Abstract
Suberin is a cell wall lipid polyester found in the cork cells of the periderm offering protection against dehydration and pathogens. Its biosynthesis and assembly, as well as its contribution to the sealing properties of the periderm, are still poorly understood. Here, we report on the isolation of the coding sequence CYP86A33 and the molecular and physiological function of this gene in potato (Solanum tuberosum) tuber periderm. CYP86A33 was down-regulated in potato plants by RNA interference-mediated silencing. Periderm from CYP86A33-silenced plants revealed a 60% decrease in its aliphatic suberin load and greatly reduced levels of C18:1 ω-hydroxyacid (approximately 70%) and α,ω-diacid (approximately 90%) monomers in comparison with wild type. Moreover, the glycerol esterified to suberin was reduced by 60% in the silenced plants. The typical regular ultrastructure of suberin, consisting of dark and light lamellae, disappeared and the thickness of the suberin layer was clearly reduced. In addition, the water permeability of the periderm isolated from CYP86A33-silenced lines was 3.5 times higher than that of the wild type. Thus, our data provide convincing evidence for the involvement of ω-functional fatty acids in establishing suberin structure and function.
Periderm, the boundary tissue that replaces the epidermis in the secondary organs of plants, provides efficient protection against dehydration, UV radiation, and pathogens (Esau, 1965). Periderm is regularly found in the bark of woody plants, but herbaceous plants may also form a well-developed periderm in roots, tubers, and the oldest portions of stem. The periderm has been widely studied in potato (Solanum tuberosum) tubers because of the latter's great agronomic significance (Schmidt and Schönherr, 1982; Vogt et al., 1983; Lulai and Freeman, 2001; Sabba and Lulai, 2002). Shrinkage and flaccidity occur in tubers if the protection afforded by the periderm against water loss is compromised (Lulai et al., 2006). Suberin and waxes embedded into the suberin matrix are considered essential for periderm imperviousness (Franke and Schreiber, 2007). Chemically, suberin is a complex lipid polymer consisting of a fatty acid-derived domain (aliphatic suberin) cross-linked by ester bonds to a polyaromatic lignin-like domain (aromatic suberin; Kolattukudy, 2001; Bernards, 2002; Franke and Schreiber, 2007). Aliphatic suberin has been widely analyzed in potato periderm (Kolattukudy and Agrawal, 1974; Graça and Pereira, 2000; Schreiber et al., 2005). The main monomers released from potato aliphatic suberin are a mixture of ω-hydroxyacids and α,ω-diacids with chain lengths ranging from C16 to C28 (mainly C18), together with glycerol. Small amounts of monofunctional fatty acids, alcohols, and ferulic acid are also released. Waxes are complex mixtures of lipids extractable with chloroform that in potato periderm consist mostly of linear very-long-chain aliphatics up to C32 (Schreiber et al., 2005). Suberin is deposited in the cell wall as a continuous deposit or secondary cell wall that overlays the polysaccharide primary cell wall from the inside (Esau, 1965). These suberin deposits appear under the transmission electron microscope (TEM) as regularly alternating opaque and translucent lamellae (Schmidt and Schönherr, 1982). Although several molecular models for suberin have been proposed (Kolattukudy, 1980; Bernards, 2002; Graça and Santos, 2007), how the suberin and wax components are organized in the lamellated suberin secondary cell wall is a matter of debate (Graça and Santos, 2007). Moreover, to what extent suberin and wax deposition and composition determine sealing properties of periderm still remains unclear (Schreiber et al., 2005). Several studies confirm the importance of waxes for the diffusion barrier (Soliday et al., 1979; Vogt et al., 1983; Schreiber et al., 2005), but the significance of aliphatic suberin has hardly been investigated at all. Interestingly, an Arabidopsis (Arabidopsis thaliana) knockout mutant for the GLYCEROL-3-PHOSPHATE ACYLTRANSFERASE5 gene (GPAT5) with altered suberin showed higher tetrazolium salt permeability in the seed coat (Beisson et al., 2007).
ω-Hydroxylation of fatty acids, a reaction carried out in plants by cytochrome P450 monooxygenases, is a crucial step in the biosynthesis of plant biopolymers (Kolattukudy, 1980; Nawrath, 2002). The Arabidopsis mutant lacerata, which shows phenotype defects compatible with a cutin deficiency, is defective in CYP86A8 encoding a fatty acid ω-hydroxylase (Wellesen et al., 2001). The aberrant induction of type three genes1 (att1) mutant, showing an altered cuticle ultrastructure and a higher transpiration rate than wild type, is defective in CYP86A2 and contains reduced amounts of ω-functionalized cutin monomers (Xiao et al., 2004). Moreover, a genome-wide study of cork oak (Quercus suber) bark highlighted a member of the cytochrome P450 of the CYP86A subfamily as a strong candidate gene for aliphatic suberin biosynthesis (Soler et al., 2007); and a role for CYP86A1 in the biosynthesis of suberin has recently been confirmed in the primary root of Arabidopsis knockout mutants (Li et al., 2007; Hofer et al., 2008). However, how the lack of fatty acid ω-hydroxylase activity may affect the structural features and sealing properties of suberin in periderm cell walls has not been documented.
To provide experimental evidence of the role of CYP86A genes in periderm fine structure and water transpiration properties, especially quantitative permeability studies so far unexplored in Arabidopsis, we followed a strategy based on the potato (cv Desirée). The potato is a model of choice for such studies because transgenic plants can be produced in reasonable time and sufficient amounts of periderm can easily be obtained from tubers for chemical and physiological studies (Vogt et al., 1983; Schreiber et al., 2005). Based on the CYP86A gene that was highlighted in cork oak periderm as a strong suberin candidate for aliphatic suberin biosynthesis, we isolated the putative ortholog in potato and used a reverse genetic approach to analyze the effects of down-regulation on the chemical and ultrastructural features of suberin and on the physiological properties of the tuber periderm. Our findings indicate that down-regulation of CYP86A33, as this gene was designated, results in a strong decrease of the ω-functionalized monomers in aliphatic suberin, which are necessary for the suberin typical lamellar organization and for the periderm resistance to water loss.
RESULTS
Cloning and Phylogenetic Analysis of Potato CYP86A33 and Cork Oak CYP86A32
We obtained the full-length coding sequence of a cork oak CYP86A gene (accession no. EU293406) highlighted by prior works as a strong candidate for suberin biosynthesis (Soler et al., 2007, 2008). This gene, henceforth referred to as CYP86A32, was then used as a query to identify the potato homologous sequence in The Institute for Genomic Research (TIGR; http://www.tigr.org) database. Highest homology (86% similarity) was for potato tentative consensus TC148994, which was assembled from tuber and root ESTs. Although TC148994 covered a complete coding sequence, no true open reading frame was obtained. Thus, cDNA prepared from potato tuber skin was used to isolate the complete coding sequence (accession no. EU293405), referred to as CYP86A33. The amino acid sequences of CYP86A32 and CYP86A33 displayed high similarity (88% and 89%, respectively) to Arabidopsis CYP86A1.
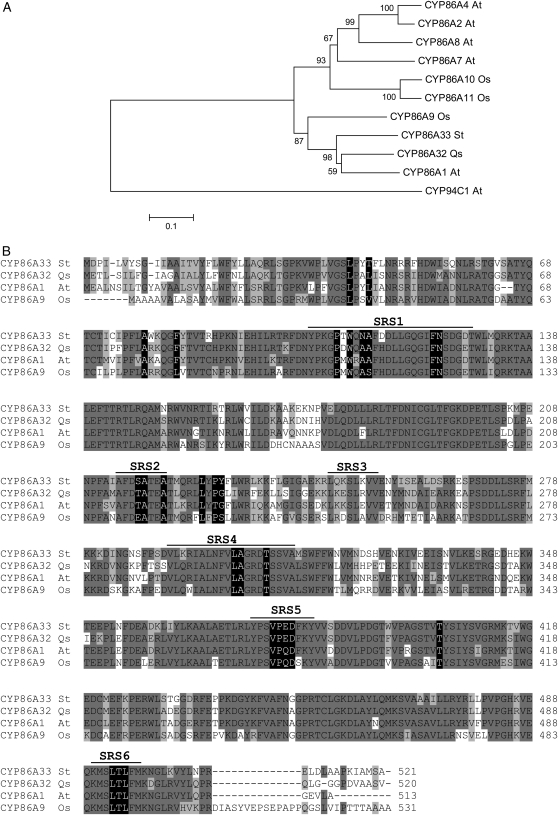
A phylogenetic study with the CYP86A subfamily in Arabidopsis and rice (Oryza sativa) was carried out to trace the phylogenetic position of CYP86A32 and CYP86A33 among the CYP86A subfamily. All Arabidopsis proteins included in the study demonstrated in vitro ω-hydroxylase activity (Benveniste et al., 1998; Kandel et al., 2007; Rupasinghe et al., 2007) and also—for CYP94C1—a capacity to yield α,ω-diacids (Kandel et al., 2007). A multiple amino acid sequence alignment generated by ClustalW was used to generate two unrooted phylogenetic trees using, respectively, the neighbor-joining and the unweighted pair group method with arithmetic mean algorithms. Both returned identical clade distributions with similar bootstrap values (Fig. 1A). Arabidopsis CYP86A1, cork oak CYP86A32, and potato CYP86A33 (bootstrap value ≥ 980/1,000) together with rice CYP86A9 (bootstrap value ≥ 870/1,000) fell into the same subclade. All the other members of the CYP86A subfamily grouped according to paralogous relationships as shown by prior phylogenetic analyses (Nelson et al., 2004; Duan and Schuler, 2005). CYP94C1 was excluded from the CYP86A clade. The amino acid sequence alignment of CYP86A32, CYP86A33, and the two most homologous proteins, CYP86A1 and CYP86A9, showed high sequence conservation (Fig. 1B), including residues predicted to contact oleic acid in Arabidopsis CYP86A proteins (Rupasinghe et al., 2007). Conservation was more pronounced in the sequence region ranging from the substrate recognition site 4 (SRS4) to the C-terminal end.
Figure 1.
Phylogenetic analysis of CYP86A subfamily members. A, Phylogenetic analysis of CYP86A32 and CYP86A33. Amino acid sequences of Q. suber (Qs) CYP86A32 and S. tuberosum (St) CYP86A33 were aligned by ClustalW with the CYP86A protein subfamily of Arabidopsis (At) CYP86A1, CYP86A2, CYP86A4, CYP86A7, and CYP86A8 and rice (Oryza sativa; Os) CYP86A9, CYP86A10, and CYP86A11. A CYP94 family member (CYP94C1) was also added. Alignment was used to construct a neighbor-joining tree using the MEGA package, version 3.1. Identical clade distribution and similar bootstrap values were obtained using an unweighted pair group method with arithmetic mean algorithm. Bootstrapped values indicating the level of significance (%) by the separation of the branches are shown. The branch length indicates the extent of the difference according to the scale at the bottom. Note that CYP86A1, CYP86A32, CYP86A33, and CYP86A9 are grouped within the same subclade, although CYP86A9 is separated from the rest. CYP86A and CYP94 are clearly separated in two major clades as previously reported. B, Amino acid sequence alignment of the related proteins CYP86A32, CYP86A33, CYP86A1, and CYP86A9. Amino acids that are identical or similar are shaded in dark or light gray, respectively. SRSs are underlined, and the residues, which it is predicted will contact the oleic acid substrate, are shaded in black.
CYP86A33 Tissue Specificity and Down-Regulation in Potato
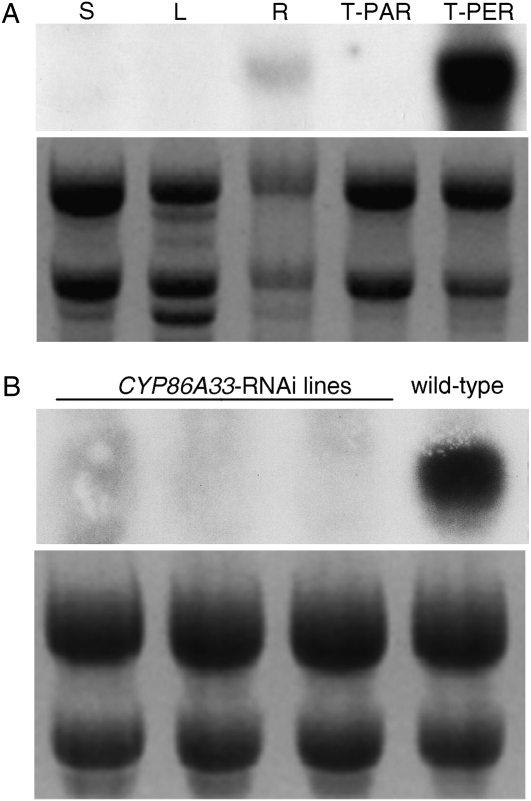
A northern-blot analysis to verify the expression pattern of CYP86A33 in potato tissues prior to RNA interference (RNAi) silencing revealed marked tissue and organ specificity (Fig. 2A). The transcript accumulated heavily in the periderm (tuber skin) and in smaller amounts in the primary root, with both containing suberin. No expression could be detected in stem, leaf, or tuber parenchyma.
Figure 2.
Northern-blot analysis of CYP86A33 in potato organs and tuber tissues. Northern-blot membranes and ethidium bromide-stained gels are shown in the upper and lower panels of each image, respectively. A, Transcript profile in potato: stem (S), leaf (L), root (R), tuber parenchyma (T-PAR), and tuber periderm (T-PER). Note that the CYP86A33 transcript accumulates only in suberized tissues: roots and tuber periderm. B, Transcript accumulation in tuber periderms of three independent CYP86A33-RNAi transgenic potato plants in comparison to wild type. Periderms from these three lines were selected for chemical and water permeability analyses.
To prevent the silencing of off-target genes, the CYP86A33 RNAi-mediated silencing in potato was performed using a nonconserved region of 224 bp, identified by BLASTN analysis (Altschul et al., 1990) against the most similar sequences in TIGR potato database. Double insertion of this gene region into the pBIN19RNAi vector using Gateway LR clonase generated an inverted repeat construct capable of producing a hairpin chimera that targets the CYP86A33 mRNA. Transgenic plants were produced by Agrobacterium-mediated transformation. Twenty-nine kanamycin-resistant lines from independent transformation events were grown in the greenhouse until tuber harvesting. Northern-blot analysis of the tuber skin identified 14 of these lines (48%) as effectively silenced. Gene silencing was reconfirmed in three lines (Fig. 2B) that were propagated to produce enough tubers to analyze the chemical composition and water permeability of the periderm. For data presented here, tubers were harvested from 8-week-old plants and stored for 21 d at room temperature before periderm analysis.
To identify putative unintended targets of CYP86A33 silencing, a BLASTN analysis in TIGR potato database (http://compbio.dfci.harvard.edu/tgi/cgi-bin/tgi/gimain.pl?gudb=potato) was performed using the CYP86A33-RNAi fragment as a query. The most homologous ESTs are TC175254 and BQ514437 (64% similarity), which have maximal similarity to Arabidopsis CYP86A2 and CYP96A9, respectively (Supplemental Table S1). According to microarray experiments in TIGR Solanaceae Genomics Resource (http://www.tigr.org/tdb/sol), these two genes are highly expressed in tuber skin and the CYP86A9 putative ortholog is also induced during tuber onset just as CYP86A33. On the other hand, TC175254 and BQ514437 alignment with the CYP86A33-RNAi fragment (Supplemental Fig. S1A) shows that BQ514437 has a longer sequence with high identity (92% identity from 174–200 nucleotides of RNAi fragment) and therefore a higher probability of being RNAi off-target exists. To check whether the transcripts of this gene could be off-targets of CYP86A33 short-interfering RNA, its mRNA level was analyzed by means of a reverse transcription (RT)-PCR analysis in parallel with those of ACTIN and CYP86A33. Results showed that, whereas the levels of CYP86A33 transcript are lower in CYP86A33-silenced lines, those of BQ514437 are not affected by silencing (Supplemental Fig. S1B). Taking into account this result and the information available in potato databases, there is no cross-silencing and therefore we can assume that the phenotype observed in CYP86A33-silenced lines must be a consequence of CYP86A33 deficiency.
CYP86A33 Down-Regulation and Suberin Fine Structure
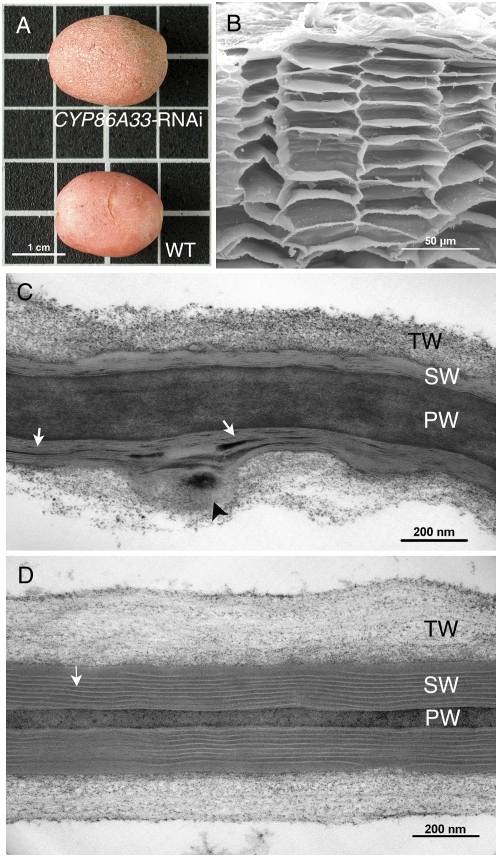
Potato plants silenced for CYP86A33 developed normally either in vitro or in pots. No phenotypic changes could be observed in leaves, shoots, roots, or developing tubers when compared to wild-type plants. Neither when freshly harvested nor during postharvesting storage at room temperature did the tubers from CYP86A33-silenced plants show a different phenotype in comparison to wild type. Only when the period of storage was greater than 2 months did tubers deficient in CYP86A33 appear somewhat more wrinkled than wild type (Fig. 3A). The anatomy and fine structure of the periderm was analyzed in 21-d-stored tubers. Examining the periderm using light and fluorescence microscopy did not reveal obvious differences in the general cellular architecture between wild-type and CYP86A33-silenced tubers. Neither did a more detailed observation using scanning electron microscopy (SEM) show any obvious difference (Fig. 3B). However, for CYP86A33-silenced periderm TEM analysis revealed a pronounced reduction in the thickness of the suberin or secondary wall and a deep alteration in the suberin ultrastructure (Fig. 3C) as compared to wild type (Fig. 3D). In CYP86A33-silenced periderm cell walls, the typical regular lamellation of the suberin layer disappeared, whereas the electron-translucent and electron-dense material formed prominent clumps and thick lamellar deposits (Fig. 3C, white arrows). The surface of the suberin layer in contact with the tertiary polysaccharide wall appeared uneven. Some cell walls, but not all, showed bulges or folds affecting the suberin layer and the tertiary wall (Fig. 3C, arrowhead).
Figure 3.
Anatomy and fine structure of periderms of CYP86A33-RNAi tubers. A, Wild-type (WT) and CYP86A33-silenced tubers. B, SEM sections of the down-regulated periderm reveals a normal organization of the cell layers as in wild-type periderm (not shown). C, TEM micrograph of the suberized cell wall of CYP86A33-RNAi peridermal cells. D, TEM micrograph of the suberized cell wall of wild-type peridermal cells. PW, Primary wall; SW, secondary suberin wall; TW, tertiary wall; white arrows, suberin lamellation; black arrowhead, atypical structures consisting of bulges or folds in the suberin wall. [See online article for color version of this figure.]
CYP86A33 Down-Regulation and Periderm Lipid Composition
The chemical composition of the soluble (wax) and insoluble (suberin) aliphatic fractions was analyzed by gas chromatography (GC) in periderm from the three independent CYP86A33 down-regulated lines (Fig. 2B). Periderm was enzymatically isolated as detailed in “Materials and Methods.” For chemical analysis, the wax fraction was extracted by organic solvent and the remaining suberin polyester analyzed after transesterification.
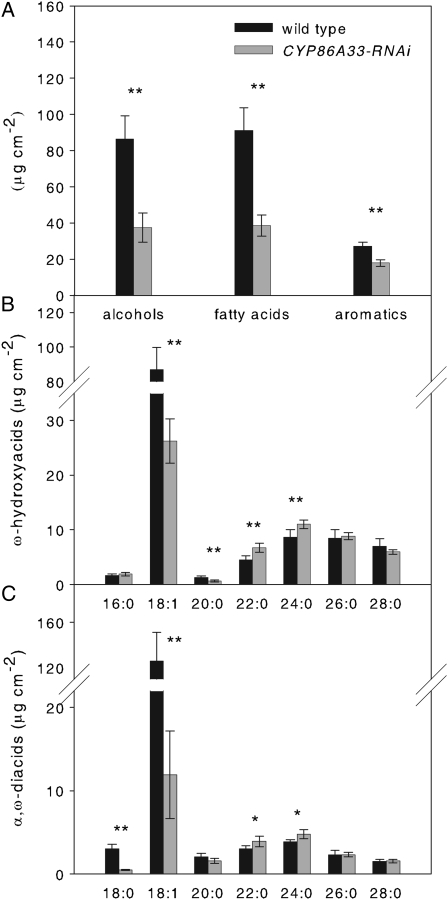
Enzymatically isolated periderm from CYP86A33-silenced tubers had a more fragile appearance, breaking easily when handled and, once dried, it was about 50% lighter than wild type (CYP86A33-RNAi 1.80 ± 0.23 mg cm−2, wild-type 2.69 ± 0.23 mg cm−2). The aliphatic suberin, which is the main lipid fraction accounting for 96% of the total lipids in wild-type periderm, was greatly affected by CYP86A33 deficiency. As measured in periderm from 21-d-stored tubers, total aliphatic suberin amounted to 462.27 ± 69.93 μg cm−2 in wild type, but only 180.88 ± 22.38 μg cm−2 in CYP86A33-silenced periderm, a reduction of 60%. All monomers showed a significant decrease (Fig. 4). The greatest reduction was in ω-hydroxyacids (Fig. 4B) and α,ω-diacids as a whole (Fig. 4C). Specifically, C18:1 α,ω-diacid and ω-hydroxyacid were reduced by 90% and 70%, respectively; C20:0 ω-hydroxyacid and C18:0 α,ω-diacid were also significantly reduced; C22:0 and C24:0 ω-hydroxyacids and α,ω-diacids showed a very minor, but statistically significant, increase. Glycerol was reduced by 60% (from 63.57 ± 19.79 μg cm−2 in wild type to 25.89 ± 13.84 μg cm−2 in CYP86A33 down-regulated periderm). In contrast with suberin, the wax fraction (4% of total lipids in wild type) was increased by a factor of 2.4 in the CYP86A33-RNAi periderm (Table I). The increase was higher in free fatty acids and ferulic acid esters (a 3.4-fold increase), whereas alkanes and alcohols showed only minor changes (Table I).
Figure 4.
Aliphatic suberin chemical composition of wild-type and CYP86A33-RNAi periderms from 21-d-stored tubers. A, Total amounts of primary alcohols, fatty acids, and aromatic compounds. B, All ω-hydroxyacid monomers identified. C, All α,ω-diacid monomers identified. Asterisks denote a significant difference between CYP86A33-RNAi (n = 6) and wild-type (n = 4) periderms (t test; *, P < 0.05; **, P < 0.01). Data are represented as an average of three independent CYP86A33-RNAi lines. Data represent the mean ± sd.
Table I.
Wax monomer content (μg cm−2) of wild-type (n = 4) and CYP86A33-RNAi periderms (n = 6) from 21-d-stored tubers
The soluble aliphatic fraction was obtained by treating the enzymatically isolated periderm with 1:1 chloroform:methanol. Absolute amounts of released monomers and the total amount of each substance class are given. Asterisks denote a significant increase (↑) in CYP86A33-RNAi periderms compared to wild type (t test; *, P < 0.05; **, P < 0.01). Data are represented as an average of three independent CYP86A33-RNAi lines. Data represent the mean ± sd.
| Wax Chemical Composition
|
||
|---|---|---|
| Wild Type | CYP86A33-RNAi | |
| μg cm−2 | μg cm−2 | |
| Alkanes | ||
| C25 | 3.67 ± 2.70 | 2.92 ± 1.33 |
| C26 | 0.25 ± 0.19 | 0.11 ± 0.07 |
| C27 | 1.00 ± 0.25 | 2.88 ± 0.52 **↑ |
| Total | 4.93 ± 2.98 | 5.92 ± 1.11 |
| Alcohols | ||
| C26 | 0.20 ± 0.09 | 0.48 ± 0.12 **↑ |
| C28 | 3.31 ± 0.87 | 2.86 ± 0.37 |
| C30 | 0.61 ± 0.19 | 1.31 ± 0.55 *↑ |
| Total | 4.12 ± 0.98 | 4.65 ± 0.42 |
| Fatty acids | ||
| C22:0 | 0.13 ± 0.10 | 0.15 ± 0.03 |
| C24:0 | 0.11 ± 0.05 | 0.31 ± 0.03 **↑ |
| C26:0 | 0.11 ± 0.05 | 0.84 ± 0.18 **↑ |
| C28:0 | 0.39 ± 0.18 | 1.70 ± 0.45 **↑ |
| C30:0 | 0.46 ± 0.22 | 1.07 ± 0.25 **↑ |
| Total | 1.20 ± 0.43 | 4.06 ± 0.88 **↑ |
| Ferulic acid esters | ||
| C18 | 0.34 ± 0.20 | 0.63 ± 0.15 *↑ |
| C20 | 0.16 ± 0.09 | 0.51 ± 0.10 **↑ |
| C21 | 0.28 ± 0.09 | 2.51 ± 0.45 **↑ |
| C22 | 0.08 ± 0.03 | 0.79 ± 0.21 **↑ |
| C23 | 0.38 ± 0.09 | 4.93 ± 0.89 **↑ |
| C24 | 0.13 ± 0.04 | 1.71 ± 0.47 **↑ |
| C25 | 0.16 ± 0.05 | 0.69 ± 0.12 **↑ |
| C26 | 0.31 ± 0.10 | 1.75 ± 0.34 **↑ |
| C27 | 0.14 ± 0.09 | 0.33 ± 0.05 **↑ |
| C28 | 1.36 ± 0.53 | 3.64 ± 0.41 **↑ |
| C29 | 0.71 ± 0.31 | 2.08 ± 0.41 **↑ |
| C30 | 3.34 ± 0.91 | 7.61 ± 0.85 **↑ |
| C31 | 0.56 ± 0.35 | 1.16 ± 0.30 *↑ |
| Total | 7.96 ± 2.33 | 28.32 ± 3.39 **↑ |
| Total waxes | 18.21 ± 5.02 | 42.95 ± 3.08 **↑ |
To exclude the possibility that the changes in suberin and wax composition of CYP86A33 down-regulated plants could be due to a delay in the periderm maturation process, freshly harvested (0-d-stored) and 60-d-stored tubers were analyzed for comparison. For both, the general variation in aliphatic suberin composition between CYP86A33-silenced and wild-type tubers (Supplemental Fig. S2) was similar to that observed in 21-d-stored tubers (Fig. 4). Moreover, the aliphatic suberin load was in both freshly harvested and 60-d-stored tubers reduced by 60% and the greatest reductions corresponded to C18:1 ω-hydroxyacids and α,ω-diacids. As regards the wax fraction, the effects of CYP86A33 knockdown in wax amount and chemical composition were similar in freshly harvested and 21-d-stored tubers. For 60-d-stored tubers, the total wax load showed no statistically significant differences compared with wild type.
CYP86A33 Down-Regulation and Periderm Water Permeability
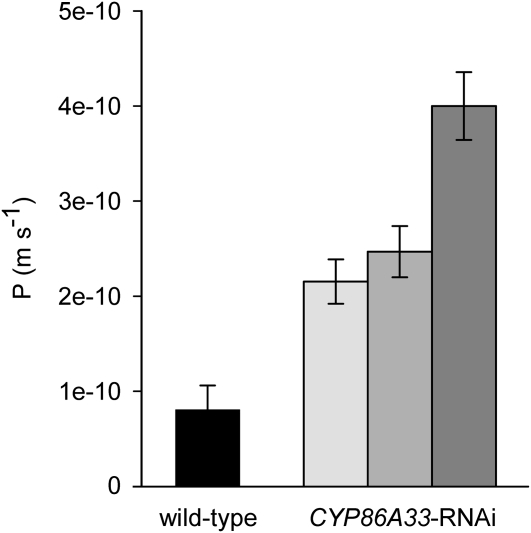
The water permeability of isolated periderm was measured by a gravimetric method in wild type and in the three independent CYP86A33 down-regulated lines. Water permeance in silenced periderms showed on average a 3.5-fold increase compared with that of wild-type periderm (Fig. 5). Permeance increased significantly from 0.81 ± 0.26 × 10−10 m s−1 (mean ± sd) in wild type to an average of 2.82 ± 0.838 × 10−10 m s−1. Similar results were obtained using periderm from 60-d-stored tubers (Supplemental Fig. S3).
Figure 5.
Water permeances of isolated periderms of CYP86A33-RNAi and wild type from 21-d-stored tubers. CYP86A33 silencing impairs periderm barrier properties and increases water permeance (P; m s−1) in periderms from three independent CYP86A33-RNAi lines (n = 3, n = 3, and n = 4) compared to wild type (n = 22). Data represent the mean ± sd.
DISCUSSION
Results presented here suggest that CYP86A33 is critical for the aliphatic suberin biosynthesis in potato periderm. Decrease on ω-functional fatty acids has strong consequences in the ultrastructural organization and water-sealing properties of suberin. The results are consistent with the fact that CYP86A33 transcript accumulates only in tissues where suberin is deposited (Fig. 2A). Moreover, our findings agree with the pattern of transcript accumulation during the stolon-to-tuber transition because accumulation begins at tuberization onset, as shown by the microarray hybridization data stored in TIGR Solanaceae genomics resource (http://www.tigr.org/tigr-scripts/tdb/sol/study/sol_expression.pl).
Significance of ω-Functional Fatty Acids in Suberin Biosynthesis
In wild-type potato periderm, the aliphatic suberin and wax chemical composition was comparable with previous analysis (Schreiber et al., 2005). The most obvious general result of CYP86A33 silencing was the reduction by 60% of total aliphatic suberin affecting all monomers, but first and foremost C18:1 ω-hydroxyacid (70% decrease) and α,ω-diacid (90% decrease), which are the main monomers in aliphatic potato suberin. This fact, together with a marked drop in C20:0 ω-hydroxyacid and C18:0 and C20:0 α,ω-diacid, emphasizes the importance of CYP86A33 for ω-hydroxylation in periderm, taking into account that ω-hydroxyacids are precursors for the synthesis of α,ω-diacids (Agrawal and Kolattukudy, 1977; Dean and Kolattukudy, 1977). The very minor increases in C22 and C24 ω-hydroxyacid and α,ω-diacid, observed only in 21-d-stored tubers, were not sufficient to compensate for the drop in ω-functionalized fatty acids. Nonetheless, as a consequence of CYP86A33 silencing, all substance classes other than ω-hydroxyacids and α,ω-diacids were also reduced (Fig. 4). Of note is that glycerol was reduced by 60%. This fact may be a consequence of the interaction between suberin metabolic pathways and/or an impaired capacity to esterify such monomers due to the drop in α,ω-functional groups. On the other hand, neither C18:1 ω-hydroxyacid nor α,ω-diacid was totally omitted in suberin from CYP86A33-silenced tubers. This low accumulation of products may be due to residual activity of CYP86A33 or more probably to redundant ω-hydroxylases. The latter explanation seems more probable when we consider that knockout mutants of CYP86A1 (Li et al., 2007; Hofer et al., 2008) and CYP86A2 (Xiao et al., 2004) showed low levels of ω-functionalized products in suberin and cutin, respectively. As far as the periderm wax fraction is concerned, representing only 4% of periderm aliphatics, CYP86A33 down-regulation produced opposite effects in comparison with aliphatic suberin. The total extracted amount of wax was greatly increased. Most free fatty acids, primary alcohols, and ferulate esters were augmented, but not all (Table I; Supplemental Table S2). Such increases may result from the accumulation of some aliphatic precursors that cannot be incorporated into the suberin. However, other mechanisms controlling the synthesis, degradation, and redirection of monomers to other pathways should also be active in periderm.
All members of the CYP86A subfamily in Arabidopsis analyzed so far have a demonstrated fatty acid ω-hydroxylase activity in vitro (Benveniste et al., 1998; Rupasinghe et al., 2007). A fatty acid ω-hydroxylase activity may also be expected for potato CYP86A33 based on the changes that its deficiency produces in the aliphatic suberin composition that mainly concern the ω-functionalized monomers. The fact that aliphatic suberin compounds other than ω-hydroxyacids and diacids were also altered by CYP86A33 silencing is not in contradiction with a ω-hydroxylase activity for CYP86A33 because the Arabidopsis cyp86a1 mutant also produces similar alteration in suberin composition (Li et al., 2007; Hofer et al., 2008). The similarity in suberin phenotype between CYP86A33- and CYP86A1-deficient plants, in conformity with a high amino acid sequence similarity (89%) between both genes (CYP86A33 and CYP86A1), together with the careful selection of an unconserved region for the CYP86A33-hpRNAi construct, strengthens the hypothesis that CYP86A33 deficiency is responsible of the alterations observed in CYP86A33-RNAi periderm in comparison to wild type. To unequivocally prove that CYP86A33 has ω-hydroxylase activity, we overexpressed the gene in yeast (Saccharomyces cerevisiae) and in Nicotiana benthamiana and measured the enzymatic activity in isolated microsomes using lauric acid as substrate. However, none of these systems allowed detection of any ability of the gene product to metabolize free fatty acids. Of note is that several authors point out that substrates other than free fatty acids, such as fatty acids activated, linked to glycerol or to the polymer, could be the natural substrates of CYP86A proteins (Cahoon et al., 2002; Beisson et al., 2007; Li et al., 2007; Pollard et al., 2008). Moreover, the possibility that CYP86A33 could also catalyze the complete oxidation of the methyl to the carboxyl group, as reported for CYP94A5 (Le Bouquin et al., 2001) and CYP94C1 (Kandel et al., 2007), cannot be excluded.
ω-Functional Fatty Acids Are Required for the Periderm Fine Structure and Water Barrier Function
Our analysis allowed the measurement of the effects of CYP86A33 silencing in the sealing properties of periderm cell walls. Both the thickness and the ultrastructure of the suberin layer (Fig. 3, C and D) were greatly affected by CYP86A33 deficiency, as might be expected from the dramatic changes in the amount of periderm lipid and its composition. These results generally conform to the effects of CYP86A2 deficiency in cuticles as shown by the Arabidopsis att1 mutant, which presents 70% less cutin and a loose cuticle ultrastructure in comparison with wild type (Xiao et al., 2004). A very significant alteration in the suberin ultrastructure in CYP86A33-silenced periderm can be deduced from the loss of the typical regular lamellation and the formation of a few short irregular lamellae and conspicuous clumps of electron-dense and electron-translucent material (Fig. 3, C and D). Although it is generally accepted that translucent lamellae correspond to aliphatic zones and dense lamellae to aromatic rich zones (Schmutz et al., 1993, 1996; Graça and Santos, 2007), suberin macromolecular organization has not been clarified (Franke and Schreiber, 2007). There is some evidence to support the hypothesis that the glycerol-α,ω-diacid-glycerol unit is the base for suberin's three-dimensional structure and that the ω-hydroxyacids are pivotal for the connection of the several translucent lamellae of the suberin polyester and the linkage to the aromatics (Arrieta-Baez and Stark, 2006; Graça and Santos, 2006). According to this hypothesis, the leading cause of the changes observed in the suberin ultrastructure of CYP86A33-silenced periderm seems to be the great reduction experienced by C18:1 ω-functionalized monomers. An impairment in the formation of glycerol-α,ω-diacid-glycerol units is consistent with a significant decrease in glycerol and thus with the reduction observed in the lamellation and thickness of the suberin wall. Fewer linkages of ω-hydroxyacids to aromatics agrees with a decrease in esterified aromatic monomers and hence with the formation of clumps of dense material and the loss of the typical regular lamellation. However, clarifying the respective role of diacids and ω-hydroxyacids in assembling the suberin macromolecular structure would require a model in which only one type of ω-functional fatty acids was reduced.
The main function of periderm is the protection it affords against water loss. However, the relationship between the periderm lipid coverage and the water transpiration properties is not fully understood (Schreiber et al., 2005). The crucial role of waxes in water-sealing properties of the periderm has been reported by several authors (Soliday et al., 1979; Vogt et al., 1983; Schreiber et al., 2005), but the periderm of CYP86A33-RNAi plants provides clear evidence that aliphatic suberin is also relevant for the water permeability of periderm. Although in CYP86A33-RNAi periderm the wax load increases by 2-fold, the permeability to water experiences a 3.5-fold raise, which can be attributed to the marked reduction in aliphatic suberin monomers (a 60% decrease) and the altered ultrastructure of the suberin. Both the monomer's load and the structural organization of the lipid polyester determine the suberin sealing properties. Our results are in general agreement with cutin mutants in which a 60% to 70% reduction in cutin load, accompanied by a distortion in cutin ultrastructure, leads to an increase (from 2- to 4-fold) in permeability to water (Xiao et al., 2004; Li et al., 2007). A relationship between the aliphatic suberin load and permeability to water was suggested by increased permeability to tetrazolium salt of the seed coat of gpat5 defective in suberin (Beisson et al., 2007). The seed coats of gpat5 displayed a more fragile appearance and break easily when handled as occurs in periderm from CYP86A33-silenced plants. That not only the lipid amount, but also the structural features of the polymer, should be significant for the sealing properties is supported by observations in the cuticle of plants co-overexpressing GPAT5/CYP86A1, which shows a higher load of aliphatic monomers, but also increased permeability to water (Li et al., 2007).
MATERIALS AND METHODS
Plant Material and Growth Conditions
Potato (Solanum tuberosum ‘Desirée’) plants were propagated in vitro and tubers were grown in the greenhouse. For in vitro propagation, stem cuttings were cultured in Murashige and Skoog medium (Duchefa) supplemented with 2% (w/v) Suc and grown in growth cabinets under a light/dark photoperiod cycle of 16/8 h at 22°C and 67 μmol m−2 s−1. In vitro plants were transferred to soil and grown for about 2 months in the greenhouse for tuber production. Tubers were harvested from 8-week-old plants and stored at room temperature before analysis.
Cloning of the Full-Length CYP86A32 and CYP86A33 Sequences
The full-length of CYP86A32 (accession no. EU293406) was obtained from the cork oak ESTs EE743846, EE745211, and EE743708 (Soler et al., 2007) by 5′- and 3′-RACE (Invitrogen) according to the manufacturer's protocols. Gene-specific primers 5′-TCAGGGTCAAACAGAAGGA-3′ and 5′-GGGTGACATGTGACAGTGAA-3′ were used to amplify the 5′-end and 5′-AGCGAAGTGGATCGAGAAG-3′ to amplify the 3′-end sequences. The PCR products were cloned into pCR4-TOPO (Invitrogen) and sequenced using the BigDye Terminator 3.1 kit (Applied Biosystems). The full-length CYP86A33 (accession no. EU293405) was obtained by TBLASTX analysis at the TIGR potato database (http://www.tigr.org) using the cork oak CYP86A32 sequence as a query. Tentative consensus TC114700 was the best match. The CYP86A33 sequence was PCR amplified from a cDNA tuber skin, using the gene-specific primers 5′-CACATTGAAAATTACAACAAAGCA-3′ and 5′-TCCTCTCTACTTCACTTAAACCAAAA-3′. First-strand cDNA was synthesized using the SuperScript II reverse transcriptase (Invitrogen) and an oligo(dT)16 primer. PCR was carried out using Advantage Polymerase (Promega). CYP86A32 and CYP86A33 were named according to the P450 Nomenclature Committee (http://drnelson.utmem.edu/CytochromeP450.html).
Plasmid Construction
The silencing of CYP86A33 in potato plants was carried out by means of a 224-bp fragment that encompasses nucleotides 1220 to 1443. This fragment was PCR amplified from the BQ513101 clone (Arizona Genomics Institute) using primers 5′-TTTACTCGGTGGGGAGAATG-3′ and 5′-CGGTAATAGCCGGTAACGAA-3′ bearing at their 5′-ends the attB1 and attB2 recombinant sequences, respectively. The PCR product was cloned into the donor plasmid pDONR207 (Invitrogen) by BP clonase II recombination (Gateway Technology; Invitrogen). The binary destination vector (pBIN19RNAi) was obtained by subcloning the Gateway RNAi cassette from pH7GWIWG2(II) (www.psb.rug.ac.be/gateway; Karimi et al., 2002) into the pBIN19 vector. For this purpose, the RNAi cassette was excised by a partial XbaI digestion and a HindIII digestion, and inserted into the XbaI-HindIII sites of the pBIN19 plasmid. This cassette included a chloramphenicol resistance marker (CmR) and two ccdB genes flanked by recombinant attR1 and attR2 sequences in inversed orientations, separated by an intron. The CYP86A33 fragment from pDONR207 was transferred into the binary destination vector using LR clonase II (Invitrogen). The PCR insert and vector were incubated for 5 min at 65°C before the clonase was added to improve cloning efficiency and incubated overnight at room temperature. Recombination replaced the ccdB genes by the CYP86A33 fragment, yielding a hairpin construct able to trigger CYP86A33 mRNA degradation. Restriction enzyme digestion was used to verify the recombinant construct.
Plant Transformation for RNAi-Mediated Silencing
Potato leaves were infected with the Agrobacterium tumefaciens strain GV2260 transformed with the RNAi recombinant plasmid in accordance with Hofgen and Willmitzer (1988). Potato cv Desirée plants were transformed as previously described by Banerjee et al. (2006). Kanamycin-resistant plants were regenerated and grown until tuber development and analyzed for CYP86A33 mRNA accumulation in the tuber skin.
RNA Isolation and Northern Analysis
Total RNA was isolated from potato tissues using the guanidine hydrochloride method (Logemann et al., 1987). Samples (20 μg/lane) were separated in a 1.5% formaldehyde-agarose gel (w/v) and transferred onto a nylon membrane (Hybond-N; GE Healthcare). Hybridization was carried out in accordance with Amasino (1986). Filters were hybridized at 42°C and washed in 3× SSC and 0.5% SDS at 65°C. The CYP86A33-RNAi fragment was used to obtain a CYP86A33 probe that was 32P-labeled (GE Healthcare) by the random-primed method (Roche).
RT-PCR with Incremental Cycle Numbers
We followed the procedure described by Soler et al. (2007). For each tissue analyzed, first-stranded cDNA was synthesized from 0.5 μg of total RNA using Superscript III (Invitrogen) in a 20-μL reaction and then 2.5-fold diluted. The total RNA used was previously treated with TURBO DNase (Ambion) to prevent genomic contamination and a further purification step was performed with the CleanUp protocol of RNeasy plant mini kit (Qiagen). For PCRs to be compared, we used 60-μL reactions with equal amounts of cDNA as a template and forward and reverse gene-specific primers, respectively, for the housekeeping ACTIN (TC119084; 5′-CCTTGTATGCTAGTGGTCG-3′ and 5′-GCTCATAGTCAAGAGCCAC-3′), for CYP86A33 (5′-TCTACTGGGGTATCCGCAAC-3′ and 5′-TTTGGTGAAAGGGTTTCAGG-3′) and for BQ514437 (5′-CAGGGCGTGATACGACTAGC-3′ and 5′-TACGAAGGCTCGTGCCTAAT-3′). Aliquots of 15 μL were taken every three cycles from cycle 30 to 36 and analyzed by agarose gel electrophoresis stained by ethidium bromide. To discard possible genomic DNA contaminations, the actin primers were designed complementary to two exons flanking an intron.
Isolation of Periderm Membranes
Freshly harvested tubers between 3 and 8 cm long were rinsed in tap water and stored for 21 and 60 d at room temperature, in the dark, if necessary. Periderms were isolated enzymatically as described (Vogt et al., 1983; Schreiber et al., 2005). Periderm discs were punched out from tubers using a cork borer (1-cm diameter) and immersed in a mixture of 2% (v/v) cellulase (Celluclast; Novo Nordiskand) and 2% (v/v) pectinase (Trenolin Super DF; Erbslöh) in 10−2 m citric buffer, pH 3.0 (adjusted with KOH). Sodium azide (Sigma) was added to prevent bacterial growth. During the 4-d incubation, the solution was changed two to three times until it was clear. Isolated periderm membranes were washed twice in 2 × 10−2 m boric buffer, pH 9.0, for 24 h and then carefully washed with deionized water. All incubations were carried out at room temperature with shaking at 30 rpm. Isolated periderms were dried and stored at room temperature until used.
In a strict sense, potato periderm is composed of three distinct tissues: the suberized tissue (phellem), the cambial layer (phellogen), and the phelloderm. During this enzymatic isolation, only the suberized phellem tissue is obtained, whereas phellogen and phelloderm are removed. However, we use the term periderm instead of phellem as a number of different authors have done in the past (Vogt et al., 1983; Stark et al., 1994; Schreiber et al., 2005).
Measurement of Peridermal Permeance
Peridermal permeability was measured using a gravimetric method previously described (Schönherr and Lendzian, 1981; Schreiber et al., 2005). Isolated periderm membranes were soaked overnight in deionized water, laid flat on plastic sheets, and left to dry at room temperature for 24 h. To avoid the effect of lenticels on water permeability measurements, small chambers (diameter 6 mm) and periderm fragments free of lenticels were used. Periderm membranes were mounted on water-filled transpiration chambers (300 μL) made of stainless steel with the physiological inner side of the isolated periderm facing the inner side of the chambers. Once mounted, the transpiration chambers were turned upside down to ensure direct contact between water and periderm. Chambers were kept in closed polyethylene boxes containing dry silica gel and stored at 25°C and the weight loss caused by evaporation of water across the periderm to the silica gel was determined at regular intervals using an analytical balance (Analytic AC210S; Sartorius). Transpiration kinetics was obtained by plotting the amounts of water (g), which had diffused across the periderm, against time (s). Permeances (P; m s−1) were calculated from the slopes (F; g s−1) of the linear regression lines and fitted to the transpiration kinetics using the following equation: P = F × (A × Δc)−1, where A (2.83 × 10−3 m2) corresponds to the exposed area and Δc (106 g m−3) represents the driving force, provided by the concentration of water in the chamber (Schreiber et al., 2005).
GC Analysis
Wax and aliphatic suberin extraction and GC-mass spectrometry (MS) and GC-flame ionization detector (FID) analysis were carried out as previously described (Schreiber et al., 2005). The total amount of periderm material used for each analysis ranged from 2 to 3 mg. Three independent CYP86A33 down-regulated lines were used for these studies. Wax was extracted from isolated periderms at room temperature for 18 h in a mixture (1:1 [v/v]) of chloroform and methanol. Chloroform-methanol extracts were used for wax analysis without further purification. For the depolymerization of aliphatic suberin, wax-free periderms were dried for at least 24 h in a desiccator containing silica gel, and subsequently transesterified by incubation at 70°C for 18 h with methanol-boron trifluoride (approximately 10% BF3 in methanol; Fluka; Kolattukudy and Agrawal, 1974; Zeier and Schreiber, 1998). Monomers released after transesterification and wax extracts obtained by chloroform-methanol extraction were analyzed as trimethylsilyl (TMS) derivatives by means of GC-MS and GC-FID. TMS derivatives were prepared after complete solvent evaporation (N2 gas) by adding 100 μL of chloroform, and derivatized with a 1:1 (v/v) mixture of 20 μL pyridine (GC grade; Merck) and 20 μL of N,N-bis-trimethylsilyltrifluoroacetamide (Macherey-Nagel) for 40 min at 70°C. Monomers were quantified using a GC-FID (HP 5890 Series II; Hewlett-Packard) and analyzed using HPChemStation software (Hewlett-Packard) by comparison with an internal standard (2 μg of tetracosane for wax and 10 μg dotriacontane for suberin). Identification of the compounds was carried out by GC and a quadrupole mass selective detector HP 5971A (Hewlett-Packard) as described previously (Schreiber et al., 2005).
Determination of Glycerol
The glycerol content in suberin was enzymatically quantified based on the method reported in the past (Schmutz et al., 1996; Moire et al., 1999). Prior to analysis, periderm membranes were treated with a chloroform-methanol mixture as described above. Then wax-free periderm membranes were transesterified at 70°C for 18 h with methanol-boron trifluoride (approximately 10% BF3 in methanol; Fluka) to release the aliphatic suberin compounds. The hydrolyzed cell wall residues were rinsed once with chloroform. The methanolysate were neutralized with saturated sodium hydrogen carbonate and the aqueous phase selected for the determination of glycerol. Free glycerol was quantified at 540 nm using the free glycerol reagent (Sigma) and a glycerol standard solution (triolein; Sigma). All three independent CYP86A33 down-regulated lines were used for this study.
Sequence Alignment and Phylogenetic Analysis
Amino acid sequence alignments were performed using the ClustalW program at the European Bioinformatics Institute (EBI; http://www.ebi.ac.uk/Tools/clustalw). The alignment was edited using the BioEdit Sequence Alignment Editor 7.0.1 (Hall, 1999). CYP86A SRSs and the amino acidic residues predicted to contact the oleic acid substrate were identified in accordance with Rupasinghe et al. (2007). Phylogenetic analyses were conducted using MEGA, version 3.1 (Kumar et al., 2004).
Periderm Microscopy
For light and fluorescence microscopy, small fragments of tuber periderm and isolated periderm discs were included in fresh potato blocks and cut using a rotation microtome (Anglia Scientific). Sections were mounted on slides in water and observed in bright field and epifluorescence on a Leica DMR-XA optical microscope (Leica Microsystems).
For SEM, small fragments of tuber periderm were fixed under vacuum with 4% formaldehyde in phosphate-buffered saline (pH 7.5) at room temperature for at least 48 h. Fragments were dehydrated with an increasing ethanol concentration series exchanged through amyl-acetate and critical point dried. The pieces were mounted on copper stubs and coated with gold. Specimens were observed using a Zeiss DSM 960A SEM (Zeiss). Digital images were collected and processed using the Quartz PCI 5.10 (Quartz Imaging Corporation).
For TEM, sections of periderm dissected into 1 × 1 mm2 were immediately placed in 100 mm sodium cacodylate buffer (pH 7.0) containing 2.5% (w/v) glutaraldehyde and 2% (w/v) paraformaldehyde for 2 to 4 h. A vacuum was applied until samples were submerged. Tissues were washed three times with 100 mm sodium cacodylate buffer (pH 7.0) and subsequently fixed overnight in 100 mm sodium cacodylate buffer (pH 7.0) containing 1% (w/v) osmium tetroxide. Samples were then washed with 100 mm sodium cacodylate buffer (pH 7.0), dehydrated in an acetone series (30%–100% by 10% steps, 10 min each step), and infiltrated with Spurr's epoxy resin (1:2, 1:1, and 2:1 resin:acetone [v/v] and pure resin for 4 h, overnight, 3 and 5 h, respectively). Infiltrated tissues were placed in molds and incubated at 60°C for 2 d. Embedded materials were thin sectioned using an ultramicrotome RMC MT-XL Sections were collected onto 200-mesh copper grids, stained with 2% (w/v) uranyl acetate for 15 min and rinsed for 30 min before being observed and photographed with the TEM ZEISS EM910 (Germany) at an accelerating voltage of 60 kV. Images were obtained on Kodak Electron Microscope film 4489 and scanned by HP 6100C (Hewlett-Packard).
All microscopic analyses were performed by the microscopy service of the University of Girona.
CYP86A32 (accession no. EU293406) and CYP86A33 (accession no. EU293405) have been isolated in this work. Three cork oak ESTs (accession nos. EE743846, EE745211, and EE743708) were used to obtain the full-length sequence of CYP86A32. The sequences CYP86A1 (accession no. NP_200694), CYP86A2 (accession no. NP_191946), CYP86A4 (accession no. NP_171666), CYP86A7 (accession no. NP_176558), CYP86A8 (accession no. NP_182121), CYP86A9 (accession no. AP003442), CYP86A10 (accession no. AP004139), CYP86A11 (accession no. AL606687) and CYP94C1 (accession no. NP_180337) were used to construct the phylogenetic tree.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Analysis of the putative off-target EST BQ514437 in CYP86A33-RNAi periderm.
Supplemental Figure S2. CYP86A33-RNAi aliphatic suberin chemical composition at different stages of tuber maturation.
Supplemental Figure S3. Water permeances of isolated periderms of CYP86A33-RNAi and unsilenced or control periderms from 60-d-stored tubers.
Supplemental Table S1. List of the potato ESTs most homologous to CYP86A33-RNAi fragment.
Supplemental Table S2. Wax chemical composition of CYP86A33-RNAi periderm at different stages of tuber maturation.
Supplementary Material
Acknowledgments
We would like to thank to Jordi Blavia and Carme Carulla (Serveis Tècnics de Recerca, Universitat de Girona) for their highly skilled work with TEM and SEM; Dr. Pilar Fontanet (Institut de Biologia Molecular de Barcelona) for helpful advice on potato treatments; and Imma Guardiola and Sara Gómez (Departament de Biologia, Universitat de Girona) for taking care of the transgenic plants.
This work was supported by the Spanish Ministerio de Ciencia y Tecnología (grant no. AGL2003–00416); the Ministerio de Educación y Ciencia (grant no. AGL2006–07342, an FPI grant, and two mobility grants to O.S.); the Departament d'Universitats, Investigació i Societat de la Informació of Catalonia and European Social Fund (FI grant to M.S.); and the Deutsche Forschungsgemeinschaft.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Mercè Figueras (merce.figueras@udg.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open access articles can be viewed online without a subscription.
References
- Agrawal VP, Kolattukudy PE (1977) Biochemistry of suberization—ω-hydroxyacid oxidation in enzyme preparations from suberizing potato-tuber disks. Plant Physiol 59 667–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215 403–410 [DOI] [PubMed] [Google Scholar]
- Amasino RM (1986) Acceleration of nucleic-acid hybridization rate by polyethylene-glycol. Anal Biochem 152 304–307 [DOI] [PubMed] [Google Scholar]
- Arrieta-Baez D, Stark RE (2006) Using trifluoroacetic acid to augment studies of potato suberin molecular structure. J Agric Food Chem 54 9636–9641 [DOI] [PubMed] [Google Scholar]
- Banerjee AK, Prat S, Hannapel DJ (2006) Efficient production of transgenic potato (S. tuberosum L ssp andigena) plants via Agrobacterium tumefaciens-mediated transformation. Plant Sci 170 732–738 [Google Scholar]
- Beisson F, Li Y, Bonaventure G, Pollard M, Ohlrogge JB (2007) The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. Plant Cell 19 351–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste I, Tijet N, Adas F, Philipps G, Salaun JP, Durst F (1998) CYP86A1 from Arabidopsis thaliana encodes a cytochrome P450-dependent fatty acid omega-hydroxylase. Biochem Biophys Res Commun 243 688–693 [DOI] [PubMed] [Google Scholar]
- Bernards MA (2002) Demystifying suberin. Can J Bot 80 227–240 [Google Scholar]
- Cahoon EB, Ripp KG, Hall SE, McGonigle B (2002) Transgenic production of epoxy fatty acids by expression of a cytochrome P450 enzyme from Euphorbia lagascae seed. Plant Physiol 128 615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean BB, Kolattukudy PE (1977) Biochemistry of suberization—incorporation of [1-14C]oleic acid and [1-14C]acetate into aliphatic components of suberin in potato-tuber disks (Solanum tuberosum). Plant Physiol 59 48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Schuler MA (2005) Differential expression and evolution of the Arabidopsis CYP86A subfamily. Plant Physiol 137 1067–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau K (1965) Plant Anatomy, Ed 2. John Wiley & Sons, New York, pp 366–381
- Franke R, Schreiber L (2007) Suberin—a biopolyester forming apoplastic plant interfaces. Curr Opin Plant Biol 10 252–259 [DOI] [PubMed] [Google Scholar]
- Graça J, Pereira H (2000) Suberin structure in potato periderm: glycerol, long-chain monomers, and glyceryl and feruloyl dimers. J Agric Food Chem 48 5476–5483 [DOI] [PubMed] [Google Scholar]
- Graça J, Santos S (2006) Linear aliphatic dimeric esters from cork suberin. Biomacromolecules 7 2003–2010 [DOI] [PubMed] [Google Scholar]
- Graça J, Santos S (2007) Suberin: a biopolyester of plants' skin. Macromol Biosci 7 128–135 [DOI] [PubMed] [Google Scholar]
- Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41 95–98 [Google Scholar]
- Hofer R, Briesen I, Beck M, Pinot F, Schreiber L, Franke R (2008) The Arabidopsis cytochrome P450 CYP86A1 encodes a fatty acid ω-hydroxylase involved in suberin monomer biosynthesis. J Exp Bot 59 2347–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofgen R, Willmitzer L (1988) Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res 16 9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel S, Sauveplane V, Compagnon V, Franke R, Millet Y, Schreiber L, Werck-Reichhart D, Pinot F (2007) Characterization of a methyl jasmonate and wounding-responsive cytochrome P450 of Arabidopsis thaliana catalyzing dicarboxylic fatty acid formation in vitro. FEBS J 274 5116–5127 [DOI] [PubMed] [Google Scholar]
- Karimi M, Inze D, Depicker A (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7 193–195 [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE (1980) Biopolyester membranes of plants—cutin and suberin. Science 208 990–1000 [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE (2001) Polyesters in higher plants. Adv Biochem Eng Biotechnol 71 1–49 [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE, Agrawal VP (1974) Structure and composition of aliphatic constituents of potato-tuber skin (suberin). Lipids 9 682–691 [Google Scholar]
- Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5 150–163 [DOI] [PubMed] [Google Scholar]
- Le Bouquin R, Skrabs M, Kahn R, Benveniste I, Salaun JP, Schreiber L, Durst F, Pinot F (2001) CYP94A5, a new cytochrome P450 from Nicotiana tabacum is able to catalyze the oxidation of fatty acids to the ω-alcohol and to the corresponding diacid. Eur J Biochem 268 3083–3090 [DOI] [PubMed] [Google Scholar]
- Li Y, Beisson F, Koo AJK, Molina I, Pollard M, Ohlrogge J (2007) Identification of acyltransferases required for cutin biosynthesis and production of cutin with suberin-like monomers. Proc Natl Acad Sci USA 104 18339–18344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann J, Schell J, Willmitzer L (1987) Improved method for the isolation of RNA from plant tissues. Anal Biochem 163 16–20 [DOI] [PubMed] [Google Scholar]
- Lulai EC, Freeman TP (2001) The importance of phellogen cells and their structural characteristics in susceptibility and resistance to excoriation in immature and mature potato tuber (Solanum tuberosum L) periderm. Ann Bot (Lond) 88 555–561 [Google Scholar]
- Lulai EC, Weiland JJ, Suttle JC, Sabba RP, Bussan AJ (2006) Pink eye is an unusual periderm disorder characterized by aberrant suberization: a cytological analysis. Am J Potato Res 83 409–421 [Google Scholar]
- Moire L, Schmutz A, Buchala A, Yan B, Stark RE, Ryser U (1999) Glycerol is a suberin monomer. New experimental evidence for an old hypothesis. Plant Physiol 119 1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C (2002) The biopolymers cutin and suberin. In C Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, doi/101199/tab0021 [DOI] [PMC free article] [PubMed]
- Nelson DR, Schuler MA, Paquette SM, Werck-Reichhart D, Bak S (2004) Comparative genomics of rice and Arabidopsis. Analysis of 727 cytochrome P450 genes and pseudogenes from a monocot and a dicot. Plant Physiol 135 756–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard M, Beisson F, Li Y, Ohlrogge JB (2008) Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci 13 236–246 [DOI] [PubMed] [Google Scholar]
- Rupasinghe SG, Duan H, Schuler MA (2007) Molecular definitions of fatty acid hydroxylases in Arabidopsis thaliana. Proteins 68 279–293 [DOI] [PubMed] [Google Scholar]
- Sabba RP, Lulai EC (2002) Histological analysis of the maturation of native and wound periderm in potato (Solanum tuberosum L) tuber. Ann Bot (Lond) 90 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HW, Schönherr J (1982) Fine-structure of isolated and non-isolated potato-tuber periderm. Planta 154 76–80 [DOI] [PubMed] [Google Scholar]
- Schmutz A, Buchala AJ, Ryser U (1996) Changing the dimensions of suberin lamellae of green cotton fibers with a specific inhibitor of the endoplasmic reticulum-associated fatty acid elongases. Plant Physiol 110 403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz A, Jenny T, Amrhein N, Ryser U (1993) Caffeic acid and glycerol are constituents of the suberin layers in green cotton fibres. Planta 189 453–460 [DOI] [PubMed] [Google Scholar]
- Schönherr J, Lendzian K (1981) A simple and inexpensive method of measuring water permeability of isolated plant cuticular membranes. Z Pflanzenphysiol 102 321–327 [Google Scholar]
- Schreiber L, Franke R, Hartmann K (2005) Wax and suberin development of native and wound periderm of potato (Solanum tuberosum L) and its relation to peridermal transpiration. Planta 220 520–530 [DOI] [PubMed] [Google Scholar]
- Soler M, Serra O, Molinas M, Garcia-Berthou E, Caritat A, Figueras M (2008) Seasonal variation in transcript abundance in cork tissue analyzed by real time RT-PCR. Tree Physiol 28 743–751 [DOI] [PubMed] [Google Scholar]
- Soler M, Serra O, Molinas M, Huguet G, Fluch S, Figueras M (2007) A genomic approach to suberin biosynthesis and cork differentiation. Plant Physiol 144 419–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliday CL, Kolattukudy PE, Davis RW (1979) Chemical and ultrastructural evidence that waxes associated with the suberin polymer constitute the major diffusion barrier to water-vapor in potato-tuber (Solanum tuberosum L). Planta 146 607–614 [DOI] [PubMed] [Google Scholar]
- Stark RE, Sohn W, Pacchiano RA, Albashir M, Garbow JR (1994) Following suberization in potato wound periderm by histochemical and solid-state 13C nuclear-magnetic-resonance method. Plant Physiol 104 527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt E, Schönherr J, Schmidt HW (1983) Water permeability of periderm membranes isolated enzymatically from potato-tubers (Solanum tuberosum L). Planta 158 294–301 [DOI] [PubMed] [Google Scholar]
- Wellesen K, Durst F, Pinot F, Benveniste I, Nettesheim K, Wisman E, Steiner-Lange S, Saedler H, Yephremov A (2001) Functional analysis of the LACERATA gene of Arabidopsis provides evidence for different roles of fatty acid omega-hydroxylation in development. Proc Natl Acad Sci USA 98 9694–9699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Goodwin SM, Xiao Y, Sun Z, Baker D, Tang X, Jenks MA, Zhou JM (2004) Arabidopsis CYP86A2 represses Pseudomonas syringae type III genes and is required for cuticle development. EMBO J 23 2903–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeier J, Schreiber L (1998) Comparative investigation of primary and tertiary endodermal cell walls isolated from the roots of five monocotyledoneous species: chemical composition in relation to fine structure. Planta 206 349–361 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.