloma: results of a pilot study including biological aspects. Bone Marrow Transplant. 2005;36:771–779. doi: 10.1038/sj.bmt.1705138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haddad JJ. Cytokines and related receptor-mediated signaling pathways. Biochem Biophys Res Commun. 2002;297:700–713. doi: 10.1016/s0006-291x(02)02287-8. [DOI] [PubMed] [Google Scholar]
- 44.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, editors. SAS system for mixed models. Cary, N.C: SAS Institute, Inc; 1996. Spatial variability; pp. 303–330. [Google Scholar]
- 45.Busca A, Locatelli F, Marmont F, Ceretto C, Falda M. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2007;82:45–52. doi: 10.1002/ajh.20752. [DOI] [PubMed] [Google Scholar]