Abstract
As pollen tubes grow toward the ovary, they are in constant contact with the pistil extracellular matrix (ECM). ECM components are taken up during growth, and some pistil molecules exert their effect inside the pollen tube. For instance, the Nicotiana alata 120-kD glycoprotein (120K) is an abundant arabinogalactan protein that is taken up from the ECM; it has been detected in association with pollen tube vacuoles, but the transport pathway between these compartments is unknown. We recently identified a pollen C2 domain-containing protein (NaPCCP) that binds to the carboxyl-terminal domain of 120K. As C2 domain proteins mediate protein-lipid interactions, NaPCCP could function in intracellular transport of 120K in pollen tubes. Here, we describe binding studies showing that the NaPCCP C2 domain is functional and that binding is specific for phosphatidylinositol 3-phosphate. Subcellular fractionation, immunolocalization, and live imaging results show that NaPCCP is associated with the plasma membrane and internal pollen tube vesicles. Colocalization between an NaPCCP∷green fluorescent protein fusion and internalized FM4-64 suggest an association with the endosomal system. NaPCCP localization is altered in pollen tubes rejected by the self-incompatibility mechanism, but our hypothesis is that it has a general function in the transport of endocytic cargo rather than a specific function in self-incompatibility. NaPCCP represents a bifunctional protein with both phosphatidylinositol 3-phosphate- and arabinogalactan protein-binding domains. Therefore, it could function in the transport of pistil ECM proteins in the pollen tube endomembrane system.
Angiosperm sexual reproduction requires pollen transfer to a receptive stigma followed by its hydration, germination, and pollen tube growth. Pollen tubes grow through the stigma and style toward the ovule, where the sperm cells are discharged for fertilization. Pollen tubes do not divide; rather, they extend through tip growth while periodically producing callose plugs, separating highly vacuolated distal regions from the actively growing tip (Taylor and Hepler, 1997). The tip region shows strong zonation. An apical region or clear zone, a subapical, organelle-rich zone, a nuclear zone, and a distal vacuolated zone or plug region that may extend several centimeters are easily recognized (Mascarenhas, 1993). Proper deposition of wall material and rapid tube extension require coordination between GTPase-regulated trafficking pathways, the cytoskeleton, signaling pathways, and oscillatory ion and water fluxes (Li et al., 1999; Fu et al., 2001; Zonia et al., 2002; Camacho and Malhó, 2003; Chen et al., 2003; de Graaf et al., 2005; Gu et al., 2005).
Pollen tube endomembrane system dynamics are critical for growth: wall materials are deposited by exocytosis, and the membrane is recovered by endocytosis (Picton and Steer, 1983; Cheung and Wu, 2008). Exocytosis of material synthesized in the Golgi occurs near the tip (Cheung et al., 2002). Additional wall material is produced by membrane-bound callose synthase, but this occurs behind the tip (Brownfield et al., 2007). Distinct endocytosis zones have been identified by pulse-chase membrane labeling, observations of charged nanoparticles, and electron microscopy (Derksen et al., 1995; Moscatelli et al., 2007; Zonia and Munnik, 2008). Clathrin-independent endocytosis occurs at the pollen tube apex; endocytic vesicles clearly contribute to vesicle populations in the clear zone once thought to be composed entirely of exocytic vesicles (Moscatelli et al., 2007; Bove et al., 2008; Zonia and Munnik, 2008). Inhibitor studies suggest that clathrin-dependent endocytosis occurs in the organelle-rich zone a few micrometers back from the tip (Moscatelli et al., 2007). Furthermore, coated vesicles have been observed from 6 to 15 μm from the tip by electron microscopy (Derksen et al., 1995).
Pollen-pistil interactions influence pollen tube growth either positively or negatively. Positive effects are evident from the observation that pollen tubes grow as much as 10 times faster and achieve much greater lengths in planta than in culture (Cheung et al., 2000). Self-incompatibility (SI) systems provide the best understood examples of negative effects. In SI, pollen-pistil interactions cause rejection of closely related pollen tubes (de Nettancourt, 2001).
Arabinogalactan proteins (AGPs) secreted into the pistil extracellular matrix (ECM) play key roles in both positive and negative interactions, but the underlying molecular interactions with pollen tubes are just beginning to be understood. The transmitting tract-specific (TTS) glycoprotein (Cheung et al., 1995; Wu et al., 1995, 2000) and the 120-kD glycoprotein (120K; Hancock et al., 2005) are pistil AGPs implicated in pollination in Nicotiana. Both are abundant components of the pistil ECM (Cheung et al., 1995; Lind et al., 1996) and share a conserved Cys-rich C-terminal domain (CTD). TTS was first described in Nicotiana tabacum (i.e. NtTTS) as a pollen tube attractant. Pollen tubes grow toward TTS in culture, and its glycosylation levels progressively increase closer to the ovary (Cheung et al., 1995). Pollen tubes deglycosylate TTS, which suggests that TTS may act as a nutritive factor (Wu et al., 1995) and, thus, positively affect pollen tube growth.
120K is implicated in SI in Nicotiana alata (Cruz-Garcia et al., 2005; Hancock et al., 2005), a species that displays S-RNase-based gametophytic SI (McClure et al., 1989). In SI, compatibility is controlled by the polymorphic S-locus; pollen is rejected if its S-haplotype matches either of the two S-haplotypes in the diploid pistil (de Nettancourt, 2001). Each S-haplotype is unique and encodes separate pollen- and pistil-specificity genes (Kao and Tsukamoto, 2004). S-RNases determine specificity on the pistil side and directly inhibit the growth of closely related pollen tubes (McClure et al., 1989). S-locus F-box proteins (SLF/SFB) control specificity on the pollen side (Sijacic et al., 2004). SLF/SFB proteins bind S-RNase in vitro and appear to form several distinct complexes with other pollen proteins (Qiao et al., 2004; Hua and Kao, 2006; Huang et al., 2006). SI, therefore, is a clear example of inhibitory pollen-pistil interactions: interaction between a pistil protein, S-RNase, and a pollen protein, SLF/SFB, determines compatibility. However, other pistil factors are also required for SI (McClure et al., 1999; Hancock et al., 2005; McClure and Franklin-Tong, 2006). 120K, for example, is required for SI but does not directly contribute to S-specificity (Hancock et al., 2005).
120K was first identified as an abundant component of the transmitting tract ECM that contains both arabinogalactan and extensin-like carbohydrate moieties (Lind et al., 1994). 120K is an S-RNase-binding protein that is taken up by growing pollen tubes (Lind et al., 1996; Cruz-Garcia et al., 2005; Goldraij et al., 2006). Immunolocalization studies show 120K in the pollen tube cytoplasm and associated with pollen tube tonoplast membranes (Lind et al., 1996; Goldraij et al., 2006). Goldraij et al. (2006) also found S-RNase in the lumen of pollen tube vacuoles. In many cases, S-RNase was found in vacuoles with 120K apparently embedded in the surrounding membrane. S-RNase is also found in vacuoles of incompatible pollen tubes, but the breakdown of these vacuoles late in SI and the concomitant release of S-RNase may contribute to the rejection mechanism. Other pistil proteins are also taken up by growing pollen tubes; for example, endocytosis of biotinylated stigma/style Cys-rich adhesin has been reported in lily (Lilium longiflorum) pollen tubes (Kim et al., 2006). Although the uptake of pistil proteins such as 120K and S-RNase has not been well characterized, it is likely that endocytosis and retrograde transport of ECM components occurs on a large scale. Thus, it is important to identify pollen proteins that interact with endocytic cargo from the pistil ECM and that could participate in transport through the pollen tube endomembrane system.
We recently described a pollen-specific C2 domain-containing protein, NaPCCP, that interacts with the CTD of the potential cargo proteins, NaTTS and 120K. NaPCCP consists of a short N-terminal domain, an 80-residue C2 domain, and a 79-residue C-terminal region. In vitro pull-down assays showed that the C-terminal region of NaPCCP is sufficient for binding the AGP CTDs (Lee et al., 2008b). Originally implicated in binding mammalian protein kinase C to phosphatidylserine in a calcium-dependent manner (Bazzi and Nelsestuen, 1987, 1990; Brose et al., 1992), C2 domains are now known to contribute to transient membrane association of a variety of proteins with functions that include vesicular transport, lipid modification, GTPase regulation, ubiquitylation, and protein phosphorylation (Coussens et al., 1986; Clark et al., 1991; Brose et al., 1992; Cullen et al., 1995; Dunn et al., 2004). Calcium-independent lipid binding of C2 domain-containing proteins has also been reported (Damer and Creutz, 1994; Fukuda et al., 1994).
Here, we report the lipid-binding properties of NaPCCP and its association with the pollen tube endomembrane system. Lipid overlay and liposome-binding experiments show that NaPCCP specifically binds to phosphatidylinositol 3-phosphate (PI3P). Immunolocalization and live imaging studies of compatible pollen tubes show that NaPCCP is associated with the pollen tube plasma membrane (PM) and with punctate structures in the cytoplasm. In SI, incompatible pollen tubes show altered NaPCCP distributions. We speculate that NaPCCP is involved in the uptake and transport of proteins from the ECM.
RESULTS
NaPCCP Binds PI3P
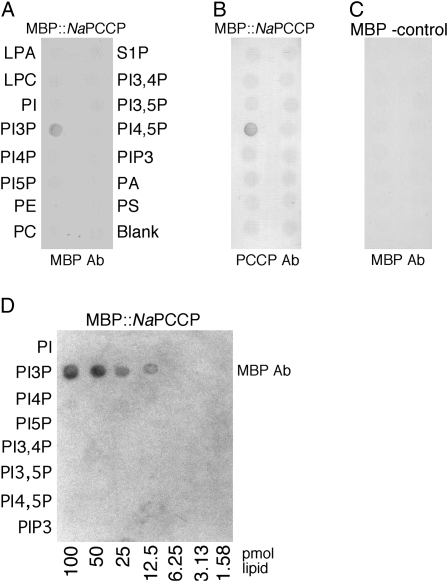
The lipid-binding function of the NaPCCP C2 domain was tested using lipid overlay and liposome-binding assays. NaPCCP was expressed in Escherichia coli as a maltose-binding protein (MBP) fusion (Lee et al., 2008b) and then used to survey potential lipid binding with PIP strips. Figure 1 shows that MBP∷NaPCCP specifically binds PI3P but not other phosphatidylinositol mono-, di-, or triphosphates or the other phospholipids tested. Binding is robust and can be detected with either MBP- or NaPCCP-specific antibodies (Fig. 1, A and B). MBP alone showed no binding (Fig. 1C). Binding could be detected with as little as 12.5 pmol of PI3P (Fig. 1D).
Figure 1.
NaPCCP binds PI3P. Lipid PIP strips (A–C) or PIP arrays (D) were incubated with MBP∷NaPCCP or an MBP-only control, as indicated at the top of each panel. Binding was detected with anti-MBP (A, C, and D) or anti-PCCP (B) antibody (Ab), as indicated below each panel. A and B, PIP strips incubated with MBP∷PCCP and detected with MBP antibody or NaPCCP antibody. C, MBP incubated with the PIP strip (negative control). D, MBP∷NaPCCP incubated with a quantitative PIP array detected with anti-MBP. LPA, Lysophosphatidic acid; LPC, lysophosphocholine; PI5P, phosphatidylinositol 5-phosphate; PE, phosphatidylethanolamine; S1P, sphingosine 1-phosphate; PI3,4P, phosphatidylinositol 3,4-diphosphate; PI3,5P, phosphatidylinositol 3,5-diphosphate; PI4,5P, phosphatidylinositol 4,5-diphosphate; PA, phosphatidic acid; PS, phosphatidylserine.
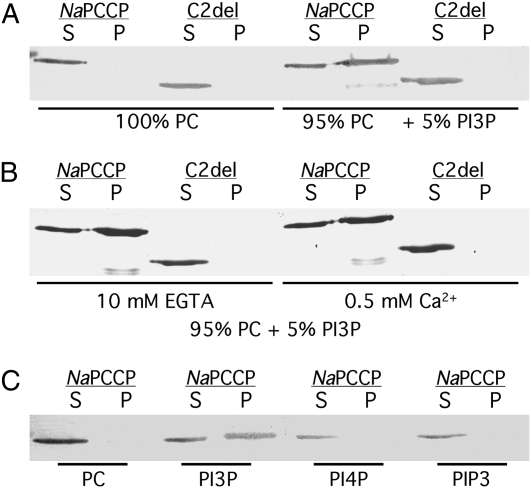
Liposome-binding experiments were conducted using MBP∷NaPCCP and MBP∷C2del (Lee et al., 2008b) to test lipid binding under more native conditions. Preliminary experiments showed approximately linear binding of MBP∷NaPCCP to phosphatidylcholine (PC) liposomes containing up to 15% PI3P and in assays containing up to 4 μg of MBP∷NaPCCP. The standard binding assay employed PC liposomes with 5% phosphatidylinositol phosphate and 2 μg of MBP∷NaPCCP. Binding was assayed by pelleting the liposomes and using protein blots to detect MBP∷NaPCCP or MBP∷C2del in the pellet and supernatant fractions. Figure 2 confirms that MBP∷NaPCCP specifically binds to PI3P. PC-only liposomes do not bind MBP∷NaPCCP, and approximately half of the MBP∷NaPCCP pelleted with 5% PI3P liposomes (Fig. 2A). The C2del fusion did not pellet with either PC or PI3P liposomes, confirming that the C2 domain is essential for lipid binding. MBP∷NaPCCP PI3P liposome binding is calcium independent and occurred in the presence of 10 mm EGTA or 0.5 mm Ca2+ (Fig. 2B). Liposomes containing 5% phosphatidylinositol-4-monophosphate (PI4P) or phosphatidylinositol 3,4,5-triphosphate (PIP3) did not significantly bind MBP∷NaPCCP (Fig. 2C).
Figure 2.
The NaPCCP C2 domain is required for binding to PI3P-containing liposomes. Phosphatidylcholine-based liposomes were incubated with MBP∷NaPCCP or MBP∷C2del and centrifuged at 100,000g, and proteins in the supernatant (S) and liposome pellet (P) fractions were detected by immunostaining with anti-MBP. A, The C2 domain is required for PI3P binding. Left, Control liposomes containing PC alone; right, 95% PC + 5% PI3P. B, Calcium is not required for binding. Left, Incubation in 10 mm EGTA; right, incubation in 0.5 mm Ca2+. C, MBP∷NaPCCP incubated with liposomes containing 100% PC or 95% PC + 5% PI3P, PI4P, or PIP3 as shown.
NaPCCP Binding to Pollen Tube Microsomes
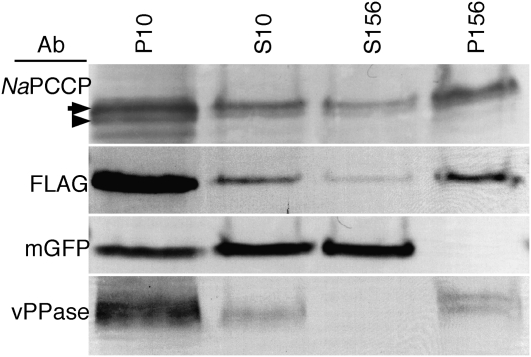
Differential centrifugation experiments tested whether NaPCCP is associated with pollen tube membranes. Since the NaPCCP anti-peptide antibody binds in the conserved C2 domain, it could cross-react with other C2 domain proteins. Therefore, FLAG-tagged NaPCCP was used to test for membrane association in pollen tubes. To provide a clear cytosol marker, transgenic (N. alata × Rastroensis) hybrids expressing NaPCCP∷FLAG were crossed with plants expressing GFP to obtain pollen expressing both NaPCCP∷FLAG and GFP. Expression of the NaPCCP∷FLAG transgene in primary transformants is shown in Supplemental Figure S1, A to C. Pollen tubes from plants expressing GFP and NaPCCP∷FLAG were homogenized and subjected to differential centrifugation. Figure 3 shows that native NaPCCP (arrowhead) and NaPCCP∷FLAG (arrow) partition between the cytosol and microsome fractions (S156 and P156, respectively). NaPCCP, NaPCCP∷FLAG, GFP, and vacuolar pyrophosphatase (vPPase) were all present in the low-speed pellet fraction (P10) due to the difficulty of fully disrupting pollen tubes. High-speed centrifugation cleanly separated the cytosolic GFP marker from vPPase, which served as a microsomal marker (S156 versus P156; Fig. 3). Native NaPCCP and NaPCCP∷FLAG were present in the cytosolic and microsomal fractions, although more protein was recovered from the latter.
Figure 3.
NaPCCP is present in cytosolic and microsomal pollen tube fractions. Pollen tubes expressing both NaPCCP∷FLAG and cytosolic GFP were grown in culture, homogenized, and subjected to differential centrifugation. P10, 10,000g pellet; S10, 10,000g supernatant; S156, 156,000g supernatant; P156, 156,000g microsomal pellet. The anti-PCCP antibody (Ab) detects native NaPCCP (arrowhead) and NaPCCP∷FLAG (arrow). GFP was used as a cytosolic marker; vPPase was used as a microsome marker.
NaPCCP Associates with Pollen Tube Membranes
Immunolocalization and live imaging studies show that NaPCCP associates with the PM and the pollen tube endomembrane system. NaPCCP∷FLAG and NaPCCP∷GFP fusions were transformed into N. tabacum and expressed from the pollen-specific LAT52 promoter (Twell et al., 1990). Pollen tubes from the transformed plants grew normally, and the transgenes were transmitted at the expected ratios (Supplemental Fig. S1, A, C, and D; Supplemental Table S1).
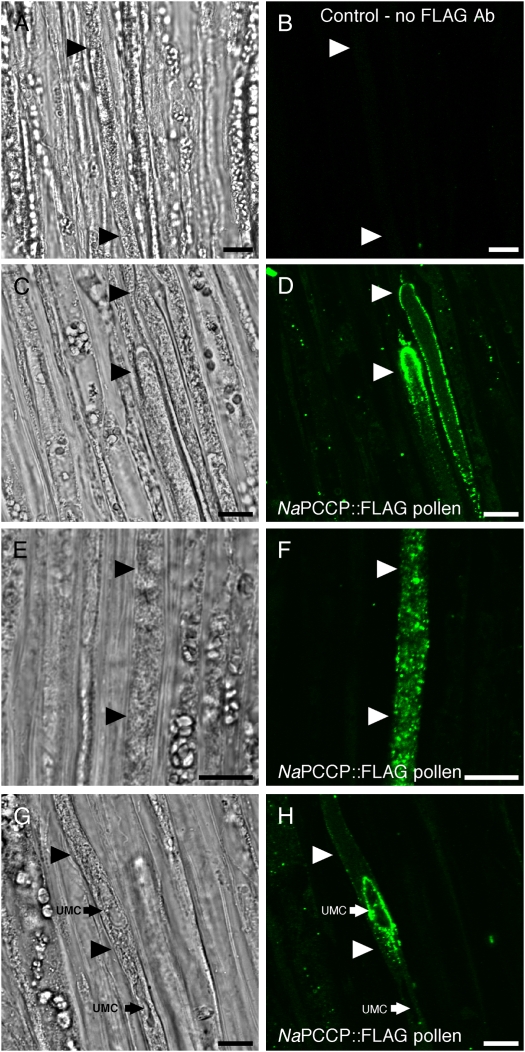
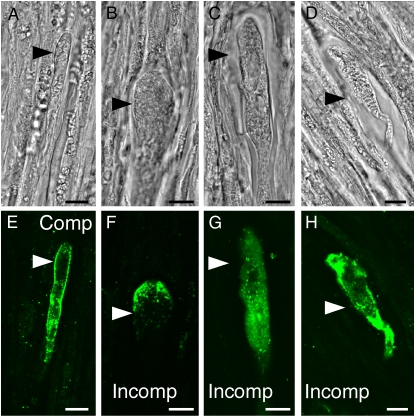
Immunolocalization studies using N. tabacum pollen expressing NaPCCP∷FLAG growing through N. tabacum pistils provide evidence for membrane association. Pollen tubes are identifiable in the transmitting tract as elongated cells with relatively dense cytoplasm laden with small vesicles (Fig. 4, arrowheads). Controls without FLAG antibody (Fig. 4, A and B) or pollinations with wild-type pollen (i.e. not expressing NaPCCP∷FLAG; Supplemental Fig. S1, E and F) showed little or no signal. Observations of apical and subapical regions of 185 pollen tubes near the pollen tube tip showed NaPCCP∷FLAG associated with the PM and punctate structures behind the tip in 80% of pollen tubes (Fig. 4, C and D). A punctate labeling pattern well behind the tip (0.1–1 mm) was observed in 50% of tubes (Fig. 4F). In 30% of the pollen tubes, intense labeling around an unknown membrane compartment (UMC; white arrows) was observed in the highly vacuolated zone of the pollen tube (Fig. 4H).
Figure 4.
NaPCCP∷FLAG is localized to pollen tube membranes. N. tabacum pistils were pollinated with NaPCCP∷FLAG pollen and prepared for immunohistochemistry after 18 h. Bright-field (left) and fluorescence (right) images are shown; arrowheads show pollen tubes. A and B, Control; no FLAG antibody. C to H, Representative NaPCCP∷FLAG localization patterns. C and D, Tip localization seen in approximately 80% of pollen tube tips. E and F, Punctate pattern seen behind the tip in approximately 50% of pollen tubes. G and H, Localization around a UMC (small arrow) seen in approximately 30% of pollen tubes. Bars = 10 μm. [See online article for color version of this figure.]
SI (N. alata × Rastroensis) hybrids expressing NaPCCP∷FLAG were used to test whether NaPCCP localization is altered in incompatible pollen tubes. Hybrids displayed normal S-specific pollen rejection in fruit set and pollen tube-staining assays (Supplemental Table S2; Supplemental Fig. S2). Compatible and incompatible pollinations on hybrid pistils with NaPCCP∷FLAG-expressing pollen were prepared for immunolocalization. Figure 5 shows that compatible pollen tubes have NaPCCP∷FLAG associated with the PM and punctate structures behind the tip, comparable to the pattern in N. tabacum (Fig. 5, A and E, versus Fig. 4). Incompatible tubes were distorted and often had swollen tips, a characteristic of S-specific pollen tube rejection. Although incompatible pollen tubes sometimes show NaPCCP∷FLAG localization near the tip PM (Fig. 5, B and F), the punctate pattern in more distal regions (Fig. 4F) is usually replaced by diffuse staining (Fig. 5, C, D, G, and H). It is not clear from the static images whether the altered localization of NaPCCP∷FLAG in incompatible pollen tubes is directly caused by the S-specific rejection mechanism or as a consequence of growth inhibition.
Figure 5.
NaPCCP∷FLAG localization in compatible and incompatible pollen tubes 24 h after pollination. Arrowheads point to pollen tubes. Bright-field (A–D) and fluorescence (E–H) images show immunolocalization of NaPCCP∷FLAG (green). A and E, Compatible pollination (N. alata × Rastroensis) SR1S105 × (N. alata × Rastroensis NaPCCP∷FLAG 14-1) SRC10SA2. B to D and F to H, Representative patterns seen after incompatible pollination (N. alata × Rastroensis) SR1S105 × (N. alata × Rastroensis NaPCCP∷FLAG 14-1) SR1S105. Bars = 10 μm. [See online article for color version of this figure.]
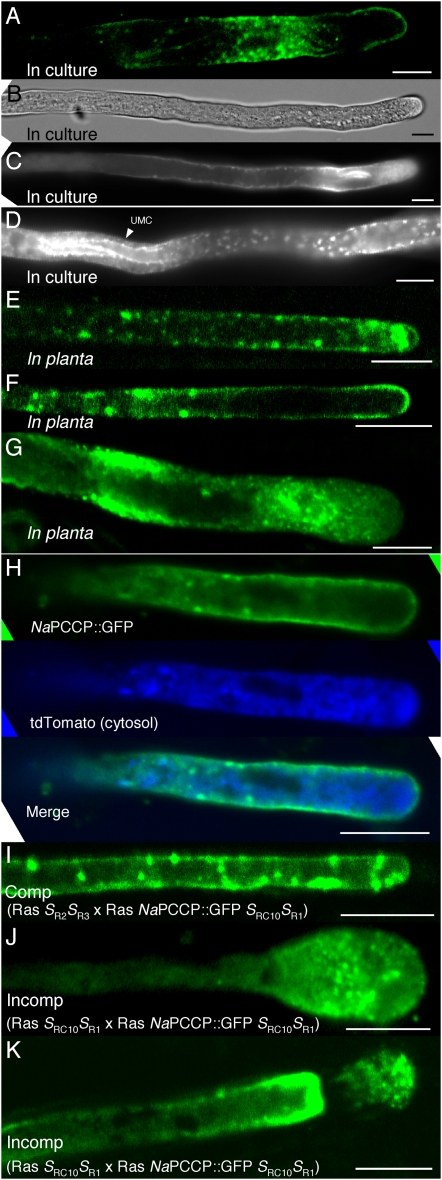
Live imaging studies of NaPCCP∷GFP-expressing pollen show localization to PM and punctate structures (Fig. 6). Live pollen tubes are most clearly visualized when grown in culture. Figure 6, A to D, show that the distribution of NaPCCP∷GFP in N. tabacum pollen tubes growing in culture closely resembles the pattern seen by immunolocalization of NaPCCP∷FLAG (Fig. 4, C–H). NaPCCP∷GFP associates with the PM and with vesicles near the tip, in more distal regions (Fig. 6, A–C), and around the margin of UMCs (Fig. 6D).
Figure 6.
Live imaging of NaPCCP∷GFP localization. A to D, N. tabacum pollen tubes expressing NaPCCP∷GFP in culture. A, Fluorescence image of a single optical section. B and C, Bright-field (B) and fluorescence (C) images of a single tube tip. D, Fluorescence image of a region distal from the tip with a UMC. E to K, Optical sections of NaPCCP∷GFP pollen tubes in planta. E to G, Tip-proximal regions of pollen tubes growing in N. tabacum pistils. H, Pollen tube expressing NaPCCP∷GFP (top; green) and cytosolic tdTomato (center; blue). Bottom, A deconvolved merged image. I to K, NaPCCP∷GFP in compatible and incompatible pollinations. I, Compatible Rastroensis SR2SR3 × Rastroensis SRC10SR1 NaPCCP∷GFP pollination. J and K, Incompatible Rastroensis SRC10SR1 × Rastroensis SRC10SR1 NaPCCP∷GFP pollinations showing distorted pollen tubes. Bars = 10 μm.
Nicotiana pistils are sufficiently thick that growing pollen tubes can be directly visualized by hand sectioning the pistil after pollination. This offers direct insights into pollen tube physiology during growth through the pistil and allows comparison with pollen tubes grown in culture. Two hundred N. tabacum pollen tubes expressing NaPCCP∷GFP were observed in this way. Tubes were scored for punctate patterns, at the tip and distal from the tip (more than 200 μm), and for PM association or diffuse cytosolic localization. Representative images are shown in Figure 6, E to H. Many tubes displayed both PM and punctate labeling. In N. tabacum, 149 of 200 (75%) NaPCCP∷GFP-labeled pollen tubes showed punctate labeling. Of these, 60% (i.e. 90 of 149) showed punctate labeling within 20 to 200 μm of the tip (Fig. 6E); 40% (59 of 149) displayed punctate patterns distally but not at the tip (Fig. 6F). More than half of all pollen tubes (55%; 111 of 200) showed PM labeling (Fig. 6F), and 82% of those (91 of 111) also displayed labeled punctate structures. Only 20 of 200 (10%) tubes displayed labeling exclusively on the PM. Diffuse cytosolic localization was observed in 31 of 200 (15%) pollen tubes (Fig. 6G). Figure 6H shows an N. tabacum pollen tube expressing both NaPCCP∷GFP and cytosolic tdTomato (i.e. a red fluorescent protein; Shaner et al., 2004) growing in planta. The top image shows NaPCCP∷GFP PM labeling patterns with heavy labeling 10 to 20 μm from the tip. tdTomato labeling (Fig. 6H, center) is in the cytosol. The merged image (Fig. 6H, bottom) shows limited mixing that is consistent with the results in Figure 3, but the punctate and PM signals from NaPCCP∷GFP are clearly distinct from the cytosol marker.
Pollinations utilizing SI Rastroensis (SRC10SR1) pollen expressing NaPCCP∷GFP were visualized in compatible (SR2SR3) and incompatible (SRC10SR1) Rastroensis pistils. Figure 6I shows that NaPCCP∷GFP distribution in living compatible Rastroensis pollen tubes closely resembles the pattern in N. tabacum NaPCCP∷GFP pollen tubes (Fig. 6E) and the patterns observed by immunolocalization (Figs. 4D and 5E). Incompatible Rastroensis pollen tubes are arrested close to the stigma and show characteristic distorted morphology and tip swelling. NaPCCP∷GFP labeling is more diffuse in incompatible pollen tubes, although punctate labeling is visible (Fig. 6, J and K). These results confirm the altered distribution of NaPCCP∷GFP in incompatible pollen tubes (Fig. 5, F–H).
NaPCCP Associates with Endosomal Membranes
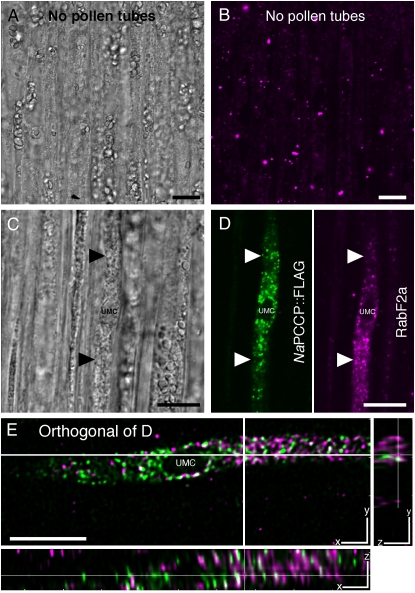
The lipid overlay and liposome-binding results (Figs. 1 and 2) show that NaPCCP binds to PI3P, a component of endosomal membranes (Czech, 2003). Therefore, association of NaPCCP with endosomes was tested using immunolocalization and FM4-64 membrane-labeling experiments. We utilized an endosome marker (anti-RabF2a; Haas et al., 2007) in dual-labeling experiments with N. tabacum pollen expressing NaPCCP∷FLAG to test for endosome association. The RabF2a antibody lightly labeled transmitting tract cells (Fig. 7B), but pollen tubes showed heavy labeling of punctate structures (Fig. 7D, magenta, right). An anti-FLAG antibody similarly labeled punctate structures in the same pollen tube (Fig. 7D, green, left), but image deconvolution shows that only a very small number of structures are labeled with both antibodies (Fig. 7E, white). Thus, NaPCCP∷FLAG is not extensively associated with the population of endosomes labeled with anti-RabF2a.
Figure 7.
Largely independent localization of NaPCCP∷FLAG- and anti-RabF2a-labeled endosomes. Unpollinated control (A and B) or N. tabacum pistils pollinated with NaPCCP∷FLAG pollen (C–E) were immunostained with anti-FLAG or anti-RabF2a (endosomal marker) antibodies. The unpollinated control images (A and B) show RabF2a-positive endosomes in transmitting tract cells. C, Bright-field image showing a NaPCCP∷FLAG pollen tube approximately 500 μm behind the tip. D, Single optical section of C showing a pollen tube with a prominent vacuole. Left, Anti-FLAG (green); right, anti-RabF2a (magenta). E, Orthogonal views of a deconvolved, merged stack from C and D. The main panel shows a single optical section (x-y plane); cross-hairs indicate where the stack was sectioned to show the y-z and x-z planes. White spots indicate rare areas of colocalization, but the NaPCCP∷FLAG (green) and RabF2a (magenta) signals are largely independent. Bars = 10 μm.
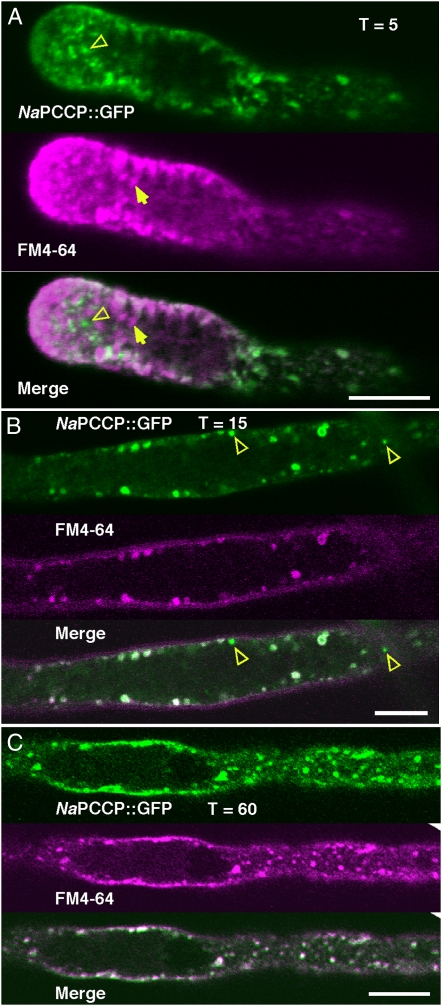
Dual-labeling experiments were conducted with NaPCCP∷GFP and FM4-64 as another test for association with endosomal membrane. FM4-64 binds to the PM and successively labels endomembrane compartments as endosomal membrane mixes with internal compartments (Bolte et al., 2004). N. tabacum pollen tubes expressing NaPCCP∷GFP were treated with FM4-64 in culture and then imaged after 5, 15, or 60 min. Figure 8A (5 min of incubation) shows FM4-64-labeled PM and vesicles streaming back from the tip (Bove et al., 2008; Zonia and Munnik, 2008). NaPCCP∷GFP labels punctate structures in this pollen tube. The merged image shows considerable overlap with the FM4-64 signal, but some puncta show labeling with only one or the other label (Fig. 8A, open or closed arrowheads). After 15 or 60 min of incubation, internalized FM4-64 has mixed with membrane populations farther from the tip (Fig. 8, B and C). While after 15 min there are a few singly labeled structures (Fig. 8B, open arrowheads), the signals show essentially complete overlap after 60 min. Thus, the punctate structures labeled by NaPCCP∷GFP contain membrane recovered from the PM and are likely endosomal compartments distinct from the population labeled with anti-RabF2a (Fig. 7E).
Figure 8.
Association of NaPCCP∷GFP with FM4-64-labeled compartments in N. tabacum. NaPCCP∷GFP (top; green), FM4-64 (center; magenta), and merge (bottom) are shown. Yellow open arrowheads indicate predominantly NaPCCP∷GFP-labeled vesicles, and yellow closed arrows indicate predominantly FM4-64-labeled vesicles. A, Pollen tube after 5 min of incubation, imaged near the tip. With a few exceptions (e.g. arrowheads), the signals are mostly mixed close to the tip. B, Pollen tube after 15 min of incubation, imaged behind the tip. C, Pollen tube after 60 min of incubation, imaged behind the tip. Bars = 10 μm.
Since some endosomal material is ultimately targeted to the vacuole, and the results in Figures 4 and 6 show clustering of NaPCCP around UMCs resembling a vacuole, we tested for colocalization of NaPCCP∷FLAG with vPPase. The results in Supplemental Figure S3 show little colocalization of these markers, suggesting that these structures are not mature vacuoles that display the vPPase marker. UMC structures with NaPCCP clustering sometimes resemble sperm cells (Fig. 4), but they do not stain with 4′,6-diamidino-2-phenylindole (DAPI; Supplemental Fig. S4).
DISCUSSION
Pollen tubes attain remarkable growth rates in planta, as high as 250 nm s−1 in lily (Messerli et al., 2000) and 400 nm s−1 in N. alata (Lee et al., 2008a), rates much faster than growth in culture. Thus, the pistil promotes rapid pollen tube growth. Results showing that pistil proteins enter pollen tubes and are processed in the endomembrane system suggest that processes occurring inside pollen tubes contribute to rapid growth in planta (Lind et al., 1996; Cheung et al., 2000; Luu et al., 2000; Goldraij et al., 2006; Kim et al., 2006). Pollen tube growth clearly involves both large-scale transport of secretory vesicles to the growing tip (Campanoni and Blatt, 2007; Cheung and Wu, 2007) as well as endocytosis that provides for retrieval and recycling of membrane (Picton and Steer, 1983; Derksen et al., 1995; Zonia and Munnik, 2008).
NaPCCP was identified in a yeast two-hybrid screen for pollen proteins that bind to NaTTS and 120K, two pistil AGPs known to interact with pollen (Cheung et al., 1995; Wu et al., 1995, 2000; Lind et al., 1996; Hancock et al., 2005). The CTDs of these pistil proteins are very similar, and while NaTTS has not been directly shown to enter pollen tubes, there is abundant evidence that 120K does so (Lind et al., 1996; Goldraij et al., 2006). Pull-down experiments demonstrated that a 79-amino acid sequence located near the NaPCCP C terminus is sufficient for binding the CTD of NaTTS and 120K (Lee et al., 2008b). Since 120K, at least, is clearly taken up by pollen tubes, NaPCCP's C2 domain lipid-binding motif and its binding to the pistil AGPs made it worthy of further investigation as a candidate for guiding pistil proteins through the pollen tube endomembrane system.
Examination of the lipid-binding capabilities of NaPCCP using PIP strips and liposome-binding experiments revealed that NaPCCP specifically binds to PI3P in a Ca2+-independent fashion (Figs. 1 and 2). C2 domains have been found to interact with phosphatidylserine, phosphatidylinositol (PI) polyphosphates, and occasionally PI monophosphates (Evans et al., 2004, 2006; Rodriguez-Alfaro et al., 2004; Kouchi et al., 2005). Kouchi et al. (2005) identified a C2 domain interaction with PI3P; however, its binding was not PI3P specific. Thus, NaPCCP's specificity for PI3P is unique. While most C2 domain proteins show Ca2+-dependent phospholipid binding (Rizo and Sudhof, 1998), Ca2+-independent binding also occurs (Ochoa et al., 2001; Shearn and Norris, 2007). Although we found no evidence for Ca2+-dependent binding to PI3P in vitro, it is possible that Ca2+ regulates NaPCCP binding to PI3P in vivo.
Consistent with its lipid binding properties in vitro, fractionation and localization experiments show that NaPCCP associates with pollen tube membranes (Figs. 3–8). The fractionation results show that most NaPCCP pellets with the high-speed microsomal pellet (Fig. 3). However, some NaPCCP and NaPCCP∷FLAG was always recovered in the cytosol. This observation could be due to a transient association or separate pools of NaPCCP.
NaPCCP binding to PI3P is significant because the intracellular side of the eukaryotic PM contains PIs that act as localized signals. Recent studies suggest that PI signals are likely to be especially important in pollen tube endomembrane system dynamics. For example, Zonia and Munnik (2004, 2008) showed that osmotic inhibition of endocytosis caused a 2-fold decrease in PI monophosphates, and conditions that favor endocytosis increased PI monophosphates. They also found that stimulating endocytosis increased PI(3,5)P2, a late endosome marker synthesized from PI3P during endosomal transition into a multivesicular body (MVB; Zonia and Munnik, 2004). In general, PM microdomains and endomembrane structures are labeled with specific PI signals that recruit sorting complexes (Czech, 2003). Specifically, PI3P is a component of endosomes, late endosomes/MVBs, and recycling endosomes (Czech, 2003). Two PI3P-binding motifs have been described: the FYVE (Gillooly et al., 2001) and PX (Kanai et al., 2001) domains. GFP fusions with the PI3P-binding FYVE domain (Gillooly et al., 2001) colocalize with endosome markers such as Rab5, EEA1, SARA, and clathrin-coated vesicles (Gillooly et al., 2003). Since PI3P recruits proteins required for the assembly of protein complexes needed for merging, sorting, and recycling of endocytic vesicles (Simonsen et al., 1998; Lawe et al., 2001; Katzmann et al., 2003; Naslavsky et al., 2003), the results showing that NaPCCP binds both PI3P (Figs. 1 and 2) and pistil AGPs (Lee et al., 2008b) are consistent with a role in the transport of pistil proteins in the pollen tube endomembrane system.
Immunolocalization and live imaging experiments with NaPCCP fusions are consistent with a role in endocytosis. Experiments using pollen tubes expressing NaPCCP fusions grown in planta revealed a punctate pattern typical of endosomes (Cheung and Wu, 2007) through much of the actively growing region (Figs. 4 and 6–8). NaPCCP fusion-labeled vesicles were often observed farther from the tip than might be expected from observations of pollen tubes grown in culture (i.e. 10 μm to hundreds of micrometers; Figs. 4D, 5A, and 6, A, E, F, and I). Perhaps, pollen tubes growing in planta may have a more extended growth region because of their much faster growth rates (Cheung et al., 2000; Lee et al., 2008a). Nevertheless, culture-grown tubes expressing NaPCCP∷GFP displayed similar patterns to tubes grown in planta (Fig. 6, A–D), albeit over a more compressed region. In either condition, the pattern is similar to the subapical endocytosis pattern described by Zonia and Munnik (2008). Some NaPCCP∷FLAG pollen tubes contained heavily decorated UMCs that appear vacuole like. UMCs were variable in size, usually located more than 200 μm from the tip, and associated with many small, NaPCCP-labeled vesicles. NaPCCP-decorated UMCs were typically observed much closer to the tip in pollen tubes grown in culture than when grown in planta (Fig. 6D versus Fig. 4, G and H). Although these structures resemble germ cells, DAPI staining shows that they do not contain DNA (Supplemental Fig. S4). We have preliminary evidence for distinct classes of vacuole-like compartments in pollen tubes (S.K. and B.M., unpublished observations), and it is possible that NaPCCP-associated UMCs represent one of these compartments.
FM4-64 experiments (Bolte et al., 2004) and immunolocalization with anti-RabF2a provided more specific tests of whether NaPCCP is present on endosomes or compartments derived from the PM. Anti-Rab2Fa labels endosomes delivered to a vacuole through the MVB pathway (Kotzer et al., 2004; Haas et al., 2007). Our results show very little colocalization between anti-RabF2a and NaPCCP∷FLAG (Fig. 7E). However, experiments with FM4-64 clearly show that NaPCCP∷GFP-labeled vesicles contain PM-derived material (Fig. 8). This is consistent with separation between RabF2a-labeled endosomes and the endocytic pathway involving NaPCCP. Using different approaches, both Moscatelli et al. (2007) and Zonia and Munnik (2008) found evidence for multiple endocytic pathways in the tip and subapical regions of pollen tubes. Moscatelli et al. (2007) propose a clathrin-dependent endocytic route that transports material from the subapical PM to the trans-Golgi network (TGN). Based on the association of FM4-64 and the TGN marker VHA-a1, Dettmer et al. (2006) also found support for a PM-to-TGN pathway. Our observation that NaPCCP shows only slight colocalization with vesicles labeled with anti-RabF2a and extensive colocalization with FM4-64 at the PM and in internal vesicle populations strongly supports the existence of multiple endocytic pathways in pollen tubes. However, as in other studies, our results are consistent with a degree of overlap between pathways, since we observed limited colocalization between NaPCCP and the RabF2a-labeled population and between NaPCCP and the vPPase-labeled vacuoles.
NaPCCP was originally identified in the SI species N. alata (Lee et al., 2008b). Therefore, we examined the localization of NaPCCP∷FLAG and NaPCCP∷GFP in both compatible and incompatible backgrounds. Compatible pollen tubes display NaPCCP localization patterns that we regard as typical: strong labeling of the apical PM and punctate labeling behind the tip (Figs. 5E and 6I). However, in rejected pollen tubes, NaPCCP fusions display patterns consistent with major physiological perturbations: more cytosolic labeling is observed, but small vesicles in the swollen pollen tube tips are also labeled (Figs. 5, F–H, and 6, J and K). Although NaPCCP binds to 120K, a factor required for SI, it also binds NaTTS, an AGP that promotes tube growth and is not known to be involved in SI or taken into pollen tubes. Moreover, NaPCCP-like sequences in closely related SI and self-compatible Nicotiana species are extremely conserved (Lee et al., 2008b). This too is more consistent with a general role in pollen tube biology than a SI-specific role.
We interpret the localization differences in rejected tubes as SI-related effects on the pollen tube endomembrane system rather than as a result of an SI-specific effect directly targeting NaPCCP function. Nevertheless, transport of pistil proteins in the pollen tube endomembrane system is clearly important in SI, and the interaction between NaPCCP and 120K, which also binds S-RNase (Cruz-Garcia et al., 2005), may be important. S-RNase is transported to the vacuole in compatible pollen tubes (Goldraij et al., 2006). However, by analogy to cytotoxins such as ricin (Ferrini et al., 1995), we speculate that some S-RNase also moves to the endoplasmic reticulum (ER), where it could exit to the cytosol (McClure, 2006). Like S-RNase, most of the ricin that enters the cell moves to the vacuole; ricin that is diverted to the ER first passes through the Golgi (Di Cola et al., 2005). Our observation that NaPCCP localization is similar to PM-to-TGN transport pathways suggests that it could help divert 120K (and any associated S-RNase) away from the bulk pathway that ends in the vacuole and toward the TGN and ER. This is potentially important in SI because S-RNase in the ER could exit to the cytosol for interaction with SLF. Furthermore, 120K is also known to interact with an E3 ubiquitin ligase, NaSBP1 (Lee et al., 2008b), whose Petunia ortholog (Sims and Ordanic, 2001; Hua and Kao, 2006) is capable of forming SLF-S-RNase complexes in pollen and polyubiquitylates S-RNase in vitro (Hua and Kao, 2008). Thus, although the bifunctional character of NaPCCP, with its PI3P- and AGP-binding domains, suggests a general role in the transport of pistil ECM materials through the pollen tube endomembrane system, it could also be important for the S-RNase-SLF interaction that mediates compatibility.
MATERIALS AND METHODS
Plants and Growth Conditions
Nicotiana alata (SC10SC10, S105S105, and SA2SA2 genotypes) and Nicotiana tabacum ‘Praecox’ were described previously (Murfett et al., 1994; Beecher and McClure, 2001). The SI putative species Rastroensis was collected in Brazil by Dr. Tim Holtsford (University of Missouri). A voucher specimen is housed in the Dunn-Palmer Herbarium at the University of Missouri (UMO 190742). Rastroensis (SRC10SR1) and N. tabacum were transformed with Agrobacterium tumefaciens strain LBA4404 harboring LAT52-GFP (a gift from Dr. A. Cheung, University of Massachusetts-Amherst), NaPCCP∷GFP, or NaPCCP∷FLAG constructs in pBIN19 (Bevan, 1984). Transformations of Rastroensis followed the Nicotiana plumbaginifolia protocol described previously (Beecher, 1999), except that 2-isopentyladenine was the cytokinin, cocultivation plates used Suc rather than Glc, and cefotaxime and vancomycin (50 μg mL−1) were added to inhibit bacterial growth. Transformed and wild-type Rastroensis (SRC10SR1) was crossed with N. alata to produce N. alata × Rastroensis hybrids as needed. For the fractionation experiment shown in Figure 3, an SR1SA2 (N. alata × Rastroensis) hybrid expressing cytosolic GFP from the LAT52 promoter was crossed with Rastroensis NaPCCP∷FLAG (SRC10SR1) to create progeny expressing both NaPCCP∷FLAG and GFP in the pollen tubes. N. tabacum expressing NaPCCP∷GFP was crossed to N. tabacum expressing the red fluorescent protein variant tdTomato (Shaner et al., 2004) in the pollen tube cytosol to produce the NaPCCP∷GFP/tdTomato plant shown in Figure 6H. All Nicotiana plants were grown in soil under greenhouse conditions (14-h days at approximately 24°C and 10-h nights at approximately 20°C).
NaPCCP-Tag Constructs
MBP∷NaPCCP and MBP∷C2del constructs were described previously (Lee et al., 2008b). The NaPCCP∷FLAG and NaPCCP∷GFP fusions were engineered as follows. NaPCCP was amplified from the MBP∷NaPCCP construct using primers that introduced an NcoI site and a linker with a PstI site: [PCCP-G sense (NcoI), 5′-GCAACCATGGAGTCATCACCAAAAACACCAGACAGCAAAG-3′; PCCP-G antisense (PstI), 5′-GCAACTGCAGCTGAAGCAGATGCCAAAATCTTACTGCCAGGAAG-3′]. The A206K mutation preventing dimerization of fluorescent proteins (Zacharias et al., 2002) was introduced by amplifying GFP from the LAT52-GFP construct. The strategy required amplification of 5′ and 3′ segments of GFP using primers GFP 5′ sense (PstI) (5′-GCAACTGCAGCTATGGTGAGCAAGGGCGAGGAGCTGTTC-3′), GFP 5′ A206K antisense (5′-GGGGTCTTTGCTCAGGGCGGACTGGGTGCTC-3′), GFP 3′ A206K sense (5′-GAGCACCCAGTCCGCCCTGAGCAAAGACCCC-3′), and GFP 3′ antisense (SalI) (5′-GCAAGTCGACTTACTTGTACAGCTCGTCCATGCCGAGAG-3′). The 5′ and 3′ segments were combined to form mGFP on a PstI-SalI fragment, ligated to the LAT52 promoter (Twell et al., 1990), and sequence verified. To create NaPCCP∷FLAG, PCCP-G sense (NcoI) and PCCP-F antisense (SalI) (5′-GCAAGTCGACCTACTTGTCGTCATCGTCTTTGTAGTCCATCAAAATCTTACTGCCAGGAA-3′) primers were used to amplify NaPCCP∷FLAG from MBP∷NaPCCP. The sequence was verified and ligated to the LAT52 promoter. Both constructs were transferred to pBIN19 for Agrobacterium-mediated transformation.
Antibodies
Mouse anti-FLAG M2 antibody (F-1804) and rabbit anti-GFP (G-1544) were purchased from Sigma. Rabbit anti-MBP was purchased from New England Biolabs (E8030S). The rabbit NaPCCP antibody is an affinity-purified polyclonal rabbit antibody to the peptide KLKTRVIKKDINPEWNEEL produced by Bethyl Labs. Guinea pig anti-SR1-RNase was prepared with the peptide DLNRSPYDSKKEQNF and affinity purified by Biosynthesis, Inc. The Alexa Fluor (488 and 568) goat anti-rabbit and anti-mouse antibodies were from Molecular Probes. The RabF2a antibody used to detect Ara6/7-labeled endosomes was a generous gift from Dr. Erik Nielsen and is described by Haas et al. (2007). Other antibodies have been described (Murfett et al., 1996; Goldraij et al., 2006).
Subcellular Fractionation
Approximately 100 anthers from plants expressing NaPCCP∷FLAG and cytosolic GFP were harvested and collected in a 1.5-mL tube and then shaken free of pollen. One milliliter of pollen tube growth medium (PTGM), modified from Read et al. (1993; i.e. without copper, casein acid hydrolysate, or rifampicin), was added to the anthers and vortexed. After overnight incubation at room temperature, pollen tubes were collected by centrifugation, ground under liquid nitrogen, and then stored at −80°C. Frozen, ground pollen tubes were further homogenized on ice with a Powergen 700 (Fisher Scientific) for 3 min at setting 4 in extraction buffer (50 mm Tris, pH 7.5, 0.25 m mannitol, 2 mm EDTA, 5 mm ascorbic acid, 40 μL 2 mL−1 protease inhibitor cocktail [PIC-P9599, Sigma], and 1 mm phenylmethylsulfonyl fluoride). Homogenized samples were centrifuged at 1,000g in an HB-6 rotor at 4°C for 5 min. The supernatant was subsequently centrifuged at 10,000g in an HB-6 rotor at 4°C for 20 min. The pellet (P10) was saved for analysis, and the supernatant (S10) was further centrifuged at 156,000g in a TL100.3 rotor (Beckman Coulter) at 4°C for 1 h. The top 1 mL of the supernatant (S156) was saved for analysis. The high-speed pellet (P156) was washed in extraction buffer and pelleted a second time at 156,000g for 30 min.
Protein/Lipid Overlays and Liposome Experiments
MBP∷NaPCCP and MBP∷C2del were expressed and purified as described (Lee et al., 2008b). PIP strips and PIP arrays (Echelon Biosciences) were incubated in Tris-buffered saline with Tween 20 (TBST; 10 mm Tris, pH 8.0, 150 mm NaCl, and 0.1% [v/v] Tween 20) with 1% milk for 1 h, followed by incubation with 500 ng mL−1 MBP or MBP∷NaPCCP in 10 mL of TBST with milk for 1 h. Membranes were washed three times with TBST and milk, immunostained with a 1:10,000 dilution of anti-MBP or a 1:5,000 dilution of anti-NaPCCP, and then visualized with alkaline phosphatase-conjugated secondary antibodies.
For liposome preparation, PC was purchased from Sigma (P7443), while PI3P (P-3016), PI4P (P-4016), and PIP3 (P-3916) were purchased from Echelon Biosciences. Lipids were solubilized in CHCl3:methanol (95:5), mixed as needed, dried slowly under a stream of N2 gas, vacuum dried for 1 h, and then rehydrated in liposome resuspension buffer (25 mm Tris-HCl, pH 7.5, and 100 mm NaCl). Unilaminar liposomes were made by passing lipid resuspensions 15 times through a 0.2-μm polycarbonate membrane in a mini-extruder (Avanti Polar Lipids). Liposomes were centrifuged at 100,000g in a TS-55 rotor (Beckman) for 20 min at room temperature, collected, and resuspended in binding buffer (25 mm Tris-HCl, pH 7.5, 100 mm NaCl, and 1 mm MgCl2). In control experiments, approximately linear binding was observed with liposomes containing up to 15% PI3P, and no aggregation of MBP∷NaPCCP occurred below 4 μg of added protein. Standard room temperature binding reactions included a 1-h incubation of 0.51 μmol of liposomes containing 5% PI3P with 2.0 μg of MBP∷NaPCCP or MBP∷C2del in 100 μL. Binding was assessed after centrifugation at 100,000g in a TS-55 rotor at room temperature for 30 min. The top 50 μL of the supernatant was collected for analysis. Pelleted liposomes were washed with 500 μL of binding buffer and pelleted a second time. The liposome pellet was resuspended in 4× loading buffer (0.25 m Tris, pH 6.8, 8% SDS [w/v], 40% glycerol [w/v], 200 mm dithiothreitol, and 0.004% bromphenol blue [w/v]). Equal amounts of the pellet (1/4 sample) and supernatant (1/2 sample) were analyzed by SDS-PAGE and immunoblotting. Experiments examining the effects of Ca2+ on MBP∷NaPCCP liposome binding replaced MgCl2 in the binding buffer with 0.5 mm Ca2+ or 10 mm EGTA. Liposome-binding experiments were conducted in triplicate using fresh protein preparations.
Preparation of Plant Materials/Microscopy
For pollen tube growth studies, N. tabacum pistils were collected at 12, 18, 24, and 30 h after pollination. N. alata × Rastroensis hybrid pistils were collected after 24 or 36 h. Squashes were prepared as described (Kho and Baer, 1968), and pollen tubes were measured by viewing with a Leica MZFLIII stereoscope (Meyer Instruments).
For immunolocalization, pollinations were performed and harvested after 18 h. Pistils were trimmed, fixed under vacuum in 4% paraformaldehyde, 50 mm PIPES, pH 6.9, 1 mm MgCl2, and 0.5 mm EGTA for 90 min, washed, dehydrated, infiltrated with Steedman's wax (Vitha et al., 1997), and sectioned to 8 μm onto poly-l-Lys-coated slides. Pistil sections were dewaxed and incubated in primary antibody and A-488- and A-568-conjugated secondary antibodies as described (Paris and Rogers, 1996; Goldraij et al., 2006). Anti-FLAG and anti-Rab2Fa were used at dilutions of 1:100. Anti-vPPase was used at a 1:50 dilution.
NaPCCP∷GFP-expressing tubes were grown on coverslips in 30 μL of PTGM in a humid chamber and visualized 8 h after germination. In the FM4-64 experiments, pollen tubes expressing NaPCCP∷GFP were grown in culture for 6 h. FM4-64 (12 μm final) was added, and pollen tubes were visualized immediately and then again after 15 or 60 min of incubation. In live imaging experiments, pollinations were performed 18 h prior to imaging. In planta NaPCCP∷GFP and NaPCCP∷GFP/tdTomato tubes were visualized by bisecting pistils after 18 h of growth. Sliced pistils were placed cut side down in 50 μL of water on coverslips and covered with filter paper wet with PTGM.
Images were obtained with a Zeiss LSM510 Meta inverted scanning confocal microscope fitted with a 40× C Apochromat water-immersion objective. Laser levels, gain, and offset were optimized and then kept constant for each laser. GFP and A488 were excited with a 488-nm laser and detected between 500 and 545 nm. FM4-64, A568, and tdTomato were excited with a 543-nm laser and detected between 565 and 615 nm. Sections stained with DAPI (1 μg mL−1) were excited with a 405-nm laser and imaged between 415 and 490 nm with a Zeiss 5 Live confocal microscope (63× water objective). Bright-field images were obtained by detecting laser light transmitted from the specimen. Some images were deconvolved (Autodeblur 9.1; Autoquant, Inc.) using 10 constrained iterations. Levels were adjusted in Photoshop 7.0 (Adobe Systems). Orthogonal projections were generated with Imaris 4.2 (Bitplane, Inc.). Pollen tubes expressing NaPCCP∷GFP in culture were also visualized on an Olympus IX70 inverted microscope fitted with a 40× U Apo/340 oil-immersion objective and a HQ fluorescein isothiocyanate filter (41001 filter; Chroma).
Plant Protein Extracts, Protein Gels, and Immunostaining
Styles were weighed, ground in 4× loading buffer, boiled for 5 min, and centrifuged for 3 min at 16,000g. Supernatant equivalent to 1 mg fresh weight was loaded in each lane. Pollen extracts were similarly made by boiling anthers from a single flower in 100 μL of 4× loading buffer for 5 min and centrifuging at 16,000g for 3 min; 10 μL of the supernatant was loaded in each lane. All proteins were separated on 10% Tris-Tricine gels (Schägger and von Jagow, 1987), blotted, and immunostained as described (Murfett et al., 1996).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. NaPCCP∷FLAG qualification.
Supplemental Figure S2. S-specific pollen rejection of N. alata × Rastroensis hybrids.
Supplemental Figure S3. Largely independent localization of NaPCCP∷FLAG- and vPPase-labeled vacuoles.
Supplemental Figure S4. NaPCCP∷FLAG localization in the nuclear region.
Supplemental Table S1. Segregation analysis for NaPCCP transgenics.
Supplemental Table S2. S-specific pollen rejection in N. alata × Rastroensis hybrids reflected by fruit set.
Supplementary Material
Acknowledgments
We thank Dr. Katsuhiko Kondo for helpful comments during both the experimental phase and the writing. We thank Melody Kroll for editorial assistance. Dr. Alice Cheung provided the LAT52-GFP plasmid. Dr. Erik Nielsen provided the RabF2a antibody. Dr. Zhanyuan Zhang and Dr. Joseph Ringbauer assisted with plant transformations. Dr. Tim Holtsford provided the Rastroensis plants. Ben Troutwine and Dr. G. Esteban Fernandez provided much help with image processing.
This work was supported by the National Science Foundation (grant no. IOB 0614962).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Bruce McClure (mcclureb@missouri.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Bazzi MD, Nelsestuen GL (1987) Association of protein kinase C with phospholipids vesicles. Biochemistry 26 115–122 [DOI] [PubMed] [Google Scholar]
- Bazzi MD, Nelsestuen GL (1990) Protein kinase C interaction with calcium: a phospholipids-dependent process. Biochemistry 29 7624–7630 [DOI] [PubMed] [Google Scholar]
- Beecher B (1999) Role of RNase activity in interspecific pollen rejection in Nicotiana. PhD thesis. University of Missouri, Columbia, MO
- Beecher B, McClure BA (2001) Effects of RNases on rejection of pollen from Nicotiana tabacum and N. plumbaginifolia. Sex Plant Reprod 14 69–76 [Google Scholar]
- Bevan M (1984) Binary Agrobacterium vectors for plant transformations. Nucleic Acids Res 12 8711–8721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S, Talbot C, Boutte Y, Catrice O, Read ND, Satiat-Jeunemaitre B (2004) FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J Microsc 214 159–173 [DOI] [PubMed] [Google Scholar]
- Bove J, Vaillancourt B, Kroeger J, Hepler PK, Wiseman PW, Geitmann A (2008) Magnitude and direction of vesicle dynamics in growing pollen tubes using spatiotemporal image correlation spectroscopy and fluorescence recovery after photobleaching. Plant Physiol 147 1646–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose N, Petrenko AG, Südhof TC, Jahn R (1992) Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science 256 1021–1025 [DOI] [PubMed] [Google Scholar]
- Brownfield L, Ford K, Doblin MS, Newbigin E, Read S, Bacic A (2007) Proteomic and biochemical evidence links the callose synthase in Nicotiana alata pollen tubes to the product of the NaGSL1 gene. Plant J 52 147–156 [DOI] [PubMed] [Google Scholar]
- Camacho L, Malhó R (2003) Endo/exocytosis in the pollen tube apex is differentially regulated by Ca2+ and GTPases. J Exp Bot 54 83–92 [DOI] [PubMed] [Google Scholar]
- Campanoni P, Blatt MR (2007) Membrane trafficking and polar growth in root hairs and pollen tubes. J Exp Bot 58 65–74 [DOI] [PubMed] [Google Scholar]
- Chen CY, Cheung AY, Wu HM (2003) Actin-depolymerizing factor mediates Rac/Rop GTPase-regulated pollen tube growth. Plant Cell 15 237–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Chen CY, Glaven RH, de Graaf B, Vidali L, Hepler PK, Wu HM (2002) Rab2 GTPase regulates vesicle trafficking between the endoplasmic reticulum and the Golgi bodies and is important to pollen tube growth. Plant Cell 14 945–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Wang H, Wu HM (1995) A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 82 383–393 [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wu HM (2007) Structural and functional compartmentalization in pollen tubes. J Exp Bot 58 75–82 [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wu HM (2008) Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu Rev Plant Biol 59 547–572 [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wu HM, di Stilio V, Glaven R, Chen C, Wong E, Ogdahl J, Estavillo A (2000) Pollen-pistil interactions in Nicotiana tabacum. Ann Bot (Lond) (Suppl A) 85 29–37 [Google Scholar]
- Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, Milona N, Knopf JL (1991) A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell 65 1043–1051 [DOI] [PubMed] [Google Scholar]
- Coussens L, Parker PJ, Rhee L, Yang-Feng TL, Chen E, Waterfield MD, Francke U, Ullrich A (1986) Multiple, distinct forms of bovine and human protein kinase C suggest diversity in cellular signaling pathways. Science 233 859–866 [DOI] [PubMed] [Google Scholar]
- Cruz-Garcia F, Hancock CN, Kim D, McClure BA (2005) Stylar glycoproteins bind to S-RNase in vitro. Plant J 42 295–304 [DOI] [PubMed] [Google Scholar]
- Cullen PJ, Hsuan JJ, Truong O, Letcher AL, Jackson TR, Dawson AP, Irvine RF (1995) Identification of a specific Ins(1,3,4,5)P4-binding protein as a member of the GAP1 family. Nature 376 527–530 [DOI] [PubMed] [Google Scholar]
- Czech MP (2003) Dynamics of phosphoinositides in membrane retrieval and insertion. Annu Rev Physiol 65 791–815 [DOI] [PubMed] [Google Scholar]
- Damer CK, Creutz CE (1994) Synergistic membrane interactions of the two C2 domains of synaptotagmin. J Biol Chem 269 31115–31123 [PubMed] [Google Scholar]
- de Graaf BHJ, Cheung AY, Andreyeva T, Lavasseur K, Kieliszewski M, Wu HM (2005) Rab11 GTPase-regulated membrane trafficking is crucial for tip-focused pollen tube growth in tobacco. Plant Cell 17 2564–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nettancourt D (2001) Incompatibility and Incongruity in Wild and Cultivated Plants. Springer-Verlag, New York
- Derksen J, Rutten T, Lichtscheidl IK, De Win AHN, Pierson ES, Rongen G (1995) Quantitative analysis of the distribution of organelles in tobacco pollen tubes: implications for exocytosis and endocytosis. Protoplasma 188 267–276 [Google Scholar]
- Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumaker K (2006) Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18 715–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cola A, Frigerio L, Lord JM, Roberts LM, Ceriotti A (2005) Endoplasmic reticulum-associated degradation of ricin A chain has unique and plant-specific features. Plant Physiol 137 287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn R, Klos DA, Adler AS, Hicke L (2004) The C2 domain of the Rsp5 ubiquitin ligase binds membrane phosphoinositides and directs ubiquitination of endosomal cargo. J Cell Biol 165 135–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JH, Gerber SH, Murray D, Leslie CC (2004) The calcium binding loops of the cytosolic phospholipase α2 C2 domain specify targeting to Golgi and ER in live cells. Mol Biol Cell 15 371–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JH, Murray D, Leslie CC, Falke JJ (2006) Specific translocation of protein kinase Cα to the plasma membrane requires both Ca2+ and PIP2 recognition by its C2 domain. Mol Biol Cell 17 56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrini JB, Martin M, Taupiac MP, Beaumelle B (1995) Expression of functional ricin B chain using the baculovirus system. Eur J Biochem 233 772–777 [DOI] [PubMed] [Google Scholar]
- Fu Y, Wu G, Yang Z (2001) Rop GTPase-dependent dynamics of tip-localized F-actin control tip growth in pollen tubes. J Cell Biol 152 1019–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Aruga J, Niinobe M, Aimoto S, Mikoshiba K (1994) Inositol-1,3,4,5-tetrakisphosphate binding to C2B domain of IP4BP/synaptotagmin II. J Biol Chem 269 29206–29211 [PubMed] [Google Scholar]
- Gillooly DJ, Raiborg C, Stenmark H (2003) Phosphatidylinositol 3-phosphate is found in microdomains of early endosomes. Histochem Cell Biol 120 445–453 [DOI] [PubMed] [Google Scholar]
- Gillooly DJ, Simonsen A, Stenmark H (2001) Cellular functions of phosphatidylinositol 3-phosphate and FYVE domain proteins. Biochem J 355 249–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldraij A, Kondo K, Lee CB, Hancock CN, Sivaguru M, Vazquez-Santana S, Kim S, Phillips TE, Cruz-Garcia F, McClure BA (2006) Compartmentalization of S-RNase and HT-B degradation in self-incompatible Nicotiana. Nature 439 805–810 [DOI] [PubMed] [Google Scholar]
- Gu Y, Fu Y, Dowd P, Li S, Vernoud V, Gilroy S, Yang Z (2005) A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J Cell Biol 169 127–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas TJ, Sliwinski MK, Martinez DE, Preuss M, Ebine K, Ueda T, Nielsen E, Odorizzi G, Otegui MS (2007) The Arabidopsis AAA ATPase SKD1 is involved in multivesicular endosome function and interacts with its positive regulator LYST-INTERACTING PROTEIN5. Plant Cell 19 1295–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock CN, Kent L, McClure BA (2005) The 120kDa glycoprotein is required for S-specific pollen rejection in Nicotiana. Plant J 43 716–723 [DOI] [PubMed] [Google Scholar]
- Hua Z, Kao T (2006) Identification and characterization of components of a putative Petunia S-locus F-box-containing E3 ligase complex involved in S-RNase-based self-incompatibility. Plant Cell 18 2531–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Z, Kao T (2008) Identification of major lysine residues of S3-RNase of Petunia inflata involved in ubiquitin-26S proteasome-mediated degradation in vitro. Plant J 54 1094–1104 [DOI] [PubMed] [Google Scholar]
- Huang J, Zhao L, Yang Q, Xue Y (2006) AhSSK1, a novel SKP1-like protein that interacts with the S-locus F-box protein SLF. Plant J 46 780–793 [DOI] [PubMed] [Google Scholar]
- Kanai F, Liu H, Field SJ, Akbary H, Matsuo T, Brown GE, Cantley LC, Yaffe MB (2001) The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat Cell Biol 3 675–678 [DOI] [PubMed] [Google Scholar]
- Kao T, Tsukamoto T (2004) The molecular and genetic bases of S-RNase-based self-incompatibility. Plant Cell (Suppl) 16 S72–S83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann DJ, Stefan CJ, Babst M, Emr SD (2003) Vps27 recruits ESCRT machinery to endosomes during MVB sorting. J Cell Biol 160 413–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho YO, Baer J (1968) Observing pollen tubes by means of fluorescence. Euphytica 17 299–302 [Google Scholar]
- Kim ST, Zhang K, Dong J, Lord EM (2006) Exogenous free ubiquitin enhances lily pollen tube adhesion to an in vitro stylar matrix and may facilitate endocytosis of SCA. Plant Physiol 142 1397–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzer AM, Brandizzi F, Neumann U, Paris N, Moore I, Hawes C (2004) AtRabF2b (Ara7) acts on the vacuolar trafficking pathway in tobacco leaf epidermal cells. J Cell Sci 117 6377–6389 [DOI] [PubMed] [Google Scholar]
- Kouchi Z, Shikano T, Nakamura Y, Shirakawa H, Fukami K, Miyazaki S (2005) The role of EF-hand domains and C2 domain in regulation of enzymatic activity of phospholipase Cζ. J Biol Chem 280 21015–21021 [DOI] [PubMed] [Google Scholar]
- Lawe DC, Chawla A, Merithew E, Dumas J, Carrington W, Fogarty K, Lifshitz L, Tuft R, Lambright D, Corvera S (2001) Sequential roles for phosphatidylinositol 3-phosphate and Rab5 in tethering and fusion of early endosomes via their interaction with EEA1. J Biol Chem 277 8611–8617 [DOI] [PubMed] [Google Scholar]
- Lee CB, Page L, McClure BA, Holtsford TP (2008. a) Post-pollination hybridization barriers in Nicotiana section Alatae. Sex Plant Reprod 21 183–195 [Google Scholar]
- Lee CB, Swatek KN, McClure BA (2008. b) Pollen proteins interact with the C-terminal domain of Nicotiana alata pistil arabinogalactan proteins. J Biol Chem 283 26965–26973 [DOI] [PubMed] [Google Scholar]
- Li H, Lin Y, Heath RM, Zhu MX, Yang Z (1999) Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium flux. Plant Cell 11 1731–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind JL, Bacic A, Clarke AE, Anderson MA (1994) A style-specific hydroxyproline-rich glycoprotein with properties of both extensions and arabinogalactan proteins. Plant J 6 491–502 [DOI] [PubMed] [Google Scholar]
- Lind JL, Bönig I, Clarke AE, Anderson MA (1996) A style-specific 120-kDa glycoprotein enters pollen tubes of Nicotiana alata in vivo. Sex Plant Reprod 9 75–86 [Google Scholar]
- Luu DT, Qin X, Morse D, Cappadocia M (2000) S-RNase uptake by compatible pollen tubes in gametophytic self-incompatibility. Nature 407 649–651 [DOI] [PubMed] [Google Scholar]
- Mascarenhas JP (1993) Molecular mechanisms of pollen tube growth and differentiation. Plant Cell 5 1303–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure BA (2006) New views of S-RNase-based self-incompatibility. Curr Opin Plant Biol 9 639–646 [DOI] [PubMed] [Google Scholar]
- McClure BA, Franklin-Tong V (2006) Gametophytic self-incompatibility: understanding the cellular mechanisms involved in “self” pollen tube inhibition. Planta 224 233–245 [DOI] [PubMed] [Google Scholar]
- McClure BA, Haring V, Ebert PR, Anderson MA, Simpson RJ, Sakiyama F, Clarke AE (1989) Style self-incompatibility gene products of Nicotiana alata are ribonucleases. Nature 342 955–957 [DOI] [PubMed] [Google Scholar]
- McClure BA, Mou B, Canevascini S, Bernatzky R (1999) A small asparagine-rich protein required for S-allele-specific pollen rejection in Nicotiana. Proc Natl Acad Sci USA 96 13548–13553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerli MA, Creton R, Jaffe LF, Robinson KR (2000) Periodic increases in elongation precede increases in cytosolic Ca2+ during pollen tube growth. Dev Biol 222 84–98 [DOI] [PubMed] [Google Scholar]
- Moscatelli A, Ciampolini F, Rodighiero S, Onelli E, Cresti M, Santo N, Idilli A (2007) Distinct endocytic pathways identified in tobacco pollen tubes using charged nanogold. J Cell Sci 120 3804–3819 [DOI] [PubMed] [Google Scholar]
- Murfett J, Strabala TJ, Zurek DM, Mou B, Beecher B, McClure BA (1996) S-RNase and interspecific pollen rejection in the genus Nicotiana: multiple pollen-rejection pathways contribute to unilateral incompatibility between self-incompatible and self-compatible species. Plant Cell 8 943–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murfett JM, Atherton TL, Mou B, Gasser CS, McClure BA (1994) S-RNase expressed in transgenic Nicotiana causes S-allele-specific pollen rejection. Nature 367 563–566 [DOI] [PubMed] [Google Scholar]
- Naslavsky N, Weigert R, Donaldson JG (2003) Convergence of non-clathrin- and clathrin-derived endosomes involves Arf6 inactivation and changes in phosphoinositides. Mol Biol Cell 14 417–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa WF, Garcia-Garcia J, Fita I, Corbalan-Garcia S, Verdaguer N, Gomez-Fernandez JC (2001) Structure of the C2 domain from novel protein kinase Cepsilon: a membrane binding model for Ca2+-independent C2 domains. J Mol Biol 311 837–849 [DOI] [PubMed] [Google Scholar]
- Paris N, Rogers JC (1996) The role of receptors in targeting soluble proteins from the secretory pathway to the vacuole. Plant Physiol Biochem 34 223–237 [Google Scholar]
- Picton JM, Steer MW (1983) Membrane recycling and the control of secretory activity in pollen tubes. J Cell Sci 63 303–310 [DOI] [PubMed] [Google Scholar]
- Qiao H, Wang H, Zhao L, Zhou J, Huang J, Zhang Y, Xue Y (2004) The F-box protein AhSLF-S2 physically interacts with S-RNases that may be inhibited by the ubiquitin/26S proteasome pathway of protein degradation during compatible pollination in Antirrhinum. Plant Cell 16 582–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read SM, Clarke AE, Bacic A (1993) Stimulation of growth of cultured Nicotiana tabacum W 38 pollen tubes by poly(ethylene glycol) and Cu(II) salts. Protoplasma 177 1–14 [Google Scholar]
- Rizo J, Sudhof TC (1998) C2-domains, structure and function of a universal Ca2+ binding domain. J Biol Chem 273 15879–15882 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Alfaro JA, Gomez-Fernandez JC, Corbalan-Garcia S (2004) Role of the lysine-rich cluster of the C2 domain in the phosphatidylserine-dependent activation of PKCα. J Mol Biol 335 1117–1129 [DOI] [PubMed] [Google Scholar]
- Schägger H, von Jagow G (1987) Tricine-sodium dodecylsulfate polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166 368–379 [DOI] [PubMed] [Google Scholar]
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BNG, Palmer AE, Tsien RY (2004) Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22 1567–1572 [DOI] [PubMed] [Google Scholar]
- Shearn CT, Norris FA (2007) Biochemical characterization of the type I inositol polyphosphate 4-phosphatase C2 domain. Biochem Biophys Res Commun 356 255–259 [DOI] [PubMed] [Google Scholar]
- Sijacic P, Wang X, Skirpan A, Wang Y, Dowd P, McCubbin A, Huang S, Kao T (2004) Identification of the pollen determinant of S-RNase-mediated self-incompatibility. Nature 429 302–305 [DOI] [PubMed] [Google Scholar]
- Simonsen A, Lippé R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh B-H, Murphy C, Zerial M, Stenmark H (1998) EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394 494–498 [DOI] [PubMed] [Google Scholar]
- Sims T, Ordanic M (2001) Identification of a S-ribonuclease binding protein in Petunia hybrida. Plant Mol Biol 47 771–783 [DOI] [PubMed] [Google Scholar]
- Taylor LP, Hepler PK (1997) Pollen germination and tube growth. Annu Rev Plant Physiol Plant Mol Biol 48 461–491 [DOI] [PubMed] [Google Scholar]
- Twell D, Yamaguchi J, McCormick S (1990) Pollen-specific gene expression in transgenic plants: coordinate regulation of two different tomato gene promoters during microsporogenesis. Development 109 705–713 [DOI] [PubMed] [Google Scholar]
- Vitha S, Baluska F, Mews M, Volkmann D (1997) Immunofluorescence detection of F-actin on low melting point wax sections from plant tissues. J Histochem Cytochem 45 89–95 [DOI] [PubMed] [Google Scholar]
- Wu HM, Wang H, Cheung AY (1995) A pollen tube growth stimulatory glycoprotein is deglycosylated by pollen tubes and displays a glycosylation gradient in the flower. Cell 82 395–403 [DOI] [PubMed] [Google Scholar]
- Wu HM, Wong E, Ogdahl J, Cheung AY (2000) A pollen tube growth-promoting arabinogalactan protein from Nicotiana alata is similar to the tobacco TTS protein. Plant J 22 165–176 [DOI] [PubMed] [Google Scholar]
- Zacharias DA, Violin JD, Newton AC, Tsien RY (2002) Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296 913–916 [DOI] [PubMed] [Google Scholar]
- Zonia L, Cordeiro S, Tupy J, Feijó JA (2002) Oscillatory chloride efflux at the pollen tube apex has a role in growth and cell volume regulation and is targeted by inositol 3,4,5,6-tetrakisphosphate. Plant Cell 14 2233–2249 [PubMed] [Google Scholar]
- Zonia L, Munnik T (2004) Osmotically induced cell swelling versus cell shrinking elicits specific changes in phospholipid signals in tobacco pollen tubes. Plant Physiol 134 813–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonia L, Munnik T (2008) Vesicle trafficking dynamics and visualization of zones of exocytosis and endocytosis in tobacco pollen tubes. J Exp Bot 59 861–873 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.