Abstract
The synthesis of phytochelatins (PCs) is essential for the detoxification of nonessential metals and metalloids such as cadmium and arsenic in plants and a variety of other organisms. To our knowledge, no direct evidence for a role of PCs in essential metal homeostasis has been reported to date. Prompted by observations in Schizosaccharomyces pombe and Saccharomyces cerevisiae indicating a contribution of PC synthase expression to Zn2+ sequestration, we investigated a known PC-deficient Arabidopsis (Arabidopsis thaliana) mutant, cad1-3, and a newly isolated second strong allele, cad1-6, with respect to zinc (Zn) homeostasis. We found that in a medium with low cation content PC-deficient mutants show pronounced Zn2+ hypersensitivity. This phenotype is of comparable strength to the well-documented Cd2+ hypersensitivity of cad1 mutants. PC deficiency also results in significant reduction in root Zn accumulation. To be able to sensitively measure PC accumulation, we established an assay using capillary liquid chromatography coupled to electrospray ionization quadrupole time-of-flight mass spectrometry of derivatized extracts. Plants grown under control conditions consistently showed PC2 accumulation. Analysis of plants treated with same-effect concentrations revealed that Zn2+-elicited PC2 accumulation in roots reached about 30% of the level of Cd2+-elicited PC2 accumulation. We conclude from these data that PC formation is essential for Zn2+ tolerance and provides driving force for the accumulation of Zn. This function might also help explain the mysterious occurrence of PC synthase genes throughout the plant kingdom and in a wide range of other organisms.
Both essential and nonessential metal ions can be toxic when present in excess. Zinc (Zn) ions, for instance, are used in biological systems as catalytic or structural components in a myriad of proteins (Frausto da Silva and Williams, 2001). In humans, about 10% of genes encode Zn-dependent proteins (Andreini et al., 2006) and it is reasonable to postulate similar numbers for plants. When the Zn-buffering capacity of a cell is exceeded, however, aberrant binding of Zn ions to thiols or other functional groups can occur, which disrupts the function of proteins. Also, Zn ions can displace other essential metal ions from their binding sites (Krämer and Clemens, 2005). Toxicity thresholds for Zn were found to range between 100 and 300 μg g−1 dry weight depending on plant species and physiological state (Marschner, 1995). Ions of the nonessential metal cadmium (Cd) have a high affinity for various functional groups in biological molecules, in particular thiols. When taken up into a cell they can inactivate proteins by uncontrolled binding or cause oxidative stress by depleting glutathione pools (Clemens, 2006a).
Because of the potential toxicity of metal ions, all living systems possess mechanisms to tightly regulate the distribution of metal ions and to minimize damage under conditions of excess metal supply (Eide, 2006; Grotz and Guerinot, 2006). Principally, detoxification of excess metal ions is assumed to be achieved by efflux, sequestration, and chelation. For instance, in the past few years evidence has been reported for a contribution of AtMTP1 (At2g46800) to vacuolar sequestration of Zn2+ (Kobae et al., 2004; Desbrosses-Fonrouge et al., 2005) and of AtHMA4 (At2g19110) to effluxing Zn2+ (Mills et al., 2005). Furthermore, the Arabidopsis halleri ortholog of HMA4 is essential for Zn and Cd hypertolerance (Hanikenne et al., 2008). Loss of ZIF1, a transporter of the major facilitator superfamily, results in Zn2+ hypersensitivity in Arabidopsis (Arabidopsis thaliana; Haydon and Cobbett, 2007).
The synthesis of phytochelatins (PCs), glutathione-derived metal-binding peptides, represents a major detoxification mechanism for Cd and arsenic (As) ions in various species. More recently, PCs have also been implicated in long-distance transport of Cd in the phloem (Mendoza-Cozatl et al., 2008). Formation of PCs is catalyzed by PC synthases (PCS) and genes encoding this enzyme have been cloned from plants, fungi, and nematodes (Clemens et al., 1999, 2001; Ha et al., 1999; Vatamaniuk et al., 1999, 2001). Mutant lines of Arabidopsis, Schizosaccharomyces pombe, or Caenorhabditis elegans that are deficient in PC synthesis show a severe loss of Cd and As tolerance (Clemens et al., 1999; Ha et al., 1999; Vatamaniuk et al., 2001). For other metal ions only minor effects have been reported (Cobbett and Goldsbrough, 2002). Arabidopsis cad1-3 mutant plants, which are defective in AtPCS1, showed about a 2-fold increase in copper (Cu) and mercury sensitivity and no significant increase in Zn sensitivity (Ha et al., 1999). S. pombe PCS-deficient mutants are slightly more Cu2+ sensitive than wild-type cells (Clemens et al., 1999). PC-metal complexes have been detected in plant cells only with Cd, silver, Cu, and As (Maitani et al., 1996; Schmöger et al., 2000) even though synthesis of PCs is activated by a wide range of metal ions both in vivo and in vitro (Grill et al., 1987; Vatamaniuk et al., 2000; Oven et al., 2002). Thus, the role of PC synthesis in metal detoxification has so far been seen as being confined to Cd and As (Cobbett and Goldsbrough, 2002). This, however, leaves the question as to why PCS genes are so widespread and why the enzyme is expressed constitutively throughout the plant (Rea et al., 2004). It is not clear how the sporadic need to sequester excess Cd or As ions could have provided the selective pressure to maintain PCS expression throughout the plant kingdom and beyond (Clemens, 2006b). One explanation could be the second enzymatic function of PCS, i.e. breakdown of glutathione conjugates (GS conjugates) to the corresponding β-glutamyl-Cys conjugates (Blum et al., 2007). The catalytic activity of bacterial PCS-like proteins is similar (Harada et al., 2004; Tsuji et al., 2004; Vivares et al., 2005). Another possibility is involvement of PC synthesis in essential metal homeostasis, which has been discussed occasionally (Steffens et al., 1986; Rauser, 1990; Steffens, 1990). As early as 1986, Steffens et al. suggested this based on the observation that small amounts of PCs were detectable in tomato (Solanum lycopersicum) cell cultures in the absence of excess metals. Similarly, Grill et al. reported the induction of PC synthesis by Cu and Zn ions after transfer of cultured cells into fresh medium. The amount of detectable PCs was correlated linearly with the Zn2+ concentration in the culture medium and an involvement of PC synthesis in metal homeostasis was proposed (Grill et al., 1987, 1988). However, no genetic evidence for such a role of PCs has been reported to date. Here we present evidence that PC formation indeed contributes significantly to Zn2+ detoxification in Arabidopsis and enhances Zn accumulation.
RESULTS
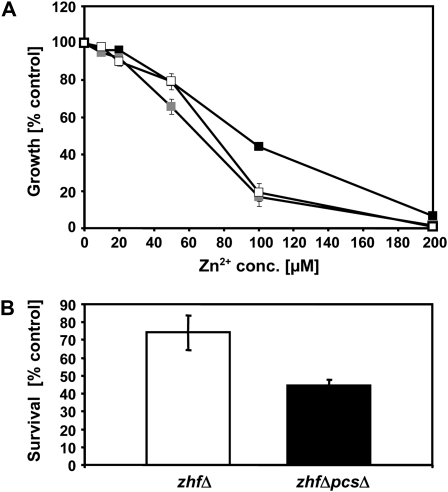
S. pombe pcsΔ mutant cells unable to synthesize PCs are hypersensitive to Cd2+. Their Zn2+ tolerance is not significantly reduced even though Zn2+ exposure elicits PC synthesis in S. pombe wild-type cells (Clemens et al., 1999; Ha et al., 1999; data not shown). This is different when the pcs gene is knocked out in zhfΔ mutant cells. Loss of Zhf (Zn homeostasis factor), an endoplasmic reticulum-localized transporter of the cation diffusion facilitator family, results in strong Zn2+ hypersensitivity (Clemens et al., 2002). Excess Zn can no longer be removed from the cytosol in zhfΔ cells (Boch et al., 2008). When we compared the growth of the zhfΔpcsΔ strain to zhfΔ cells, we found a significant decrease in Zn2+ tolerance correlated with the loss of functional PCS (Fig. 1A). In the presence of 100 μm Zn2+, zhfΔ growth was reduced by about 50%, whereas zhfΔpcsΔ cells showed a reduction in growth of about 80%. Furthermore, when exposed to a Zn2+ shock that completely inhibited growth (400 μm Zn2+ for 4 h), the survival rate of zhfΔpcsΔ cells was significantly reduced as compared to zhfΔ cells. Instead of 74% for zhfΔ only 45% of the cells were able to form colonies (Fig. 1B). Almost identical observations were made for a zhfΔhmt1Δ strain that we generated. Growth inhibition in the presence of increasing Zn2+ concentrations as well as survival rates under very high Zn2+ exposure were very similar to those measured for zhfΔpcsΔ cells (Fig. 1A; data not shown). Zn accumulation rates of zhfΔ cells are strongly reduced compared to wild-type cells (Boch et al., 2008). This difference was not further aggravated by the loss of functional Pcs or Hmt1. After incubation for 2 h in the presence of 50 μm Zn2+ zhfΔ cells contained 130 μg Zn/g dry weight (±16 μg/g dry weight), zhfΔpcsΔ cells 120 μg Zn/g dry weight (±3 μg/g dry weight), and zhfΔhmt1Δ cells 112 μg Zn/g dry weight (±11 μg/g dry weight; n = 2–4).
Figure 1.
Synthesis of PCs contributes to Zn2+ tolerance in S. pombe. A, Growth of zhfΔ (white squares), zhfΔpcsΔ (black squares), and zhfΔhmt1Δ (gray squares) S. pombe mutant cells in the presence of different Zn2+ concentrations (conc.). Growth is shown as percent of growth of control cells in the absence of added Zn2+. Error bars indicate sd, n = 4. B, For Zn shock experiments zhfΔ (white bar) and zhfΔpcsΔ (black bar) were exposed to a highly toxic Zn2+ dose (400 μm for 4 h). Zn shock tolerance is expressed as percent cell survival compared to untreated cells. Error bars indicate sd, n = 3.
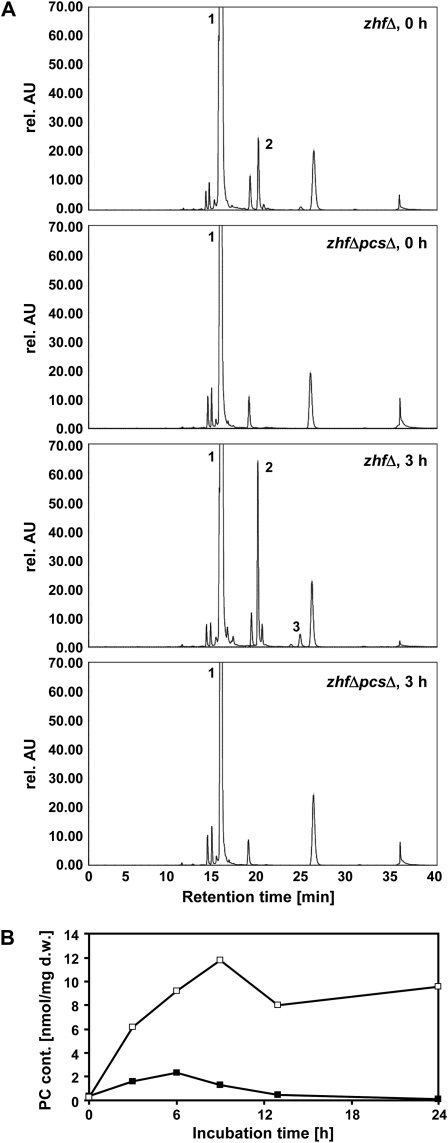
Zn2+ exposure resulted in accumulation of PC2 in zhfΔ cells. PC3 and other higher-order PCs were always near or below the detection limit when incubation times between 3 and 24 h were tested. Interestingly, even in the absence of elevated Zn2+ zhfΔ cells accumulated PC2 to a clearly detectable level (Fig. 2A). Comparison with Cd2+-treated cells showed that PC accumulation was far less pronounced in Zn2+-treated cells and more transient. Total PC content was up to 6-fold higher in cells that were grown in the presence of a Cd2+ concentration that was about as inhibitory as the Zn2+ concentration applied (please note that zhfΔ mutant cells are more Cd2+ tolerant than wild-type cells; Clemens et al., 2002; Fig. 2B). Also, total PC levels returned to control levels already after 13 h of incubation.
Figure 2.
Zn2+ exposure activates PC accumulation in zhfΔ cells. zhfΔ and zhfΔpcsΔ cells were exposed to excess Zn2+ for 3 h. Extracts were analyzed by HPLC subsequent to thiol labeling with monobromobimane. Peak 1: glutathione, peak 2: PC2, peak 3: PC3. Other peaks are either derived from monobromobimane (peaks at retention times 27 and 36 min) or unidentified. rel. AU, Relative absorption units. B, zhfΔ cells were at an OD600 of 0.6 treated with either 0.5 mm Zn2+ (black squares) or 1 mm Cd2+ (white squares) and incubated for 24 h. Aliquots were taken at different time points and analyzed by HPLC. PCs were quantified based on standard curves for PC2 and PC3. Shown are the results of a typical experiment. Three biological replicates were performed with comparable results. d.w., Dry weight.
Next, we tested the effects of PC formation on Zn2+ tolerance in cells not normally synthesizing PCs by expressing TaPCS1 in the Zn2+-hypersensitive zrc1 cot1 Saccharomyces cerevisiae strain, deficient in vacuolar Zn sequestration (MacDiarmid et al., 2000). When cells were grown in the presence of various Zn2+ concentrations, a slight but significant (P < 0.01 for 50, 100, 150, and 200 μm Zn2+) increase in Zn2+ tolerance was observed for the PC-producing cells. The 50% inhibition of initial activity value at which growth was inhibited by 50% was shifted from about 80 μm Zn2+ to about 160 μm Zn2+ (data not shown). Taken together, the data for Zn2+-sensitive yeast mutants indicated that PCs can contribute to the buffering of excess Zn2+ in the presence of a myriad of evolutionary conserved potential Zn2+-binding sites, i.e. they can effectively bind Zn2+ in vivo. Also, when more Zn is available in the cytosol under normal growth conditions due to a defect in Zn storage (Boch et al., 2008), there is elevated constitutive PC2 accumulation.
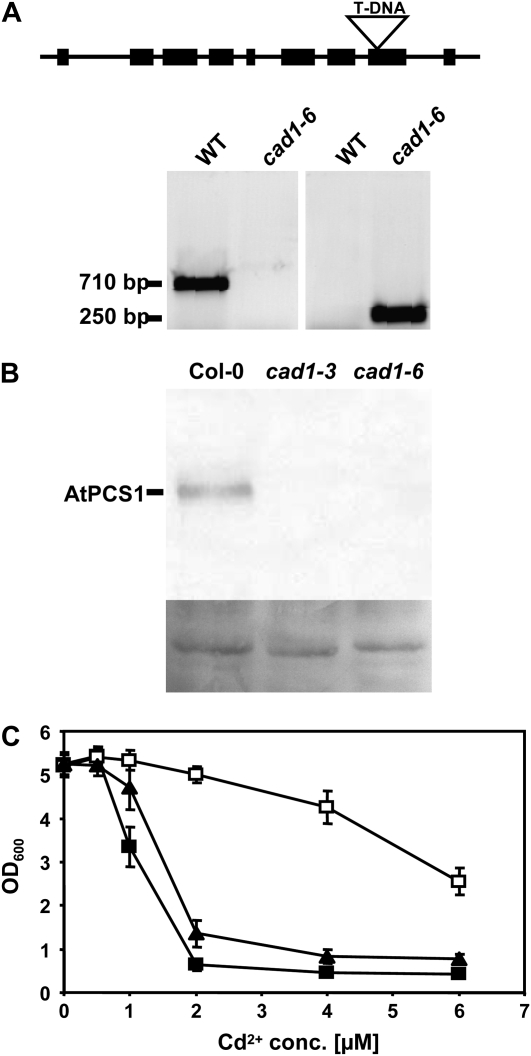
Prompted by the observations in S. pombe and S. cerevisiae, we decided to analyze Zn2+ sensitivity of PC-deficient Arabidopsis mutants. We chose for these experiments the AtPCS1 null mutant cad1-3 (Howden et al., 1995) and searched for a second strong allele. We obtained as the only available T-DNA insertion line for AtPCS1 one from the Garlic (SAIL) collection (Sessions et al., 2002) and isolated a plant homozygous for the insertion (Fig. 3A). The T-DNA insertion disrupts exon 8 of AtPCS1. We named this allele cad1-6. Reverse transcription-PCR analysis with primers either flanking the T-DNA insertion or downstream of the insertion site showed that no wild-type transcript was detectable while a truncated transcript was detected when using a primer pair upstream of the insertion site (data not shown). Western-blot analysis using a specific antiserum raised against recombinant purified AtPCS1 (Picault et al., 2006) demonstrated that AtPCS1 protein was not detectable in leaves of cad1-3 and cad1-6 plants (Fig. 3B). The effect of the T-DNA insertion on activity was investigated by constructing a corresponding AtPCS1 mutant version truncated after amino acid 409. This was expressed in the S. pombe pcsΔ mutant. Growth assays in the presence of Cd2+ indicated slight residual activity (Fig. 3C). At a concentration of 2 μm Cd2+ that did not affect growth of pcsΔ cells expressing AtPCS1, cells carrying the empty vector were inhibited by 87.8% and cells expressing the cad1-6 equivalent AtPCS1Δ409 by 74.1%. Also, residual PC accumulation was detectable in cells expressing the truncated AtPCS1 version (data not shown).
Figure 3.
Isolation of cad1-6, a homozygous T-DNA insertion mutant for AtPCS1. A, The wild-type (WT) AtPCS1 fragment of 710 bp is not detectable. Instead, a 250-bp fragment is amplified with a gene-specific primer and a T-DNA border primer (for primer sequences see “Materials and Methods”). Sequencing of this fragment confirmed the T-DNA insertion into exon 8 after bp 1229 of the coding sequence. B, Leaf extracts of Col-0, cad1-3, and cad1-6 were analyzed by SDS-PAGE, western blotting, and immunostaining with an antiserum raised against recombinant purified AtPCS1. Shown below the blot is amido black stained membrane as a loading control. C, A mutant version of AtPCS1 corresponding to the T-DNA insertion line was constructed by truncating AtPCS1 after amino acid 409. This version (AtPCS1Δ409) was expressed in the S. pombe pcsΔ mutant and growth in the presence of different Cd2+ concentrations (conc.) was compared to the mutant and the mutant expressing wild-type AtPCS1. Black squares: pcsΔ cells carrying empty vector, black triangles: AtPCS1Δ409 (cad1-6 equivalent), white squares: AtPCS1. Error bars indicate sd, n = 4.
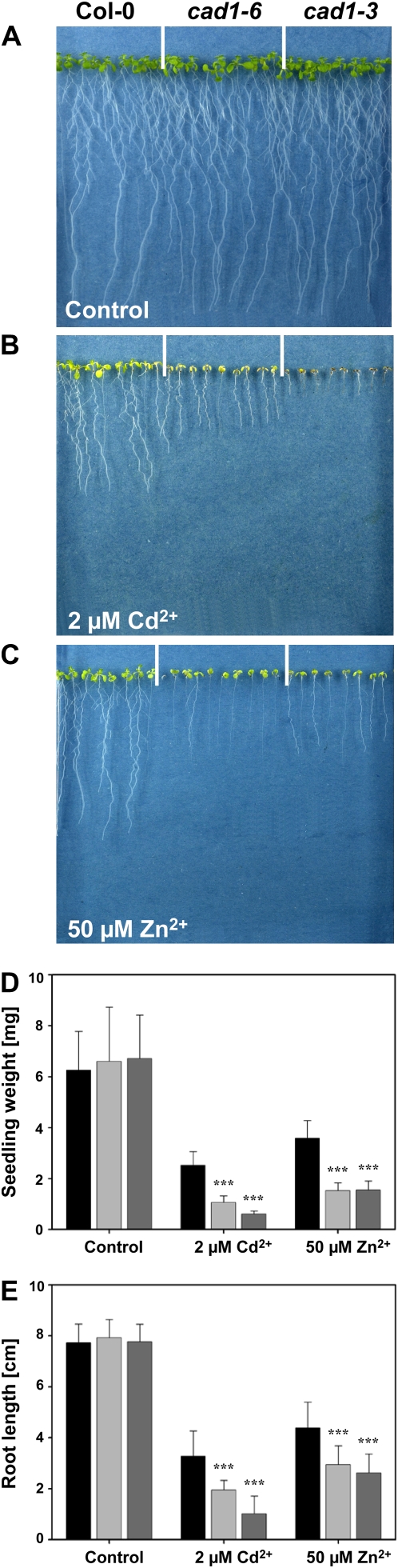
Zn2+ sensitivity of Columbia-0 (Col-0), cad1-3, and cad1-6 seedlings was assayed on a medium with low cation content and an agar that possesses less cation exchange capacity than most. This allowed assessing Zn2+ tolerance at lower concentrations because of reduced interference by other cations that are present in vast excess in many plant media and because of reduced binding of Zn2+ to the agar. Seeds were germinated and grown on vertical plates containing modified one-tenth-strength Hoagland solution. Under control conditions wild-type and mutant seedlings grew equally well (Fig. 4A). Surprisingly, we observed a pronounced Zn2+ hypersensitivity of cad1-3 and cad1-6 seedlings (Fig. 4C). The degree of increase in sensitivity was comparable to the long-known Cd2+ hypersensitivity of AtPCS1 mutants both when measuring root length and seedling weight as growth parameters. More than 200 seedlings each were analyzed in 10 independent experiments on control medium and at Cd2+ and Zn2+ concentrations close to the 50% inhibition of initial activity for Col-0 wild-type seedlings under our assay conditions (2 and 50 μm, respectively). We determined an average root growth inhibition for wild type of 57.6% at 2 μm Cd2+ and 43.2% at 50 μm Zn2+. The respective reduction in seedling weight was 59.5% and 42.4%, respectively (Fig. 4, D and E). The root growth inhibition measured for cad1-3 was 87.0% at 2 μm Cd2+ and 66.2% at 50 μm Zn2+, the seedling weight reduction was 91.0% at 2 μm Cd2+ and 77.0% at 50 μm Zn2+. Zn2+ hypersensitivity of cad1-6 was practically indistinguishable from that of cad1-3, while Cd2+ hypersensitivity was slightly less severe (Fig. 4B). Average root growth inhibition was 75.4% at 2 μm Cd2+ and 62.9% at Zn2+, average seedling weight reduction was 83.9% and 77.0%, respectively. The differences in growth between wild-type and mutant plants under conditions of Cd2+ or Zn2+ toxicity were in all cases highly significant, while growth in the presence of excess Cu2+ was not significantly different between Col and cad1-3 seedlings as reported earlier by Ha et al. (1999). The same was found for cad1-6 (data not shown).
Figure 4.
PC-deficient Arabidopsis cad1 mutants are Zn2+ hypersensitive. Seedlings of wild-type Col-0 (left), cad1-6 (center), and cad1-3 (right) were germinated and grown for 12 d on vertical agar plates with one-tenth-strength Hoagland medium either without added metal ions (A), with 2 μm Cd2+ (B), or with 50 μm Zn2+ (C) added. Root length and seedlings weight were determined. Shown in D and E are summaries for seedling weight and root length results, respectively, of 10 independent experiments and >200 seedlings each of wild-type Col-0 (black), cad1-6 (light gray), and cad1-3 (dark gray). Error bars represent sd. Data were analyzed statistically by two-way ANOVA and Tukey's honestly significant difference procedure. Asterisks indicate significant differences to the wild-type Col-0 for the two mutant lines (***, P < 0.001).
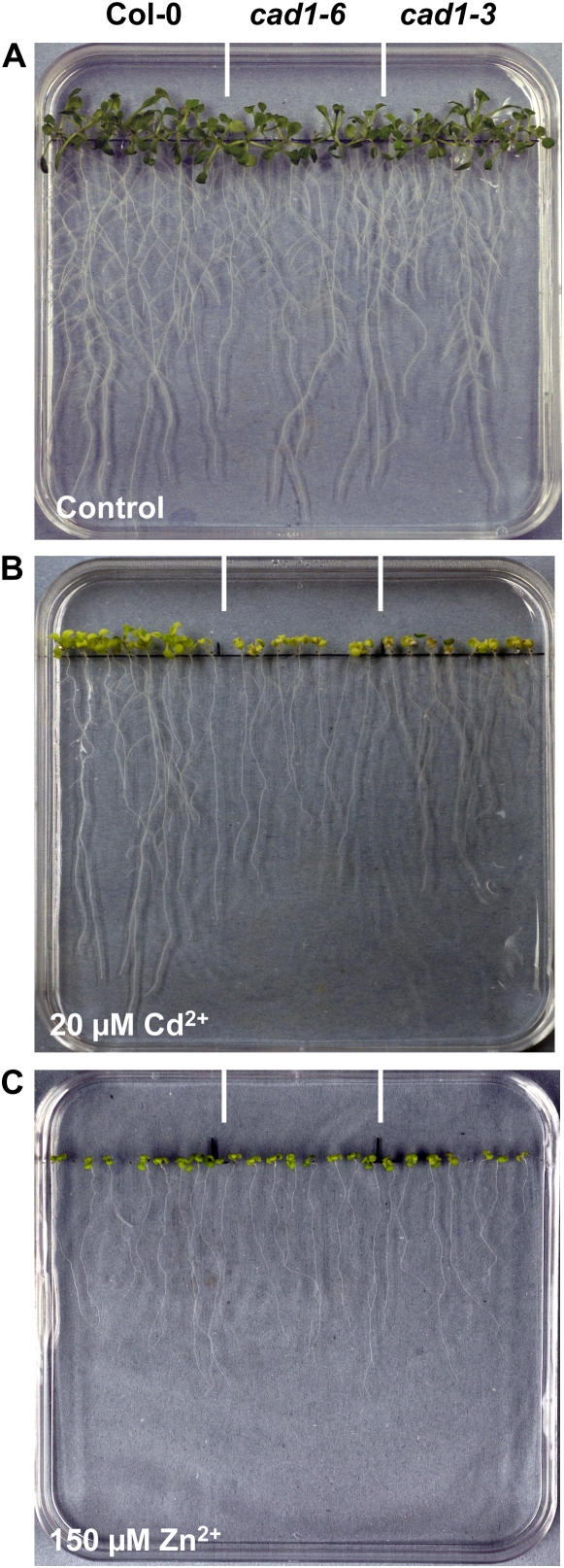
In earlier experiments with the cad1-3 mutant, no significant loss of Zn2+ tolerance had been observed (Ha et al., 1999). We therefore repeated our assays in a medium similar to the one that was used previously. One-half-strength Murashige and Skoog medium contains far higher concentrations of cations such as Ca2+ and Fe3+ and about 3-times higher Zn2+ concentrations have to be applied to cause the same reduction in root growth. In one-half-strength Murashige and Skoog medium prepared with Gelrite agar the Zn2+ hypersensitivity of cad1-3 and cad1-6 was indeed no longer detectable while Cd2+ hypersensitivity was still obvious (Fig. 5).
Figure 5.
Zn2+ hypersensitivity of PC-deficient Arabidopsis cad1 mutants cannot be detected on one-half Murashige and Skoog medium. Seedlings of wild-type Col-0 (left), cad1-6 (center), and cad1-3 (right) were germinated and grown for 10 to 12 d on vertical agar plates with one-half Murashige and Skoog medium either without added metal ions (A), with 20 μm Cd2+ (B), or with 150 μm Zn2+ (C) added.
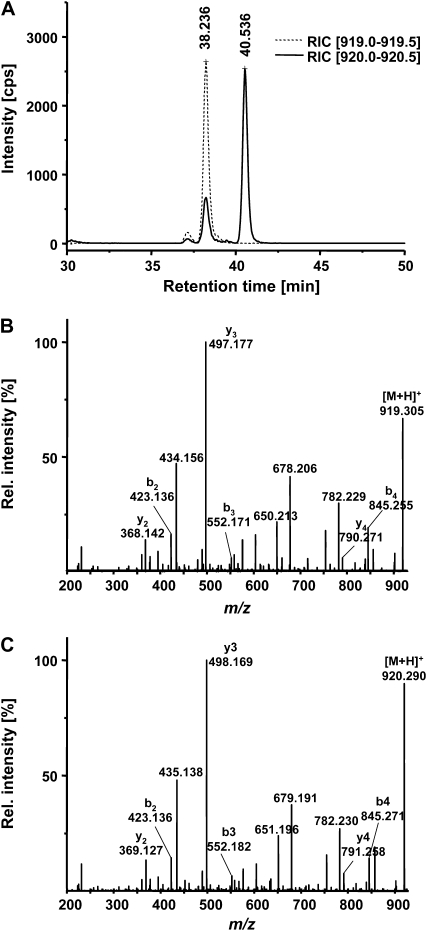
PCs have more often been analyzed in metal-challenged plant cell cultures. There are fewer reports on PC detection in plants and in most of these studies Cd2+ ions were applied as the inducer of PC synthesis. This is at least partly due to the smaller amounts accumulating in plant tissues relative to cell cultures. To be able to detect and accurately quantify PCs even when accumulation rates are low, we established a PC2 assay using separation of extracts by capillary reversed-phase chromatography coupled to electrospray ionization quadrupole time-of-flight mass spectrometry (ESI-QTOF-MS). Thiols were labeled prior to chromatographic separation to obtain better resolution and higher sensitivity. As a chemically similar internal standard, we synthesized a PC2 derivative (H-βGlu-Cys-βGlu-Cys-Gly-NH2) (= PC2-NH2). Mass difference of this amide to PC2 with a free carboxyl group is 1 D. The two labeled compounds with theoretical masses of 920.29132 (PC2-bimane2, [M+H+]) and 919.30730 (PC2-NH2-bimane2, [M+H+]) were baseline separated with retention times of around 38.1 min (PC2-NH2-bimane2) and 40.5 min (PC2-bimane2; Fig. 6A). They could be unequivocally identified based on their exact masses and CID-MSMS spectra (Fig. 6, B and C). Calibration curves were constructed for the two standards and used for quantification. The detection limit (signal-to-noise ratio of >5) was determined as 39 μg PC2/g dry weight when following the standard extraction and labeling protocol. Response was linear for at least 2 orders of magnitude.
Figure 6.
Detection and quantification of PC2 by CapLC-ESI-QTOF-MS analysis of monobromobimane-derivatized extracts. PC2-NH2 was used as an internal standard. A, The bimane derivatives of PC2 and PC2-NH2 could be baseline separated. Shown are the reconstructed ion chromatograms for the mass windows 919.0 to 919.5 (dotted line) and 920.0 to 920.5 (solid line). cps, Counts per second. B and C, PC2-NH2-bimane2 and PC2-bimane2 were unequivocally identified based on their exact masses (919.30730 and 920.29132, respectively) and CID-MSMS spectra (B, PC2-NH2-bimane2; C, PC2-bimane2).
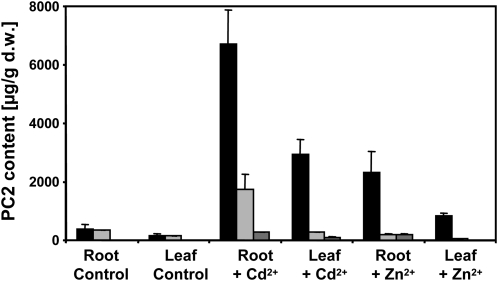
Using this assay we compared PC2 levels in hydroponically grown plants 5 d after challenging them with same-effect concentrations of Cd2+ or Zn2+ (0.5 and 20 μm, respectively; please note that the concentrations are lower than on plates because there is no cation-binding agar present). In roots and leaves of wild-type plants, considerable amounts of PC2 were detectable even in control plants (Fig. 7). The measured concentrations were about 10-times higher than the detection limit (signal-to-noise ratio >5) in roots and 5-times higher than the detection limit in leaves. Zn2+ challenge resulted in a strong increase (about 6-fold) in PC2 levels that was about 34% of what was reached in the presence of a same-effect Cd2+ concentration. In leaves the difference was similar, with PC2 levels in Zn2+-treated plants that were about 28% of those in Cd2+-treated plants. The increase in leaf PC2 levels as compared to untreated controls was about 5-fold. No PC2 was detectable in cad1-3 under control conditions, while roots and leaves of cad1-6 showed almost wild-type level PC2 accumulation in the absence of metal excess. Remarkably, PC2 was detectable in roots and leaves of Cd2+-challenged cad1-3 plants (about 5% of the concentration in wild type) and also in the roots of Zn2+-treated cad1-3 plants (about 8% of the concentration in wild type). PC2 concentrations in cad1-6 were between 8% (in leaves of Zn2+-treated plants) and 26% (in roots of Cd2+-treated plants) of the level found in Col-0.
Figure 7.
Accumulation of PC2 upon exposure to Cd2+ and Zn2+. Wild-type Col-0, cad1-3, and cad1-6 were grown hydroponically in one-tenth-strength Hoagland medium for 5 weeks before either 0.5 μm Cd2+ or 20 μm Zn2+ were added to the medium. No extra metal ions were added to control plants. After 5 d roots and leaves were harvested, frozen in liquid N2, and lyophyllized. Monobromobimane-derivatized extracts were analyzed by CapLC-ESI-QTOF-MS. Quantification of PC2 in wild-type Col-0 (black columns), cad1-3 (dark gray columns), and cad1-6 (light gray columns) is shown for control plants and those challenged with either Cd2+ or excess Zn2+. d.w., Dry weight.
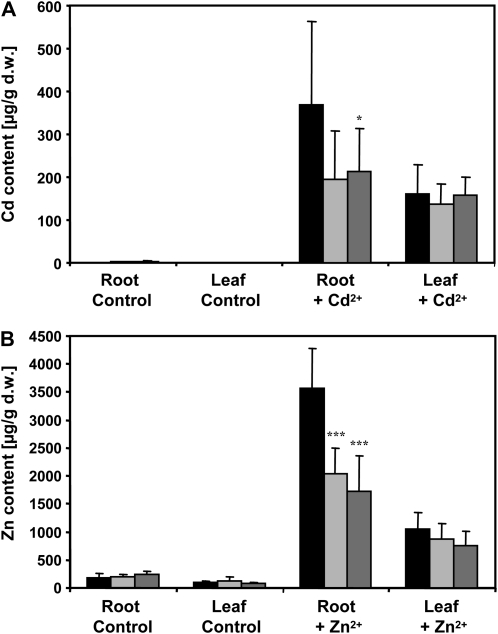
Because the uptake rates for a particular transition metal ion are positively correlated with the concentration of intracellular high-affinity binding sites for this ion (Frausto da Silva and Williams, 2001), we studied the effect of PC deficiency on accumulation and distribution of Cd and Zn. Wild type and the two mutant plant lines were grown hydroponically in one-tenth-strength Hoagland solution and after 5 weeks subjected to treatment with either 0.5 μm Cd2+ or 20 μm Zn2+ for 5 d. Roots and leaves were harvested separately and analyzed by atomic absorption spectroscopy. Cd was only detectable in treated plants. The roots of cad1-3 and cad1-6 accumulated less Cd than Col-0 (52%–58% of wild-type level). The difference was significant for cad1-6. In leaves, there was no significant difference. Also, roots of the AtPCS1 mutants accumulated only about half the amount of Zn found in wild-type plants: approximately 1.7 and 2.0 mg/g dry weight (cad1-3 and cad1-6, respectively) as compared to 3.6 mg/g dry weight (Fig. 8). In leaves the reduction was less pronounced. Wild type accumulated about 1.1 mg/g dry weight and leaves of the cad1 mutants between 0.8 and 0.9 mg/g dry weight. In a separate set of experiments, we tested whether PCS overexpression leads to elevated Zn accumulation. The cad1-3 mutant was transformed with a 35S:AtPCS1-HA construct. Resulting transgenic lines were tested for AtPCS1 expression by western analysis using the AtPCS1 antiserum and an anti-HA monoclonal antibody. One line was selected that showed clear overexpression as compared to wild-type Col-0 (Supplemental Fig. S1; Fig. 1A). This line complemented the Cd2+ hypersensitivity phenotype (data not shown). However, when grown hydroponically the transgenic line did not accumulate more Cd or Zn than Col-0 plants. Both root and leaf accumulation rates were comparable while they were lower for cad1-3 as found before (Supplemental Fig. S1; Fig. 1, B and C).
Figure 8.
PC-deficient Arabidopsis cad1 mutants show a reduced accumulation of Cd and Zn. Wild-type Col-0 (black columns), cad1-3 (light gray columns), and cad1-6 (dark gray columns) were grown hydroponically in one-tenth-strength Hoagland medium for 5 weeks before 0.5 μm Cd2+ (A) or 20 μm Zn2+ (B) were added to the medium. No Cd2+ and no extra Zn2+ was added to respective control plants. Roots and leaves of three plants per pot were harvested after 5 d and Zn content was analyzed by atomic absorption spectroscopy. Shown are the results of three independent experiments with a total of six to 10 samples per treatment and plant line. Error bars represent sd. Asterisks indicate significant differences between mutant and wild-type plants (Student's t test; *, P < 0.05; ***, P < 0.001). d.w., Dry weight.
The apparent contribution of PC synthesis to Zn accumulation at comparatively high external Zn2+ concentrations raises the question whether PC synthesis is also important for Zn2+ uptake under Zn2+-deplete conditions. We therefore tested growth of seedlings in the presence of EDTA and exposed 5-week-old hydroponically grown plants to Zn2+ deficiency. In none of these experiments was a phenotype of the cad1 mutants apparent.
DISCUSSION
The almost ubiquitous occurrence of PCS genes in the plant kingdom remains enigmatic as long as the proven functions of PCSs are confined to Cd, As, and mercury detoxification (Schat et al., 2002). Excess of these nonessential elements is a condition that during the course of evolution has been present only sporadically and in a very limited number of habitats. The question as to why the enzymes are so widely distributed and constitutively expressed had therefore been raised already soon after the discovery of PCs (Steffens, 1990) and was again emphasized a short while ago (Rea et al., 2004). At least two obvious hypotheses to explain PCS occurrence are being discussed. The more recent one states that PCSs have additional important functions in glutathione metabolism. Indeed, an additional catalytic activity for AtPCS1, the cleavage of Gly from GS conjugates, was demonstrated (Beck et al., 2003; Blum et al., 2007). This reaction is assumed to represent the first step in the turnover of GS conjugates in plants. The second hypothesis dates back to the early work on PC synthesis and proposes that PCs are involved in essential metal homeostasis (Steffens et al., 1986; Grill et al., 1988). However, no phenotypes had been described to date that would indicate a significantly reduced fitness of PC-deficient plants under conditions of either micronutrient excess or micronutrient deficiency (Howden et al., 1995; Ha et al., 1999; Cobbett and Goldsbrough, 2002). We were therefore surprised to observe a significant loss of Zn2+ tolerance in cad1-3 and the newly isolated cad1-6 allele. In fact, the hypersensitivity was comparable for Cd2+ and Zn2+ under our assay conditions and at concentrations that inhibited root growth of wild-type seedlings about half maximally. Appearance of the seedlings and data on seedling biomass indicated more pronounced toxic effects in root and leaf tissue. To determine why this phenotype had not been observed before, we repeated assays under conditions similar to the ones used previously, i.e. on Murashige and Skoog medium and typical plant tissue culture agar. Indeed, Zn2+ hypersensitivity was no longer observed while Cd2+ hypersensitivity was confirmed (Fig. 5). This demonstrates a clear influence of the growth medium on metal-related phenotypes and explains why Zn2+ hypersensitivity of PCS mutants has escaped notice so far. A detailed analysis of single media components to define the cause of this difference remains to be pursued.
In several early reports Zn2+ was seen as a weak inducer of PC synthesis in vivo. However, as Rauser (1990) already pointed out, there was often no knowledge about the effective available intracellular concentrations resulting from the treatments. Growth response can be taken as an indirect marker for intracellular metal overload. In a lot of projects this was not studied and arbitrary concentrations were used instead. We attempted to compare PC accumulation in Arabidopsis in response to Cd2+ (well known as the most potent activator) and Zn2+ by a careful quantitative analysis and at concentrations that reflected the differences in sensitivity. Under these conditions, Zn2+ exposure resulted in an accumulation of PC2 in roots that reached about 34% of that seen in Cd2+-exposed plants. For leaves the level was about 28%. We focused the analysis on PC2 because we were able to synthesize a suitable internal standard and because the PC2-bimane derivative lies in the routine scan range of our ESI-QTOF-MS (100–1,000 D). Following this approach we were not able to obtain a complete picture of total PC content and composition but gained the advantage of being able to still unequivocally detect comparatively small PC accumulation. Clearly, there was PC2 synthesis in roots and leaves in the absence of transition metal ion excess. Also, PC2 was detectable even in metal-exposed cad1-3 roots. It remains to be determined whether this PC2 synthesis indicates residual activity of the mutated AtPCS1 protein or rather activity of AtPCS2, the second PCS in Arabidopsis that at least in heterologous systems displays activity (Cazalé and Clemens, 2001).
A small yet potentially interesting dissimilarity was observed between cad1-3 and cad1-6. Both mutant lines showed equal hypersensitivity toward Zn2+ (Fig. 4C) while cad1-6 was not quite as strongly affected by Cd2+ as cad1-3. Also, there was an increase in PC2 root levels in the presence of Cd2+ only in cad1-6, which fits the data obtained for the respective truncated version of AtPCS1 in S. pombe (Fig. 3B). We interpret these data as an in vivo confirmation for the hypothesis that the C-terminal portion of PCSs is important for activation of the proteins by a broader range of metal ions including Zn2+ (Ruotolo et al., 2004). The truncated cad1-6 version can still be activated by Cd2+—conferring some degree of residual PC-dependent Cd2+ tolerance—but no longer by Zn2+ as was found in vitro for C-terminally truncated AtPCS1 (Ruotolo et al., 2004).
A critical question with respect to the observed activation of PC synthesis by Zn2+ and the loss of Zn2+ tolerance in PC-deficient mutants is the formation of PC-Zn complexes. Direct evidence is lacking (Maitani et al., 1996; Cobbett and Goldsbrough, 2002) even though Zn2+ ions clearly act as potent activators of purified recombinant PCSs. Phenotypes observed in both yeast models indicated that excess cytosolic Zn can be chelated by PCs in vivo, i.e. in the presence of a myriad of other potential binding sites (Zn-requiring proteins or low Mr chelators such as glutathione) that are evolutionary conserved among eukaryotes. Indirect evidence for PC-Zn complex formation in planta came from experiments studying the consequences of PC deficiency for Zn accumulation. It is well established that metal accumulation rates are a function of uptake activities and the availability of intracellular high-affinity binding sites (Frausto da Silva and Williams, 2001). Thus, the significant reduction of Zn accumulation observed in cad1-3 and cad1-6 indicates binding of excess Zn2+ by PCs. Clear evidence for the formation of PC-Zn complexes in vitro has been reported several times and based on different analytical approaches such as direct-infusion ESI-MS, circular dichroism, or NMR spectroscopy (Cheng et al., 2005; Kobayashi and Yoshimura, 2006; Chekmeneva et al., 2008). Stability of the complexes in vitro is lower than that of PC-Cd complexes. We still lack the techniques, however, to detect metal-ligand complexes in vivo. Tissue disruption immediately changes the chemical environment and thereby the availability of binding partners for metal ligands (Callahan et al., 2006). Thus, at this stage we are restricted to stating that effects on accumulation of and tolerance toward Zn strongly suggest formation of PC-Zn complexes. Based on our current understanding of PC complexes in S. pombe and plants, we have to assume storage of such complexes in vacuoles (Salt and Rauser, 1995; Cobbett and Goldsbrough, 2002). The comparative analysis of PC accumulation in S. pombe zhfΔ cells (Fig. 2B), however, might indicate a more transient chelation of Zn(II) by PCs. Reduced Zn accumulation in PC-deficient plants suggests that the PC synthesis pathway might represent a promising target for engineering elevated Zn accumulation in specific plant tissues even though simple constitutive overexpression of AtPCS1 did not yield plants with elevated Zn accumulation (Supplemental Fig. S1). Recently, the aspect of essential metal content of crop plants has come into focus. Zn deficiency, for instance, is very widespread (Welch and Graham, 2004) and biofortification, i.e. increasing the Zn content of edible plant parts, could be a means to help fighting this deficiency (Guerinot and Salt, 2001; Palmgren et al., 2008).
We conclude from our observations that PCs function as important chelators of excess Zn2+. This is supported by slightly elevated Zn2+ tolerance in Brassica juncea plants transformed with AtPCS1 (Gasic and Korban, 2007). What remains to be determined conclusively is whether the minor PC synthesis observed in this and other studies under normal growth conditions plays a role in the trafficking and distribution of Zn and possibly other essential micronutrients (Schat et al., 2002). Such a function could be intracellular as well as intercellular, especially since there is evidence now for long-distance transport of PC-metal complexes (Mendoza-Cozatl et al., 2008). So far, however, we have not been able to obtain any evidence for a beneficial effect of PCS genes under Zn-deplete conditions. For instance, established Zn-deficiency markers such as AtZIP9 (At4g33020) and AtNAS2 (At5g56080; Wintz et al., 2003) are not activated under normal growth conditions in cad1-3 or cad1-6 (data not shown). It will most likely be necessary to study various Zn-dependent processes in more detail to be able to answer this question.
MATERIALS AND METHODS
Plant Growth
For metal tolerance assays seeds of Arabidopsis (Arabidopsis thaliana) wild-type Col-0 and the two cad1 mutants were surface sterilized (70% ethanol for 2 min, 10 min in 10% sodium hypochlorite) and rinsed several times with sterile water. Following stratification for 2 d at 4°C seeds were inoculated onto agar (Type A, Sigma) plates with one-tenth-strength Hoagland medium without microelements, 1% Suc. Cd2+ or Zn2+ were added to the medium as chloride salts. Plates were incubated vertically under long-day conditions (16-h light/8-h dark). After 12 d root length and seedling weight were measured. For the analysis of metal accumulation and PC2 accumulation plants were grown hydroponically as described (Weber et al., 2004).
Isolation of an Insertional Mutant for AtPCS1
Seeds of an insertion line for AtPCS1 (At5g44070) were obtained from Syngenta (Garlic_650_C12.b.1a.Lb3Fa; Sessions et al., 2002) and sown on soil. T-DNA insertion in AtPCS1 was checked by PCR on genomic DNA using a T-DNA-specific primer (5′-tagcatctgaatttcataaccaatctcgatacac-3′) and a gene-specific primer (5′-tcaaaggtatgtcgccatgtcgattc-3′), amplifying a fragment of 250 bp. Plants homozygous for the insertion were isolated by testing for presence of the wild-type allele with primers AtPCS1diag-5′ (5′-cgccatgtcgattcttcaaatt-3′) and AtPCS1diag-3′ (5′-ttgggacaatgaactaataggcag-3′), which amplified a 710-bp fragment of AtPCS1. Predicted site of the insertion was confirmed by cloning and sequencing of the 250-bp fragment. A mutant version of AtPCS1, truncated C terminally corresponding to the position of the T-DNA insertion, was generated by PCR with Pfu polymerase. The cDNA coding for the first 409 amino acids of AtPCS1 was amplified with primers AtPCS1-HAC5′ (5′-cgcgctcgagaatggctatggcgag-3′) and AtPCS1KO-HAC3′ (5′-cgcgagatctctagttgcttcaggaccac-3′), digested with XhoI/BglII and ligated into pSGP72. Sequence was confirmed by automatic sequencing. AtPCS1 protein expression was assayed by SDS-PAGE, western blotting, and immunostaining with an antiserum kindly provided by Gilles Peltier (CEA Cadarache; Picault et al., 2006).
Generation of Transgenic Plants Overexpressing AtPCS1
The AtPCS1-HA gene construct used for transformation was derived from a construct tested for activity in Schizosaccharomyces pombe (Cazalé and Clemens, 2001). AtPCS1-HA was subcloned into pRT100 between the cauliflower mosaic virus 35S promoter and the cauliflower mosaic virus polyadenylation signal. The resulting cassettes were transferred as HindIII fragments into the binary plasmid vector pCB302 (Xiang et al., 1999). Agrobacterium-mediated transformation was performed by floral dip.
Yeast Strains, Cultivation of S. pombe and S. cerevisiae
The S. pombe strains employed in this study were the previously described mutant zhfΔ and the zhfΔpcsΔ double mutant (Clemens et al., 2002) as well as a newly generated zhfΔhmt1Δ strain. Cells were cultivated in Edinburgh's minimal medium supplemented appropriately. Transformation of S. pombe with various PCS genes in pSGP72 was performed according to the protocol of Bähler et al. (1998). The Saccharomyces cerevisiae strain employed was the zrc1 cot1 mutant (MacDiarmid et al., 2000), kindly provided by Dr. Ute Krämer, Heidelberg. Mutant cells were transformed with pYES2 or TaPCS1 in pYES2 as described (Clemens et al., 1999). To test metal sensitivity, S. cerevisiae and S. pombe cells grown to log phase were diluted to an OD600 of 0.05 and incubated at 30°C under shaking in the presence of different Zn2+ and Cd2+ concentrations. After 21 to 24 h cell density was measured. For Zn shock experiments overnight cultures were diluted to an OD600 of 0.1. After one cell division cells were either exposed to 400 μm Zn2+ or left untreated. Following a 4-h incubation cells were harvested, diluted, and plated onto YE5S. Colony numbers were determined after 3 d.
Atomic Absorption Spectroscopy and Inductively Coupled Plasma-Optical Emission Spectroscopy
The Zn2+ concentration in the culture medium of Arabidopsis plants grown hydroponically was increased by 20 μm or Cd2+ was added to a concentration of 0.5 μm. After 5 d roots and leaves of treated and control plants were harvested. Root tissue was washed with Millipore water and 10 mm CaCl2 several times. Dried material was digested in a microwave (microPREPA/Terminal 320, MLS GmbH Mikrowellen Laborsysteme) in 4 mL 65% HNO3 and 1 mL 30% hydrogen peroxide. Zn content was determined using a Perkin Elmer Analyst 800 (Perkin Elmer) and an iCAP 6000 ICP-OES (Thermo). Zn content of S. pombe cells was analyzed as described (Boch et al., 2008). Washed, frozen-hydrated S. pombe cells were lyophilized and subsequently digested in 4 mL 65% HNO3 and 2 mL 30% hydrogen peroxide.
PC Analysis
Root and leaf tissue of 5-week-old plants, treated for 5 d with either 20 μm Zn2+ or 0.5 μm Cd2+, was harvested, frozen in liquid nitrogen, and freeze dried. Thirty milligrams of lyophilized material were homogenized in 500 μL 0.1 m HCl spiked with 5.0 μL 10 mm PC2-NH2 (=26.9 μg) using zirconia beads and a bead beater (1 min, maximum power). Fifty microliters of the supernatant were incubated with 6 μL 20 mm Tris-(2-carboxyethyl)phosphine hydrochloride and 18 μL 4-(2-Hydroxyethyl)-piperazine-1-propanesulfonic acid/diethylenetriaminepentaacetic acid (EPPS/DTPA) buffer (200 mm EPPS, pH 9.2, 6.3 mm DTPA) for 30 min at ambient temperature in a brown eppendorf tube. Derivatization: After addition of 72 μL EPPS/DTPA buffer and 10 μL 10 mm monobromobimane (in CH3CN) the mixture was incubated for 30 min at 45°C. Through addition of 60 μL 1 m methanesulfonic acid the reaction was quenched. Before analysis by capillary liquid chromatography (CapLC) coupled to ESI-QTOF-MS the sample was filtered using a 0.45 μm PTFE syringe filter. Two microliters of labeled extract was injected onto a Phenomenex Luna C8 column (particle size 3 μm, pore size 100 Å, length 150 mm, i.d. 0.3 mm). Separation was performed using the following gradient system: solvent A = water/0.1% HCO2H; solvent B = CH3CN/0.1% HCO2H; 0 to 5 min 95% A, 5% B; 5 to 40 min linear from 5% B to 22% B, 40 to 50 min 95% B, 50 to 60 min 5% B. Flow rate was 5.5 μL/min. LC-ESI-QTOF mass spectra (positive ion mode) were recorded on an API QSTAR Pulsar Hybrid Quadrupole TOF instrument (Applied Biosystems). Ion spray voltage was +5.5 kV, detected mass range: 910 to 930 D, scan rate: 0.5 s−1, declustering potential 1: 50 V, declustering potential 2: 15 V. Each sample was analyzed twice, i.e. two extracts were run per sample. The signals for dialkylated PC2-NH2 (mass-to-charge ratio 919.0–919.5 [M+H]+) and dialkylated PC2 (mass-to-charge ratio 920.0–920.5 [M+H]+) were integrated. PC2 was quantified based on calibration curves recorded for PC2 and PC2-NH2 standards. PC2 and PC2-NH2 standards were synthesized on an Abimed Economy Peptide Synthesizer EPS 211 using N-α-Fmoc-l-Glu α-tert-butyl ester (Novabiochem). PC analysis in yeast cells was performed by precolumn derivatization with monobromobimane and HPLC as described (Clemens et al., 2002).
Statistical Analysis
Statistical analysis was performed with SigmaStat 3.5 as indicated.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Zn and Cd accumulation in plants overexpressing AtPCS1.
Supplementary Material
Acknowledgments
The expert technical assistance of Jutta Elster (peptide synthesis), Regina Weiss (sequencing), and Marina Häussler at the IPB Halle, Germany, is gratefully acknowledged. We thank Dr. Edda V. Roepenack-Lahaye (IPB Halle, Germany) for help with the MS analysis, Dr. Gilles Peltier (CEA Cadarache, France) for the AtPCS1 antiserum, and Dr. Ute Krämer (University of Heidelberg) for the S. cerevisiae strain zrc1 cot1.
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. SFB 363) and in part by the European Union through its Sixth Framework Programme for RTD (contract no. FOOD–CT–2006–016253).
The author responsible for the distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Stephan Clemens (stephan.clemens@uni-bayreuth.de).
The online version of this article contains Web-only data.
Open access articles can be viewed online without a subscription.
References
- Andreini C, Banci L, Bertini I, Rosato A (2006) Zinc through the Three Domains of Life. J Proteome Res 5 3173–3178 [DOI] [PubMed] [Google Scholar]
- Bähler J, Wu JQ, Longtine MS, Shah NG, McKenzie A, Steever AB, Wach A, Philippsen P, Pringle JR (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14 943–951 [DOI] [PubMed] [Google Scholar]
- Beck A, Lendzian K, Oven M, Christmann A, Grill E (2003) Phytochelatin synthase catalyzes key step in turnover of glutathione conjugates. Phytochemistry 62 423–431 [DOI] [PubMed] [Google Scholar]
- Blum R, Beck A, Korte A, Stengel A, Letzel T, Lendzian K, Grill E (2007) Function of phytochelatin synthase in catabolism of glutathione-conjugates. Plant J 49 740–749 [DOI] [PubMed] [Google Scholar]
- Boch A, Trampczynska A, Simm C, Taudte N, Krämer U, Clemens S (2008) Loss of Zhf and the tightly regulated Zn uptake system SpZrt1 in Schizosaccharomyces pombe reveals the delicacy of cellular Zn balance. FEMS Yeast Res 8 883–896 [DOI] [PubMed] [Google Scholar]
- Callahan DL, Baker AJ, Kolev SD, Wedd AG (2006) Metal ion ligands in hyperaccumulating plants. J Biol Inorg Chem 11 2–12 [DOI] [PubMed] [Google Scholar]
- Cazalé A, Clemens S (2001) Arabidopsis thaliana expresses a second functional phytochelatin synthase. FEBS Lett 507 215–219 [DOI] [PubMed] [Google Scholar]
- Chekmeneva E, Prohens R, Diaz-Cruz JM, Arino C, Esteban M (2008) Competitive binding of cd and zn with the phytochelatin (gamma-glu-cys) ()-gly: comparative study by mass spectrometry, voltammetry-multivariate curve resolution, and isothermal titration calorimetry. Environ Sci Technol 42 2860–2866 [DOI] [PubMed] [Google Scholar]
- Cheng YS, Yan YB, Liu JY (2005) Spectroscopic characterization of metal bound phytochelatin analogue (Glu-Cys) ()-Gly. J Inorg Biochem 99 1952–1962 [DOI] [PubMed] [Google Scholar]
- Clemens S (2006. a) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88 1707–1719 [DOI] [PubMed] [Google Scholar]
- Clemens S (2006. b) Evolution and function of phytochelatin synthases. J Plant Physiol 163 319–332 [DOI] [PubMed] [Google Scholar]
- Clemens S, Bloss T, Vess C, Neumann D, Nies D, Zur Nieden U (2002) A transporter in the endoplasmic reticulum of Schizosaccharomyces pombe cells mediates zinc storage and differentially affects transition metal tolerance. J Biol Chem 277 18215–18221 [DOI] [PubMed] [Google Scholar]
- Clemens S, Kim EJ, Neumann D, Schroeder JI (1999) Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J 18 3325–3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Schroeder J, Degenkolb T (2001) Caenorhabditis elegans expresses a functional phytochelatin synthase. Eur J Biochem 268 3640–3643 [DOI] [PubMed] [Google Scholar]
- Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Physiol Plant Mol Biol 53 159–182 [DOI] [PubMed] [Google Scholar]
- Desbrosses-Fonrouge AG, Voigt K, Schröder A, Arrivault S, Thomine S, Krämer U (2005) Arabidopsis thaliana MTP1 is a Zn transporter in the vacuolar membrane which mediates Zn detoxification and drives leaf Zn accumulation. FEBS Lett 579 4165–4174 [DOI] [PubMed] [Google Scholar]
- Eide DJ (2006) Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta 1763 711–722 [DOI] [PubMed] [Google Scholar]
- Frausto da Silva JJR, Williams RJP (2001) The Biological Chemistry of the Elements, Ed 2. Oxford University Press, New York
- Gasic K, Korban SS (2007) Expression of arabidopsis phytochelatin synthase in indian mustard (brassica juncea) plants enhances tolerance for Cd and Zn. Planta 225 1277–1285 [DOI] [PubMed] [Google Scholar]
- Grill E, Thumann J, Winnacker EL, Zenk M (1988) Induction of heavy-metal binding phytochelatins by inoculation of cell cultures in standard media. Plant Cell Rep 7 375–378 [DOI] [PubMed] [Google Scholar]
- Grill E, Winnacker EL, Zenk MH (1987) Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc Natl Acad Sci USA 84 439–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotz N, Guerinot ML (2006) Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim Biophys Acta 1763 595–608 [DOI] [PubMed] [Google Scholar]
- Guerinot ML, Salt D (2001) Fortified foods and phytoremediation. Two sides of the same coin. Plant Physiol 125 164–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha SB, Smith AP, Howden R, Dietrich WM, Bugg S, O'Connell MJ, Goldsbrough PB, Cobbett CS (1999) Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell 11 1153–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanikenne M, Talke IN, Haydon MJ, Lanz C, Nolte A, Motte P, Kroymann J, Weigel D, Krämer U (2008) Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature 453 391–395 [DOI] [PubMed] [Google Scholar]
- Harada E, von Roepenack-Lahaye E, Clemens S (2004) A cyanobacterial protein with similarity to phytochelatin synthases catalyzes the conversion of glutathione to gamma-glutamylcysteine and lacks phytochelatin synthase activity. Phytochemistry 65 3179–3185 [DOI] [PubMed] [Google Scholar]
- Haydon MJ, Cobbett CS (2007) A novel major facilitator superfamily protein at the tonoplast influences zinc tolerance and accumulation in Arabidopsis. Plant Physiol 143 1705–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R, Goldsbrough PB, Andersen CR, Cobbett CS (1995) Cadmium-sensitive, cad1 mutants of Arabidopsis thaliana are phytochelatin deficient. Plant Physiol 107 1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobae Y, Uemura T, Sato MH, Ohnishi M, Mimura T, Nakagawa T, Maeshima M (2004) Zinc transporter of Arabidopsis thaliana AtMTP1 is localized to vacuolar membranes and implicated in zinc homeostasis. Plant Cell Physiol 45 1749–1758 [DOI] [PubMed] [Google Scholar]
- Kobayashi R, Yoshimura E (2006) Differences in the binding modes of phytochelatin to cadmium(ii) and zinc(ii) ions. Biol Trace Elem Res 114 313–318 [DOI] [PubMed] [Google Scholar]
- Krämer U, Clemens S (2005) Functions and homeostasis of zinc, copper and nickel in plants. In M Tamás, E Martinoia, eds, Topics in Current Genetics 14. Springer, Heidelberg, pp 215–271
- MacDiarmid C, Gaither L, Eide D (2000) Zinc transporters that regulate vacuolar zinc storage in Saccharomyces cerevisiae. EMBO J 19 2845–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitani T, Kubota H, Sato K, Yamada T (1996) The composition of metals bound to class III metallothionein (phytochelatin and its desglycyl peptide) induced by various metals in root cultures of Rubia tinctorum. Plant Physiol 110 1145–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H (1995) Mineral Nutrition of Higher Plants, Ed 2. Academic Press Ltd., London
- Mendoza-Cozatl DG, Butko E, Springer F, Torpey JW, Komives EA, Kehr J, Schroeder JI (2008) Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus: a role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. Plant J 54 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RF, Francini A, Ferreira da Rocha PS, Baccarini PJ, Aylett M, Krijger GC, Williams LE (2005) The plant P1B-type ATPase AtHMA4 transports Zn and Cd and plays a role in detoxification of transition metals supplied at elevated levels. FEBS Lett 579 783–791 [DOI] [PubMed] [Google Scholar]
- Oven M, Page J, Zenk M, Kutchan T (2002) Molecular characterization of the homo-phytochelatin synthase of soybean Glycine max: relation to phytochelatin synthase. J Biol Chem 277 4747–4754 [DOI] [PubMed] [Google Scholar]
- Palmgren MG, Clemens S, Williams LE, Krämer U, Borg S, Schjørring JK, Sanders D (2008) Zinc biofortification of cereals: problems and solutions. Trends Plant Sci 13 464–473 [DOI] [PubMed] [Google Scholar]
- Picault N, Cazale AC, Beyly A, Cuine S, Carrier P, Luu DT, Forestier C, Peltier G (2006) Chloroplast targeting of phytochelatin synthase in Arabidopsis: effects on heavy metal tolerance and accumulation. Biochimie 88 1743–1750 [DOI] [PubMed] [Google Scholar]
- Rauser WE (1990) Phytochelatins. Annu Rev Biochem 59 61–86 [DOI] [PubMed] [Google Scholar]
- Rea PA, Vatamaniuk OK, Rigden DJ (2004) Weeds, worms, and more: papain's long-lost cousin, phytochelatin synthase. Plant Physiol 136 2463–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruotolo R, Peracchi A, Bolchi A, Infusini G, Amoresano A, Ottonello S (2004) Domain organization of phytochelatin synthase: functional properties of truncated enzyme species identified by limited proteolysis. J Biol Chem 279 14686–14693 [DOI] [PubMed] [Google Scholar]
- Salt DE, Rauser WE (1995) MgATP-dependent transport of phytochelatins across the tonoplast of oat roots. Plant Physiol 107 1293–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schat H, Llugany M, Vooijs R, Hartley-Whitaker J, Bleeker PM (2002) The role of phytochelatins in constitutive and adaptive heavy metal tolerances in hyperaccumulator and non-hyperaccumulator metallophytes. J Exp Bot 53 2381–2392 [DOI] [PubMed] [Google Scholar]
- Schmöger M, Oven M, Grill E (2000) Detoxification of arsenic by phytochelatins in plants. Plant Physiol 122 793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions A, Burke E, Presting G, Aux G, McElver J, Patton D, Dietrich B, Ho P, Bacwaden J, Ko C, et al (2002) A high-throughput Arabidopsis reverse genetics system. Plant Cell 14 2985–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens JC, Hunt D, Williams B (1986) Accumulation of non-protein metal-binding polypeptides (gamma-glutamyl-cysteinyl)n-glycine in selected cadmium-resistant tomato cells. J Biol Chem 261 13879–13882 [PubMed] [Google Scholar]
- Steffens JC (1990) The heavy metal-binding peptides of plants. Annu Rev Plant Physiol Plant Mol Biol 41 553–575 [Google Scholar]
- Tsuji N, Nishikori S, Iwabe O, Shiraki K, Miyasaka H, Takagi M, Hirata K, Miyamoto K (2004) Characterization of phytochelatin synthase-like protein encoded by alr0975 from a prokaryote, Nostoc sp. PCC 7120. Biochem Biophys Res Commun 315 751–755 [DOI] [PubMed] [Google Scholar]
- Vatamaniuk O, Bucher E, Ward J, Rea P (2001) A new pathway for heavy metal detoxification in animals: phytochelatin synthase is required for cadmium tolerance in Caenorhabditis elegans. J Biol Chem 276 20817–20820 [DOI] [PubMed] [Google Scholar]
- Vatamaniuk O, Mari S, Lu Y, Rea P (1999) AtPCS1, a phytochelatin synthase from Arabidopsis: isolation and in vitro reconstitution. Proc Natl Acad Sci USA 96 7110–7115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatamaniuk O, Mari S, Lu Y, Rea P (2000) Mechanism of heavy metal ion activation of phytochelatin (PC) synthase: blocked thiols are sufficient for PC synthase-catalyzed transpeptidation of glutathione and related thiol peptides. J Biol Chem 275 31451–31459 [DOI] [PubMed] [Google Scholar]
- Vivares D, Arnoux P, Pignol D (2005) A papain-like enzyme at work: native and acyl-enzyme intermediate structures in phytochelatin synthesis. Proc Natl Acad Sci USA 102 18848–18853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Harada E, Vess C, von Roepenack-Lahaye E, Clemens S (2004) Comparative microarray analysis of Arabidopsis thaliana and Arabidopsis halleri roots identifies nicotianamine synthase, a ZIP transporter and other genes as potential metal hyperaccumulation factors. Plant J 37 269–281 [DOI] [PubMed] [Google Scholar]
- Welch RM, Graham RD (2004) Breeding for micronutrients in staple food crops from a human nutrition perspective. J Exp Bot 55 353–364 [DOI] [PubMed] [Google Scholar]
- Wintz H, Fox T, Wu YY, Feng V, Chen W, Chang HS, Zhu T, Vulpe C (2003) Expression profiles of Arabidopsis thaliana in mineral deficiencies reveal novel transporters involved in metal homeostasis. J Biol Chem 278 47644–47653 [DOI] [PubMed] [Google Scholar]
- Xiang C, Han P, Lutziger I, Wang K, Oliver DJ (1999) A mini binary vector series for plant transformation. Plant Mol Biol 40 711–717 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.