Abstract
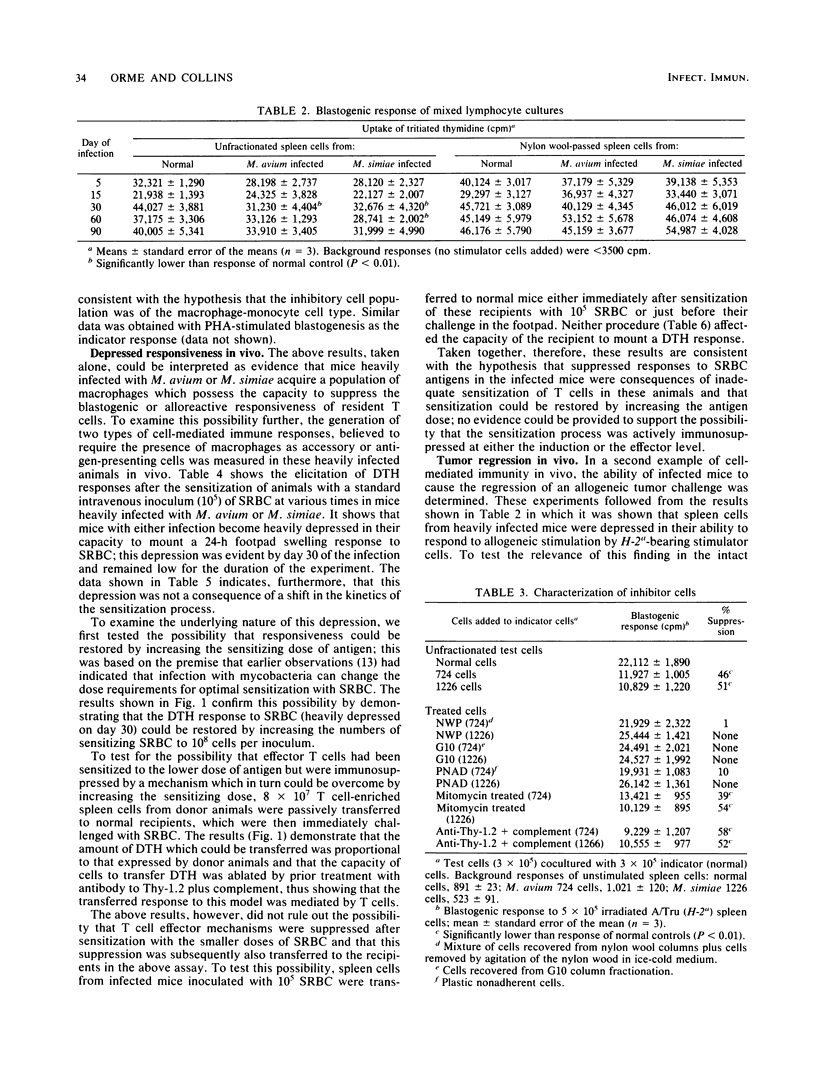
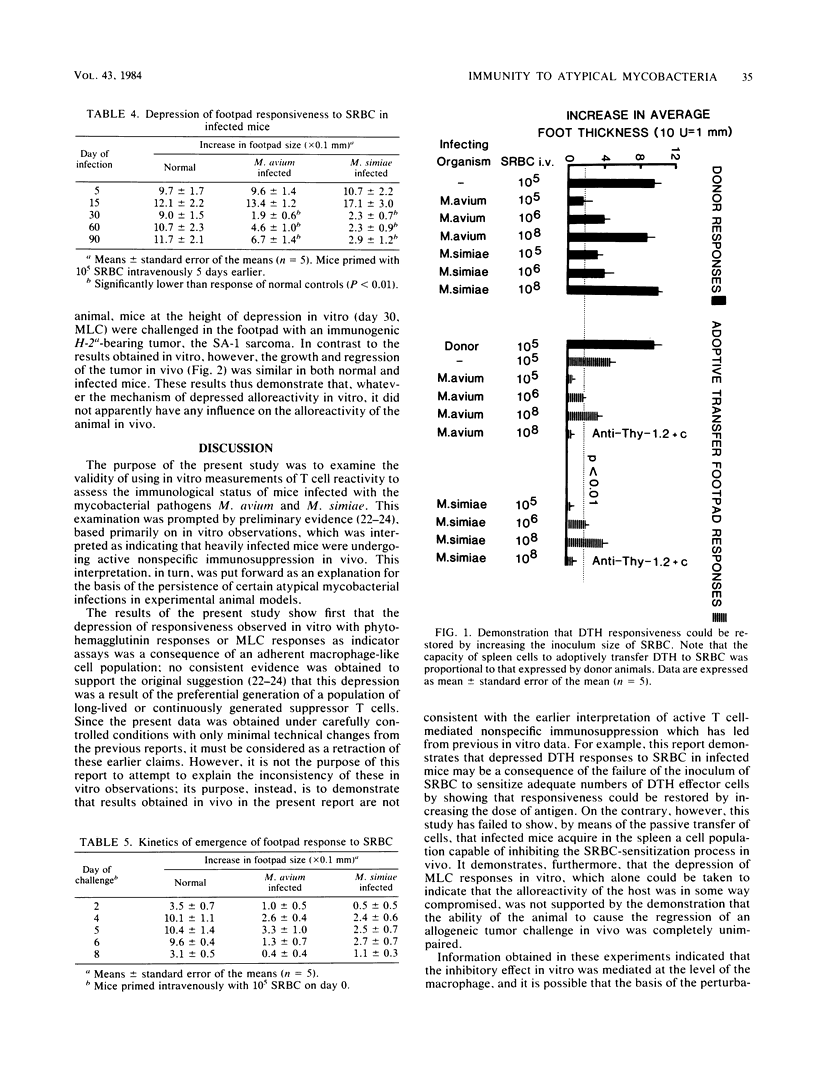
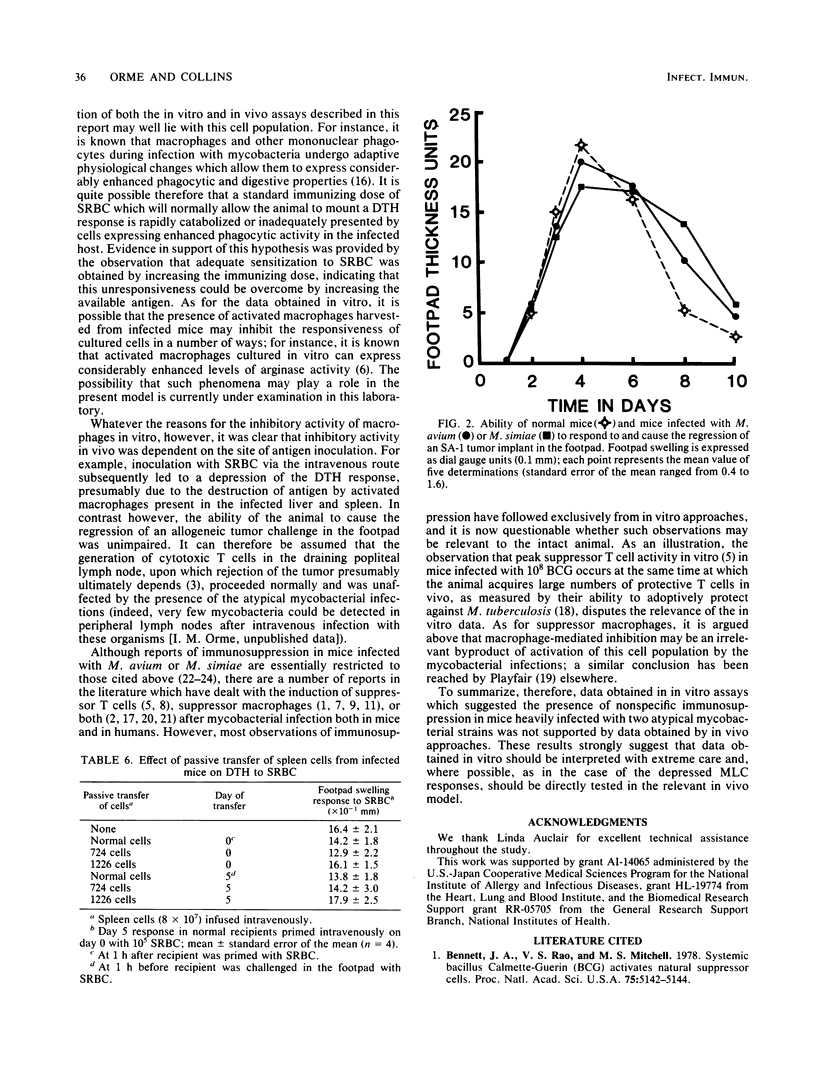
The results of in vitro experiments designed to measure the immunocompetence of mice heavily infected with the atypical mycobacterial pathogens Mycobacterium avium and Mycobacterium simiae were compared with the results of experiments which used in vivo approaches. Blastogenic responsiveness in vitro both to mitogen and to alloantigen was severely depressed in the heavily infected mice; this responsiveness could be restored by removal of an inhibitory Thy-1.2-, nylon wool-adherent cell population. No evidence was found to support the previous contention that suppressor T cells may play a role in the inhibition of this responsiveness. These results were then compared with experiments which measured the ability of the infected animal to elicit a delayed-type hypersensitivity response to sheep erythrocytes in vivo. However, although delayed-type hypersensitivity responses in vivo were also depressed, evidence was obtained which suggested that this unresponsiveness was due to inadequate sensitization of T cells, possibly due to catabolism of antigen, rather than due to the influence of an active, immunosuppressive mechanism. Finally, despite the severely depressed ability of cells from infected mice to respond to alloantigenic stimulation in vitro, infected animals were fully able to cause the regression of a tumor implant in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. A., Rao V. S., Mitchell M. S. Systemic bacillus Calmette-Guérin (BCG) activates natural suppressor cells. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5142–5144. doi: 10.1073/pnas.75.10.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock W. E., Carlson E. M., Gershon R. K. The evolution of immunosuppressive cell populations in experimental mycobacterial infection. J Immunol. 1978 May;120(5):1709–1716. [PubMed] [Google Scholar]
- Collins F. M., Morrison N. E., Montalbine V. Immune response to persistent mycobacterial infection in mice. Infect Immun. 1978 May;20(2):430–438. doi: 10.1128/iai.20.2.430-438.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Watson S. R. Suppressor T-cells in BCG-infected mice. Infect Immun. 1979 Aug;25(2):491–496. doi: 10.1128/iai.25.2.491-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie G. A. Activated macrophages kill tumour cells by releasing arginase. Nature. 1978 Jun 29;273(5665):758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- Ellner J. J. Suppressor adherent cells in human tuberculosis. J Immunol. 1978 Dec;121(6):2573–2579. [PubMed] [Google Scholar]
- Ito M., Ralph P., Moore M. A. Suppression of spleen natural killing activity induced by BCG. Clin Immunol Immunopathol. 1980 May;16(1):30–38. doi: 10.1016/0090-1229(80)90163-4. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Klimpel G. R., Henney C. S. BCG-induced suppressor cells. I. Demonstration of a macrophage-like suppressor cell that inhibits cytotoxic T cell generation in vitro. J Immunol. 1978 Feb;120(2):563–569. [PubMed] [Google Scholar]
- Lefford M. J. Transfer of adoptive immunity to tuberculosis in mice. Infect Immun. 1975 Jun;11(6):1174–1181. doi: 10.1128/iai.11.6.1174-1181.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B., Lagrange P. H., Ishibashi T. The modifying effect of BCG on the immunological induction of T cells. J Exp Med. 1974 Jun 1;139(6):1540–1552. doi: 10.1084/jem.139.6.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- North R. J. The concept of the activated macrophage. J Immunol. 1978 Sep;121(3):806–809. [PMC free article] [PubMed] [Google Scholar]
- Orbach-Arbouys S., Poupon M. F. Active suppression of in vitro reactivity of spleen cells after BCG treatment. Immunology. 1978 Mar;34(3):431–437. [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983 Jul 1;158(1):74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R. Evidence for two distinct populations of suppressor cells in the spleens of Mycobacterium bovis BCG-Sensitized mice. Infect Immun. 1981 Nov;34(2):315–322. doi: 10.1128/iai.34.2.315-322.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R., Lemieux S. Mechanisms of action of Mycobacterium bovis BCG-induced suppressor cells in mitogen-induced blastogenesis. Infect Immun. 1982 Apr;36(1):263–270. doi: 10.1128/iai.36.1.263-270.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. R., Collins F. M. Development of suppressor T cells in mice heavily infected with mycobacteria. Immunology. 1980 Mar;39(3):367–373. [PMC free article] [PubMed] [Google Scholar]
- Watson S. R., Collins F. M. Development of suppressor T-cells in Mycobacterium habana-infected mice. Infect Immun. 1979 Aug;25(2):497–506. doi: 10.1128/iai.25.2.497-506.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. R., Collins F. M. The specificity of suppressor T cells induced by chronic Mycobacterium avium infection in mice. Clin Exp Immunol. 1981 Jan;43(1):10–19. [PMC free article] [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]


