Abstract
Conventional therapies for malignant gliomas (MGs) fail to target tumor cells exclusively, such that their efficacy is ultimately limited by non-specific toxicity. Immunologic targeting of tumor-specific gene mutations, however, may allow more precise eradication of neoplastic cells.
The epidermal growth factor receptor variant III (EGFRvIII) is a consistent tumor-specific mutation that is widely expressed in MGs and other neoplasms. This mutation encodes a constitutively active tyrosine kinase that enhances tumorgenicity and migration and confers radiation and chemotherapeutic resistance. This in-frame deletion mutation splits a codon resulting in the creation of a novel glycine at the fusion junction between normally distant parts of the molecule and producing a sequence rearrangement which creates a tumor-specific epitope for cellular or humoral immunotherapy in patients with MGs.
We have previously shown that vaccination with a peptide that spans the EGFRvIII fusion junction is an efficacious immunotherapy in syngeneic murine models, but patients with MGs have a profound immunosuppression that may inhibit the ability of antigen presenting cells (APCs), even those generated ex vivo, to induce EGFRvIII-specific immune responses. In this report, we summarize our results in humans targeting this mutation in two consecutive and one multi-institutional Phase II immunotherapy trials. These trials demonstrated that vaccines targeting EGFRvIII are capable of inducing potent T- and B-cell immunity in these patients, and an unexpectedly long survival time. Most importantly, vaccines targeting EGFRvIII were universally successful at eliminating tumor cells expressing the targeted antigen without any evidence of symptomatic collateral toxicity.
These studies establish the tumor-specific EGFRvIII mutation as a novel target for humoral- and cell-mediated immunotherapy in a variety of cancers. The recurrence of EGFRvIII-negative tumors in our patients, however, highlights the need for targeting a broader repertoire of tumor-specific antigens.
Keywords: antigens, central nervous system neoplasms, epidermal growth factor receptor, immunotherapy
1. Malignant Primary Brain Tumors and Immunotherapy
Malignant primary brain tumors are more common than Hodgkin’s disease, multiple myeloma, and testicular cancer and account for more human deaths than malignant melanoma[1, 2]. Despite aggressive, image-guided tumor resection[3], high-dose external beam radiotherapy[4] or targeted brachytherapy[5], and recent advances in chemotherapy[6], patients with glioblastoma multiforme (GBM) live <15 months from the time of diagnosis[6], and patients with recurrent tumors usually survive <20 weeks[7]. Moreover, the non-specific nature of conventional therapy for brain tumors often results in incapacitating damage to surrounding normal brain and systemic tissues[8]. Thus, in order to be more effective, therapeutic strategies will have to precisely target tumor cells while minimizing collateral damage to neighboring eloquent cerebral cortex. The rationale for employing the immune system to target brain tumors is based on the premise that the inherent biologic specificity of immunologic reactivity could meet the clear need for more specific and precise therapy.
2. Immunosuppression in Patients with Malignant Gliomas
A substantial barrier to the activation of antitumor immune responses in patients with GBM is their well-documented impairment of T- and B-cell immunity[9–30]. Specifically, cutaneous anergy, lymphopenia, impaired antibody production, reduced lymphocyte protein synthesis, and diminished lymphocyte responsiveness have been documented. Importantly, there is a significant improvement in immunological parameters after surgical removal of the tumor[9, 13, 31]. One possible explanation for the impairment of cell-mediated immunity suffered by patients with primary brain tumors is the secretion by these tumors of transforming growth factor-β (TGF-β) isoforms and other immunosuppressive molecules such as interleukin (IL)-10 and PGE2. Although these and other secreted factors clearly play a direct role[11, 32–43], we have recently demonstrated that a major contributor to depressed cellular immunity in patients with GBM is an increased level of regulatory T-cells (TRegs)[44] amidst a poorly understood, but severe, CD4+ T-cell lymphopenia (Mean 168 cells/μL) that rivals that of patients with HIV/AIDS (<200 cells/μL).
TRegs potently inhibit T-cell cytokine secretion and proliferation[45–49], directly curtail the generation and expansion of endogenous or induced immune responses[50–58], and appear to play a significant role in hindering immunity to tumor-associated antigens[59, 60]. Our own studies reveal that the removal of TRegs from patient T-cells in vitro restores T-cell proliferative and cytokine responses to normal levels[44]. Inactivation of TRegs in our murine glioma model also significantly enhanced endogenous and vaccination-induced antitumor immune responses and led to complete eradication of orthotopic astrocytomas without induction of autoimmunity[61]. Similar results were obtained by us using systemic cytotoxic T-lymphocyte-associated protein (CTLA)-4 blockade which resulted in safe rejection of well-established tumors. Importantly, CTLA-4 blockade also completely reversed the CD4+ T-cell deficit and normalized the ratio of TRegs in tumor-bearing mice displaying the same deficiencies found in humans with MGs[62]. While enhancing cross-priming or eliminating the suppression of endogenous antitumor immune responses through the elimination of regulatory T-cells[44, 61] or blocking of CTLA-4 signaling[62], for example, may be effective strategies, they will run the risk of inducing uncontrollable autoimmunity[63–66].
3. Autoimmune Encephalomyelitis
Most data suggest that for immunotherapy to be effective in the context of large human tumors, a very strong and sustained antitumor immune response will be required[67]. In animal models, when such responses have been generated that target tumor-derived antigens that are shared with host cells, severe or at least clinically significant autoimmune disease has resulted[63, 68, 69]. In light of the documented expression of normal adult and fetal brain antigens on human glioma cell lines[70] and fresh tumor tissue[71–74], active immunization with unselected antigens risks inducing an uncontrolled autoimmune response against the normal central nervous system (CNS) similar to experimental allergic encephalomyelitis (EAE).
Myelin basic protein (MBP) is the most common known antigenic trigger, but myelin proteolipid protein[75, 76], myelin oligodendrocyte glycoprotein[77], glial fibrillary acidic protein, and S-100β[78] are also sufficient antigens for the induction of EAE. In animal models, EAE can be readily induced in the various species of rats, guinea pigs, mice, sheep, and monkeys after a single injection of a potent adjuvant and normal CNS tissue homogenate. More to the point, lethal EAE has been induced in non-human primates with tissue derived from human MGs[79].
The susceptibility of humans to the induction of EAE was discovered accidentally when patients were vaccinated against rabies with spinal cords from rabbits that were infected with the rabies virus[80–84]. Some concern regarding EAE is also warranted on the basis of previous active, specific immunotherapy trials in humans with brain tumors. Although no cases of EAE were reported in some human studies[85–88] and protocols have been developed for safe active, specific immunotherapy with glioma-derived cells in primates[89], careful review of the studies by Bloom et al. [90] and Trouillas[91] reveal one probable case of EAE in each study. Given the range of protocols that routinely use immunization with CNS tissue for the production of lethal EAE in non-human primates and the documented susceptibility of humans to EAE, the induction of such autoimmune responses as a result of broadly-targeted cancer immunotherapy strategies is of particular concern. Thus, the risk of EAE, or other similar and potentially lethal autoimmune responses, may severely limit the optimization and efficacy of active immunotherapy for CNS tumors if antigens are not selected carefully for tumor-specificity.
4. Tumor-specific Rejection Antigens and EGFRvIII
Most well-characterized tumor antigens are over-expressed normal proteins which have triggered immunologic tolerance to some degree. This compromises their effectiveness as tumor rejection antigens and poses a risk of autoimmunity if effectively targeted[92, 93]. Conversely, tumor-specific antigens derived from mutations in somatic genes are less prejudiced by central tolerance and less likely to be associated with autoimmunity. Some studies also suggest that the autonomous immune response to human tumors is dominated by such neoantigens[94]. These mutations, however, often arise randomly as a result of the genetic instability[95, 96] of tumors and as such tend to be patient-specific and may be incidental to the oncogenic process.
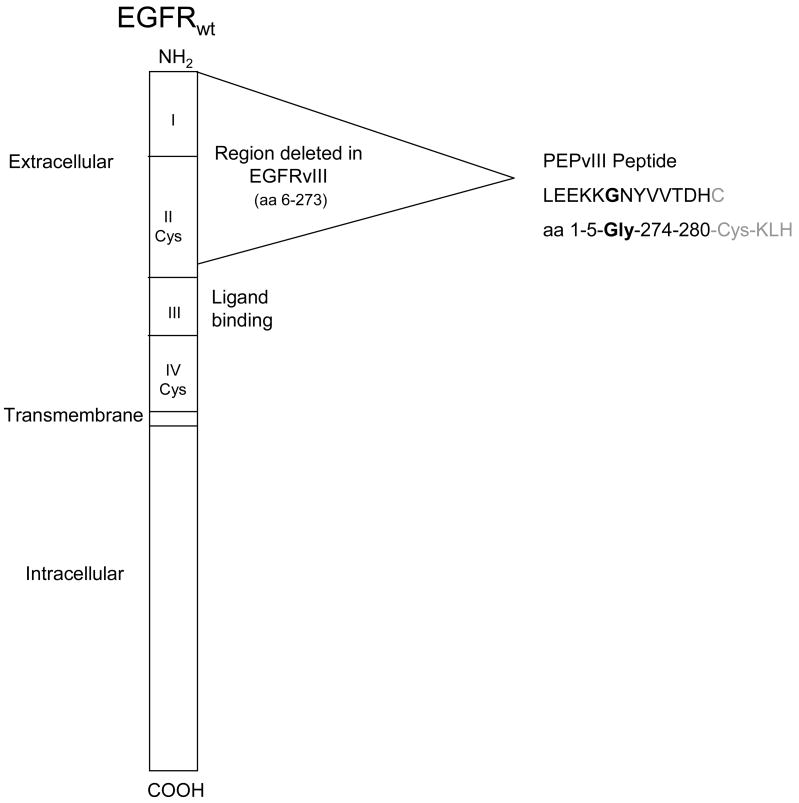
EGFRvIII (deltaEGFR, EGFR, de2-7 EGFR), however, is a frequent and consistent tumor-specific mutation (Fig. 1), central to the neoplastic process, that consists of an in-frame deletion of 801 base pairs from the extracellular domain of the EGFR that splits a codon and produces a novel glycine at the fusion junction[97, 98]. This mutation encodes a constitutively active tyrosine kinase[99, 100] that enhances tumorgenicity[100–103] and migration[104, 105] and confers radiation and chemotherapeutic resistance[106–112] to tumor cells. In addition, cells appear to become “addicted”[113] to the signaling pathways induced by EGFRvIII and die without it[114]. The EGFRvIII mutation is most frequently seen in patients with the most malignant form of primary glioma, glioblastoma multiforme (GBM)[115–121] but has been found in a broad array of other common cancers[112, 115, 120–128]. For patients with GBM, the presence of EGFRvIII-expressing cells confers a negative prognosis[121, 129]. The new glycine inserted at the fusion junction of normally distant parts of the extracellular domain results in a tumor-specific epitope not found in any normal adult tissues[130]. The exquisite tumor-specificity of EGFRvIII; its clonal expression in GBMs and other common tumors; its absence in any normal tissues; and its importance in the pathobiology of tumors make EGFRvIII an ideal target for antitumor immunotherapy[115, 123, 124, 130].
Figure 1.
Schematic diagram of the EGFR wild type protein showing the area of in-frame deletion which forms EGFRvIII. During the deletion amino acids 6 and 273 are split forming a novel glycine at the junction of amino acids 5 and 274. PEPvIII is a 13 amino acid peptide with a terminal cysteine added to facilitate conjugation to KLH.
5. Pre-Clinical Immunotherapy Studies with EGFRvIII
In pre-clinical systems EGFRvIII-expressing cell lines or an EGFRvIII-specific 14-amino acid peptide, PEPvIII (H-Leu-Glu-Glu-Lys-Lys-Gln-Asn-Tyr-Val-Val-Thr-Asp-His-Cys-OH), which has been chemically conjugated to keyhole limpet hemocyanin (KLH) (PEPvIII-KLH) has generally been used for the generation of EGFRvIII-specific antibodies[115, 130–138], induction cellular immune responses[139–141], or derivation of targeted toxins[142–145].
Murine and human chimeric[146] EGFRvIII-specific antibodies have been derived and cloned for use in diagnostic immunohistochemistry and fluorescent cytometry[115], but the most reliable results are still obtained with absorbed polyclonal sera. Monoclonal antibodies binding EGFRvIII are rapidly internalized at ambient temperatures[132, 136, 147, 148], but with modified labeling methods have been used successfully in rodent models for therapeutic radioimmunotherapy[132, 146–148].
Unarmed murine antibodies targeting EGFRvIII, however, have also shown significant antitumor activity in vitro and in vivo in rodent model systems. Using B16 melanoma transfected with murine homologue of EGFRvIII created by using cDNA sequences spanning the murine EGFR (EMBL X78987) (msEGFRvIII), we evaluated unarmed murine IgG2a (Y10) and IgG1 (L8A4) monoclonal antibodies for therapeutic efficacy[131] (Fig. 2). Both antibodies significantly inhibited subcutaneous (s.c.) tumor growth when given intraperitoneally. However, once treatments with antibodies were stopped, mice treated with the IgG1 isotype antibody suffered tumor recurrence while those treated with Y10 has longterm tumor-free survival in all mice treated (n=20; P<0.001). In vitro, Y10 was found to inhibit DNA synthesis and cellular proliferation in tumor cells expressing msEGFRvIII in the presence of the antibody. Y10 was also found to be capable of inducing autonomous, complement-mediated, and antibody-dependent cell-mediated cytotoxicity. While intraperioneal therapy with Y10 failed to increase median survival of mice with msEGFRvIII-expressing B16 melanomas in the “immunologically privileged” brain, treatment with a single intratumoral injection of Y10 increased median survival by an average 286% and produced 26% long-term survivors (n=117, P<0.001). The mechanism of action of Y10 in vivo was shown to be dependent on Fc receptors and independent of complement, granulocytes, natural killer cells, and T lymphocytes. A human chimeric antibody based on Y10 has been developed for clinical use and is also capable of inducing lysis of human EGFRvIII-expressing MG cell lines autonomously and in the presence of activated human macrophages (Fig. 3). Another murine monoclonal antibody (IgG2b) that targets EGFRvIII (mAb 806), but has some reactivity against the wild-type EGFR especially when over-expressed, has also been shown to reduce tumor growth and angiogenesis in rodents with human MG xenografts transfected with EGFRvIII[136–138]. In addition, mAb 806 has been shown to reduce EGFRvIII phosphorylation, increase tumor cell apoptosis, and down-regulate expression of the apoptotic protector Bcl-XL[138].
Figure 2.
Volume of subcutaneous B16 melanoma tumors transfected with a murine homologue of EGFRvIII, msEGFRvIII, in C57BL/6J mice treated with anti-EGFRvIII mouse MAb Y10, (IgG2a) and L8A4 (IgG1). Mice were treated i.p. on day 0 with 500 μg of anti-EGFRvIII MAbs or isotype matched control MAbs, followed by 200 μg of MAb every other day thereafter for 20 days. Treatment with isotype control MAbs failed to inhibit tumor growth. Mice treated with L8A4 remained tumor free until treatment was stopped and the tumors then grew at a rate equal to untreated tumors. Mice treated with Y10 were protected from tumor growth even after MAb therapy was stopped.
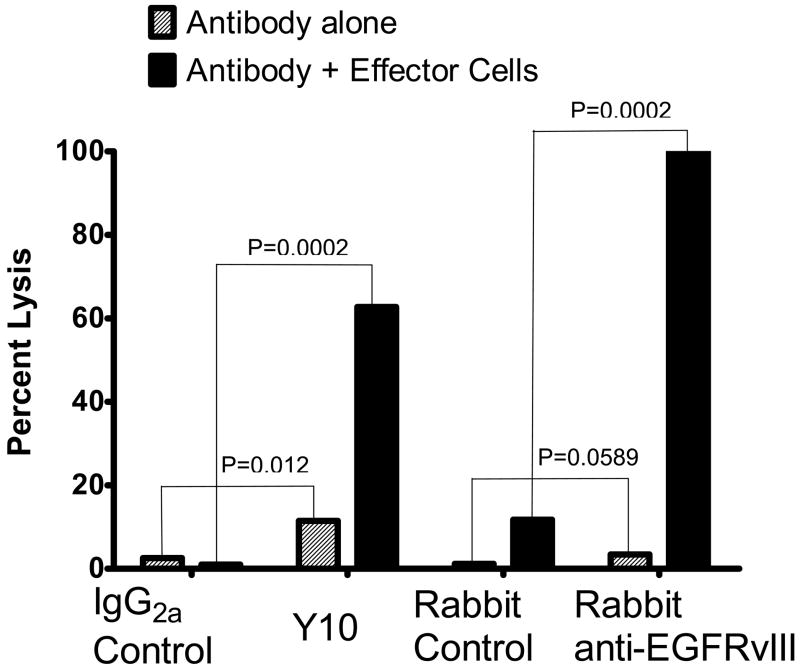
Figure 3.
Lysis of the human GBM cell line U87MG EGFR by EGFRvIII-specific MAb Y10 in the presence or absence of human macrophages. U87MG EGFR is a human GBM cell line transfected to express human EGFRvIII. Y10 is a murine IgG2a MAb that specifically recognizes EGFRvIII. Rabbit anti-EGFRvIII is a polyclonal affinity purified antiserum that specifically recognizes EGFRvIII and does not cross react with wild type EGFR. M221 is an isotype-matched control murine MAb and the rabbit control antibody is an affinity purified rabbit polyclonal antibody to an unrelated antigen. EGFRvIII specific antibodies were incubated for 72 hours with 3H thymidine labeled target cells with and without human macrophages. Antibodies were used at a concentration of 0.1 mg/mL and the macrophages were at an effector to target ratio of 100:1. Y10 produced a significantly greater lysis than M22.1 (P=0.0002).
Active immunotherapy has also been effective against syngeneic murine tumors expressing msEGFRvIII[139, 140]. Intraperitoneal vaccination with dendritic cells (DCs) mixed with PEPvIII-KLH and resuspended in saline increased median survival by >552% (>300 days, P<0.001) in C3H mice challenged with intracerebral tumors[140]. The majority of mice vaccinated using this approach survived long-term without evidence of tumor, and all survived rechallenge with tumor suggesting the development of long-lasting immunological memory. More significantly, C3H mice with well-established intracerebral tumors that received a single vaccination of PEPvIII-KLH in complete Freund’s adjuvant showed a 26% increase in median survival time with 40% of the mice surviving long-term (P=0.007)[139]. Importantly, in mice that failed to respond to PEPvIII-KLH vaccination, immunohistochemical analysis showed that 80% of tumors had lost EGFRvIII expression indicating that antigen escape variants may be a mechanism of treatment failure.
Reproducibly, mice vaccinated with PEPvIII-KLH in the presence of an appropriate adjuvant have produced EGFRvIII-specific IgG1 and IgG2a antibodies. The induction of IgG2a antibodies, however, appears to correlate with an antitumor immune response[140]. Consistent with this finding, sera from successfully vaccinated mice mediates potent antibody-dependant macrophage-mediated cytotoxicity (ADMC), and passive transfer of immune sera protects against tumor challenge[139]. In C57BL/6 (H-2b) mice, antitumor immune responses were shown to be dependent on both NK and CD8+ T-cells. Despite this, EGFRvIII-specific cellular immune responses were not detectable in tumor-naïve vaccinated C57BL/6 mice[139] although they have not been examined in C3H (H-2k)mice where the MHC Class I peptide binding sites may be more favorable[139, 140]. This suggests to us the possibility that a secondary CD8+ T-cell response may be induced which is critical to the antitumor effect, but which is not EGFRvIII-specific. While clinical evident autoimmune responses were not identified in these mice, the generation of secondary immune responses that may not be tumor-specific remains a concern.
6. Clinical Immunotherapy Studies with EGFRvIII
EGFRvIII has also been shown to be immunogenic in humans[149–151]. While we[150, 151] and others[149] have not been able to identify EGFRvIII-specific antibody titers in normal volunteers, patients with EGFRvIII-expressing breast adenocarcinomas[149] and malignant gliomas[150, 151] do appear to develop EGFRvIII-specific antibodies. Similarly, weak CTL epitopes restricted by major histocompatibility complex (MHC) class I and class II motifs have been identified by us and others[141, 149, 150] and are sufficient to induce EGFRvIII-specific lymphocyte proliferation and cytokine production[150]. Specific tetramers, however, have been difficult to generate reproducibly (Personal communication: Amy B. Heimberger, M.D.), and in our hands, MHC class II-restricted responses have generally been more potent. We have now targeted this tumor-specific mutation in one Phase I[150] and one multi-institutional Phase II[152] immunotherapy trial. These trials demonstrated that vaccines targeting EGFRvIII are capable of inducing potent T- and B-cell immunity. Most importantly, the vaccine approach was highly successful at eliminating tumor cells expressing the targeted antigen, as in the pre-clinical murine studies, without any evidence of toxicity[150, 152–155].
The first trial (VICTORI), conducted at Duke University (PI: John H. Sampson), enrolled twenty patients with histopathologically confirmed newly-diagnosed MG (WHO Grade III or IV). Following surgical resection, each patient underwent leukapheresis to obtain peripheral blood mononuclear cells (PBMCs) for autologous mature DC generation and baseline immunologic monitoring. Four of the intial 20 patients did not qualify for vaccine, as a result 3 patients with Grade III gliomas and 13 patients with GBM were vaccinated. DCs were pulsed for two hours with 500 μg of PEPvIII (Multiple Peptide Systems, San Diego CA) which was conjugated 1:1 with KLH (Biosyn, Carlsbad, CA). The first three patients received 3 x 107 mature DCs per vaccination intradermally beginning 2 weeks following completion of radiation therapy, the remaining patients received one third of their total generated DCs per injection (up to 1.1 x 108 cells). Patients were imaged every 2 months with MRI for evidence of toxicity or tumor progression.
Patients in this trial demonstrated immunologic responses[150] without any evidence of adverse events aside from Grade I-II local reactions at the vaccine injection site. There were no serious adverse events. Humoral and cellular immune responses that were specific for KLH, PEPvIII, and EGFRvIII were detected ex vivo after vaccination. Only two patients were allowed on trial with significant enhancement on their MRI at the time of vaccination. Both these patients had nearly-complete resolution of radiographic enhancement after the vaccine[150]. Although the effects of radiation cannot be excluded, one of these patients with an anaplastic astrocytoma remains alive without evidence of progression 5.4 years (65 months) after initial diagnosis and has had two children in the interim. Another patient with GBM had a much larger area of enhancement at the time of vaccination which nearly completely resolved 15 months after vaccination. She also remains alive and well 6.2 years (74.6 months) after initial diagnosis and vaccination. Two of three patients with Grade III tumors remain alive without evidence of tumor progression 66.2 and 123.7 months after vaccination while another developed progressive disease 47.7 months after vaccination. Median time-to-progression (TTP) in patients with GBM (n=13) was 46.9 weeks and median survival was 110.8 weeks. This compares well with other published reports in similar patient populations using carmustine wafers[156] or temozolomide cycles[6] where reported median survival times were 59.6 weeks and 63.3 weeks, respectively.
While the results of this Phase I trial were encouraging and demonstrated that the approach targeting EGFRvIII was promising, further development of this approach required that it be tested again and tested at different centers as early clinical study results are often difficult to replicate outside of parent institution. To accomplish this, it became necessary to simplify the manufacture of the vaccine and eliminate the cost and variability associated with autologous DC manufacture. As a result, we elected to study vaccinations without DCs employing only PEPvIII-KLH and granulocyte macrophage colony stimulating factor (GM-CSF).
The Phase II multicenter trial (ACTIVATE)[157] was conducted at Duke University (PI: John H. Sampson (n=14)) and the University of Texas M.D. Anderson Cancer Center (PI: Amy B. Heimberger (n=8)) and enrolled 22 patients with histopathologically confirmed newly-diagnosed GBM (WHO Grade IV). As in the previous trial, two weeks after completing standard external beam radiation therapy, patients received 3 vaccinations at 2 week intervals of 500 μg of PEPvIII-KLH (AnaSpec, Inc., San Jose, CA 95131) in 0.8 mL of saline with GM-CSF. Patients then underwent another leukapheresis 2 weeks after their third vaccination to provide a source of cells and serum for immunologic monitoring. Unlike in the previous trial, subsequent vaccines were then continued monthly until tumor progression was evident. Serum and PBMCs were collected prior to each vaccine for immunologic monitoring.
Symptomatic adverse events in this trial again consisted mostly of Grade I and II vaccine site reactions. The results of the multicenter Phase II study mostly confirmed the encouraging results seen in the Phase I study, but providing some interesting new findings as well. Humoral and cellular immune responses that were specific for KLH, PEPvIII, and EGFRvIII were detected ex vivo after vaccination in this trial. Radiographic responses were not sought as all patients eligible for the trial had undergone gross total resections. Median TTP in this trial was 14.2 months and not significantly different between sites (P=0.3445). These results compared favorably to an institutional historical cohort matched for eligibility criteria and adjusted for age and KPS (n = 39) where the median TTP was only 7.1 months for patients who did not receive temozolomide (n = 22) and 6.4 months for patients who did receive temozolomide at any point in their treatment (n = 17) (P=0.041)[129]. The median survival time for all 22 study subjects was 32 months which again compared well to the historical even after adjustments for age and KPS (P < 0.0001). Of the 22 patients enrolled on this trial, 5 (22.7%) lived >3 years after vaccination. Further evidence for efficacy, however, was provided by the observation that among the first 6 patients with recurrent tumors where pathologic material was obtained, when immunostaining for EGFRvIII was performed as previously described[158], all recurrent tumors (n=6) were found to no longer expressed the EGFRvIII suggesting a possible mechanism of failure involving immunologic escape. In combination, these results strongly suggest that vaccination with PEPvIII-KLH may prolong survival in at least some patients with newly-diagnosed GBM, but these results will need to be confirmed in a randomized and blinded Phase III trial.
7. Discussion
This review chronicles our development of active and passive immunotherapy strategies targeting the tumor-specific epidermal growth factor mutation, EGFRvIII. We believe that our work to date and the work of others provides evidence that tumor-specific mutations like EGFRvIII can be spontaneously immunogenic, that vaccination induced immune responses targeting conserved tumor-specific mutations like EGFRvIII can be generated in mice and humans, and that such vaccinations are efficacious in mice and may have significant therapeutic benefit in severely immunosuppressed humans with MGs as well. Although the work described above demonstrates the potential benefits of immunotherapy targeting tumor-specific antigens[159], there remain a number of issues which must be addressed to optimize this therapeutic modality.
Although tumor antigen-specific vaccination strategies may have an advantage by minimizing complications due to autoimmunity, the antigenic heterogeneity of MGs[160–163] and other tumors may limit the effectiveness of vaccinations targeting only one antigen. For example, in a study of 21 biopsy samples of human gliomas, only 47% of the tumors tested positive for EGFRvIII[164], and expression patterns even within these tumors are not homogeneous at least by IHC analysis. As such, immunotherapy strategies that target only one antigen may not target all tumors or all cells comprising a tumor, and may therefore select for the survival and proliferation of those cells that do not express the targeted antigen as may have been observed in our studies.
Although most mutations in cancer appear to be sporadic[94] they are not random and many consistent tumor-specific mutations may be identifiable[165]. Therefore, the challenge is to develop strategies that can target unique mutations without knowing their precise sequence or to derive these precise sequences in a rapid fashion with high fidelity[166]. Some hints exist as to where tumor-specific mutations might be found, however. For example, splice variants, not unlike EFGRvIII, may be a rich source to identify tumor-specific antigens as well. As an example, we have recently described a splice variant in glycoprotein nonmetastatic melanoma protein B (GPNMB) that also appears to be transcribed only in malignant tumors[167]. Similarly, mutations that lead to alternative biochemical processing of normal proteins can also produce functionally tumor-specific epitopes. A somatic mutation in the chaperone protein, Cosmc, for example, disrupts O-glycan Core 1 synthesis and produces an aberrant carbohydrate moiety that functions as a tumor-specific epitope that is immunogenic[168]. Changes in protein and lipid glycosylation are frequently seen in malignancies, and are an active area of research on malignant gliomas. Finally, mutations that lead to therapeutic resistance, including immune escape variants, may also be consistent and predictable. For example, in patients with non-small cell lung cancer expressing mutated EGFR that relapse despite an initial response to anilinoquinazoline EGFR inhibitors, a second tumor-specific point mutation has been identified. Structural modeling and biochemical studies have confirmed that this second mutation was responsible for gefitinib resistance[169]. We are currently analyzing our specimens in recurrent patients for the presence of any additional EGFR mutations to address this possibility as an explanation for the antigen escape seen in our patients. If such mutations exist, and they are predictable, which they might be as a result of the need for these cells to maintain EGFR signaling as part of their oncogenic drive, it may be possible to target these secondary mutations prophylactically.
One particular aspect of our studies that we struggled with was the analysis and significance of T-cell responses in these patients. Unlike other targeted antigens, clearly defined MHC epitopes for EGFRvIII have not been validated and tetramers cannot be reliably made[141, 149]. It also occurred to us that an over reliance on immune responses specific to certain MHC haplotypes, even if those haplotypes are relatively common in our population, negate the importance immune responses that may be restricted to the broader haplotype of a given patient and their potential importance in tumor-specific immune responses. We also struggle with understanding the importance of specific T-cell assays and their correlation with clinical results. Although the literature is replete with examples of immune response measurements including delayed-type hypersensitivity testing, ELIspot, CTL assays and cytokine flow cytometry, none of these have consistently and reliably predicted clinical responses[170, 171]. Probably, this derives from an overuse of multiple assays to analyze current clinical trials, a lack of sufficient clinical responses to active immunotherapy, and the lack of validated systems for analyzing polyfunctional responses. We believe that it is unlikely that a meaningful antigen-specific immune response can be detected when analysis is not correlated with a T-cell phenotype, and it is restricted to single functional cytokines such as γ-interferon. As a result, we are currently undertaking a thorough analysis of the trial described above, which does appear to provide tangible evidence for clinical efficacy, using a broadly applicable multicolor flow cytometry based analysis that provides polyfunctional results within T-cell phenotypic subsets to identify and validate correlations with clinical outcomes[172–174]. This analysis is based on prior work in the HIV field which can segregate patients with HIV who progress to full-blown AIDS from those who appear to suppress this progression based on T-cell responses[172–174].
It also became apparent to us on reviewing the active immunotherapy literature, that B-cell responses have been comparatively ignored. Although substantial evidence exists in the literature for the importance of T-cell responses in the eradication of cancer in animal models[175–178] and the eradication of intercellular pathogens in human infectious disease, only recently has data in humans clearly demonstrated the importance of T-cells as an anticancer strategy[68, 179, 180]. This work, however, has been predated by other strategies now that have conclusively shown efficacy in Phase III trials simply using antibody-based attacks on tumor antigens. The best example of a passive antibody approach that has provided efficacy data in Phase III cancer trials is that of trastuzumab (Herceptin™)[181, 182]. It is apparent from our work and the work of others[149] that substantial antibody responses can pre-exist in patients with EGFRvIII-expressing tumors and that these responses can be substantially enhanced by vaccination. Serum from vaccinated patients in our hands can induce potent anti-tumor ADMC responses as well. It was also evident in our animal studies that passive transfer of serum from vaccinated animals could induce anti-tumor effect. Monoclonal antibodies specific to EGFRvIII also have an anti-tumor effect in vitro and in vivo[131, 136–138]. Although passive transfer of antibodies is effective only against extra-cranial tumors in some studies[131], it remains possible that vaccination strategies that possibly induced longer-lasting antibody responses may have efficacy in the central nervous system despite an intact blood brain barrier. The best evidence to support this comes from the active vaccine trials targeting β-amyloid plaques in patients with Alzheimer’s disease. While it remains under debate whether the antibodies induced by these vaccines are responsible for any clinical responses and whether or not the antibodies entered the central nervous system to do so[183], much of the data does support this conclusion and provides support for using antibody-based approaches derived systemically against intracranial tumors[184–187].
Acknowledgments
We wish to express our sincere appreciation to our patients and families and for the assistance of Robert Schmittling, Charles Pegram, Laura Crotty, Hedi Ochiai, Shenell Summers, Denise Lally-Goss, Sharon McGehee-Norman, and Beth A. Perry, Kara Penne, Alison Paolino, Frank Tuck, and Peter Fecci.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.United States Cancer Statistics: National Program of Cancer Registries. 2003 Available from: http://apps.nccd.cdc.gov/uscs/
- 2.CBTRUS. Statistical Report: Primary Brain Tumors in the United States, 1997–2001. Central Brain Tumor Registry of the United States, 2004; 2004. [Google Scholar]
- 3.Kelly PJ. Stereotactic resection and its limitations in glial neoplasms. Stereotact Funct Neurosurg. 1992;59:84–91. doi: 10.1159/000098922. [DOI] [PubMed] [Google Scholar]
- 4.Walker MD, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303:1323–9. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]
- 5.Reardon DA, et al. Salvage radioimmunotherapy with murine iodine-131-labeled antitenascin monoclonal antibody 81C6 for patients with recurrent primary and metastatic malignant brain tumors: phase II study results. J Clin Oncol. 2006;24:115–22. doi: 10.1200/JCO.2005.03.4082. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R, et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 7.Vredenburgh JJ, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–9. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 8.Imperato JP, Paleologos NA, Vick NA. Effects of treatment on long-term survivors with malignant astrocytomas. Ann Neurol. 1990;28:818–22. doi: 10.1002/ana.410280614. [DOI] [PubMed] [Google Scholar]
- 9.Brooks WH, et al. Depressed cell-mediated immunity in patients with primary intracranial tumors. Characterization of a humoral immunosuppressive factor. J Exp Med. 1972;136:1631–47. doi: 10.1084/jem.136.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roszman TL, Brooks WH, Elliott LH. Inhibition of lymphocyte responsiveness by a glial tumor cell- derived suppressive factor. J Neurosurg. 1987;67:874–9. doi: 10.3171/jns.1987.67.6.0874. [DOI] [PubMed] [Google Scholar]
- 11.Fontana A, et al. Glioblastoma cells release interleukin 1 and factors inhibiting interleukin 2-mediated effects. J Immunol. 1984;132:1837–44. [PubMed] [Google Scholar]
- 12.Miescher S, et al. In situ characterization, clonogenic potential, and antitumor cytolytic activity of T lymphocytes infiltrating human brain cancers. J Neurosurg. 1988;68:438–48. doi: 10.3171/jns.1988.68.3.0438. [DOI] [PubMed] [Google Scholar]
- 13.Brooks WH, Caldwell HD, Mortara RH. Immune responses in patients with gliomas. Surg Neurol. 1974;2:419–23. [PubMed] [Google Scholar]
- 14.Mahaley MS, Jr, et al. Immunobiology of primary intracranial tumors. Part 1: studies of the cellular and humoral general immune competence of brain-tumor patients. J Neurosurg. 1977;46:467–76. doi: 10.3171/jns.1977.46.4.0467. [DOI] [PubMed] [Google Scholar]
- 15.Menzies CB, et al. Impaired thymus-derived lymphocyte function in patients with malignant brain tumour. Clin Neurol Neurosurg. 1980;82:157–68. doi: 10.1016/0303-8467(80)90033-5. [DOI] [PubMed] [Google Scholar]
- 16.Brooks WH, Roszman TL, Rogers AS. Impairment of rosette-forming T lymphoctyes in patients with primary intracranial tumors. Cancer. 1976;37:1869–73. doi: 10.1002/1097-0142(197604)37:4<1869::aid-cncr2820370435>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 17.Brooks WH, et al. Immunobiology of primary intracranial tumours. II Analysis of lymphocyte subpopulations in patients with primary brain tumours. Clin Exp Immunol. 1977;29:61–6. [PMC free article] [PubMed] [Google Scholar]
- 18.Braun DP, et al. Immunoregulatory cell function in peripheral blood leukocytes of patients with intracranial gliomas. Neurosurgery. 1982;10:203–9. [PubMed] [Google Scholar]
- 19.Elliott L, Brooks W, Roszman T. Role of interleukin-2 (IL-2) and IL-2 receptor expression in the proliferative defect observed in mitogen-stimulated lymphocytes from patients with gliomas. J Natl Cancer Inst. 1987;78:919–22. [PubMed] [Google Scholar]
- 20.Elliott LH, Brooks WH, Roszman TL. Inability of mitogen-activated lymphocytes obtained from patients with malignant primary intracranial tumors to express high affinity interleukin 2 receptors. J Clin Invest. 1990;86:80–6. doi: 10.1172/JCI114719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miescher S, et al. Functional properties of tumor-infiltrating and blood lymphocytes in patients with solid tumors: effects of tumor cells and their supernatants on proliferative responses of lymphocytes. J Immunol. 1986;136:1899–907. [PubMed] [Google Scholar]
- 22.Roszman TL, Brooks WH, Elliott LH. Immunobiology of primary intracranial tumors. VI. Suppressor cell function and lectin-binding lymphocyte subpopulations in patients with cerebral tumors. Cancer. 1982;50:1273–9. doi: 10.1002/1097-0142(19821001)50:7<1273::aid-cncr2820500709>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Roszman TL, Elliott LH, Brooks WH. Proliferative potential of T-cell lymphocytes from gliomas. J Neurosurg. 1992;77:820–1. doi: 10.3171/jns.1992.77.5.0820. [DOI] [PubMed] [Google Scholar]
- 24.Roszman TL. and W.H. Brooks, Immunobiology of primary intracranial tumours. III. Demonstration of a qualitative lymphocyte abnormality in patients with primary brain tumours. Clin Exp Immunol. 1980;39:395–402. [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas DG, Lannigan CB, Behan PO. Letter: Impaired cell-mediated immunity in human brain tumours. Lancet. 1975;1:1389–90. doi: 10.1016/s0140-6736(75)92308-9. [DOI] [PubMed] [Google Scholar]
- 26.Young HF, Kaplan AM. Cellular immune deficiency in patients with glioblastoma. Surg Forum. 1976;27:476–8. [PubMed] [Google Scholar]
- 27.Mahaley MS, Jr, Gentry RE, Bigner DD. Immunobiology of primary intracranial tumors. J Neurosurg. 1977;47:35–43. doi: 10.3171/jns.1977.47.1.0035. [DOI] [PubMed] [Google Scholar]
- 28.Elliott LH, Brooks WH, Roszman TL. Activation of immunoregulatory lymphocytes obtained from patients with malignant gliomas. J Neurosurg. 1987;67:231–6. doi: 10.3171/jns.1987.67.2.0231. [DOI] [PubMed] [Google Scholar]
- 29.Young HF, Sakalas R, Kaplan AM. Inhibition of cell-mediated immunity in patients with brain tumors. Surg Neurol. 1976;5:19–23. [PubMed] [Google Scholar]
- 30.Young HF, Sakalas R, Kaplan AM. Immunologic depression in cerebral gliomas. Adv Neurol. 1976;15:327–35. [PubMed] [Google Scholar]
- 31.Brooks WH, Horwitz DA, Netsky MG. Evidence for tumor-specific immune response in patients with primary brain tumors. Surg Forum. 1972;23:430–2. [PubMed] [Google Scholar]
- 32.Bodmer S, et al. Immunosuppression and transforming growth factor-beta in glioblastoma. Preferential production of transforming growth factor-beta 2. J Immunol. 1989;143:3222–9. [PubMed] [Google Scholar]
- 33.de Martin R, et al. Complementary DNA for human glioblastoma-derived T cell suppressor factor, a novel member of the transforming growth factor-beta gene family. EMBO J. 1987;6:3673–7. doi: 10.1002/j.1460-2075.1987.tb02700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wrann M, et al. T cell suppressor factor from human glioblastoma cells is a 12.5- kd protein closely related to transforming growth factor-beta. EMBO J. 1987;6:1633–6. doi: 10.1002/j.1460-2075.1987.tb02411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czarniecki CW, et al. Transforming growth factor-beta 1 modulates the expression of class II histocompatibility antigens on human cells. J Immunol. 1988;140:4217–23. [PubMed] [Google Scholar]
- 36.Zuber P, Kuppner MC, de Tribolet N. Transforming growth factor-beta 2 down-regulates HLA-DR antigen expression on human malignant glioma cells. Eur J Immunol. 1988;18:1623–6. doi: 10.1002/eji.1830181023. [DOI] [PubMed] [Google Scholar]
- 37.Kehrl JH, et al. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163:1037–50. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kehrl JH, et al. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986;137:3855–60. [PubMed] [Google Scholar]
- 39.Wahl SM, et al. Transforming growth factor-beta is a potent immunosuppressive agent that inhibits IL-1-dependent lymphocyte proliferation. J Immunol. 1988;140:3026–32. [PubMed] [Google Scholar]
- 40.Schwyzer M, Fontana A. Partial purification and biochemical characterization of a T cell suppressor factor produced by human glioblastoma cells. J Immunol. 1985;134:1003–9. [PubMed] [Google Scholar]
- 41.Rook AH, et al. Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J Immunol. 1986;136:3916–20. [PubMed] [Google Scholar]
- 42.Ranges GE, et al. Inhibition of cytotoxic T cell development by transforming growth factor beta and reversal by recombinant tumor necrosis factor alpha. J Exp Med. 1987;166:991–8. doi: 10.1084/jem.166.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Espevik T, et al. Inhibition of cytokine production by cyclosporin A and transforming growth factor beta. J Exp Med. 1987;166:571–6. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fecci PE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 45.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jonuleit H, et al. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dieckmann D, et al. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–10. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 49.Khattri R, et al. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 50.Green DR, Webb DR. Saying the 'S' word in public. Immunol Today. 1993;14:523–5. doi: 10.1016/0167-5699(93)90180-S. [DOI] [PubMed] [Google Scholar]
- 51.Sakaguchi S, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 52.Asano M, et al. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–96. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salomon B, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 54.Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25- subpopulations. J Immunol. 2000;165:3105–10. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- 55.Taguchi O, Nishizuka Y. Self tolerance and localized autoimmunity. Mouse models of autoimmune disease that suggest tissue-specific suppressor T cells are involved in self tolerance. J Exp Med. 1987;165:146–56. doi: 10.1084/jem.165.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taguchi O, et al. Tissue-specific suppressor T cells involved in self-tolerance are activated extrathymically by self-antigens. Immunology. 1994;82:365–9. [PMC free article] [PubMed] [Google Scholar]
- 57.Seddon B, Mason D. Regulatory T cells in the control of autoimmunity: the essential role of transforming growth factor beta and interleukin 4 in the prevention of autoimmune thyroiditis in rats by peripheral CD4(+)CD45RC- cells and CD4(+)CD8(−) thymocytes. J Exp Med. 1999;189:279–88. doi: 10.1084/jem.189.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bagavant H, et al. Differential effect of neonatal thymectomy on systemic and organ-specific autoimmune disease. Int Immunol. 2002;14:1397–406. doi: 10.1093/intimm/dxf105. [DOI] [PubMed] [Google Scholar]
- 59.Somasundaram R, et al. Inhibition of cytolytic T lymphocyte proliferation by autologous CD4+/CD25+ regulatory T cells in a colorectal carcinoma patient is mediated by transforming growth factor-beta. Cancer Res. 2002;62:5267–72. [PubMed] [Google Scholar]
- 60.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 61.Fecci PE, et al. Systemic Anti-CD25 Monoclonal Antibody Administration Safely Enhances Immunity in Murine Glioma without Eliminating Regulatory T Cells. Clin Cancer Res. 2006;12:4294–305. doi: 10.1158/1078-0432.CCR-06-0053. [DOI] [PubMed] [Google Scholar]
- 62.Fecci PE, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T-cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007;13:2158–67. doi: 10.1158/1078-0432.CCR-06-2070. [DOI] [PubMed] [Google Scholar]
- 63.Phan GQ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blansfield JA, et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005;28:593–8. doi: 10.1097/01.cji.0000178913.41256.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jaber SH, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol. 2006;142:166–72. doi: 10.1001/archderm.142.2.166. [DOI] [PubMed] [Google Scholar]
- 66.Attia P, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–53. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ochsenbein AF, et al. Immune surveillance against a solid tumor fails because of immunological ignorance. Proc Natl Acad Sci U S A. 1999;96:2233–8. doi: 10.1073/pnas.96.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dudley ME, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ludewig B, et al. Immunotherapy with dendritic cells directed against tumor antigens shared with normal host cells results in severe autoimmune disease. J Exp Med. 2000;191:795–804. doi: 10.1084/jem.191.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wahlstrom T, Linder E, Saksela E. Glia-specific antigens in cell cultures from rabbit brain, human foetal and adult brain, and gliomas. Acta Pathol Microbiol Scand [B] Microbiol Immunol. 1973;81:768–74. doi: 10.1111/j.1699-0463.1973.tb02274.x. [DOI] [PubMed] [Google Scholar]
- 71.Wikstrand CJ, Mahaley MS, Bigner DD. Surface antigenic characteristics of human glial brain tumor cells. Cancer Res. 1977;37:4267–75. [PubMed] [Google Scholar]
- 72.Slagel DE, Wilson CB, Simmons PB. Polyacrylamide electrophoresis and immunodiffusion studies of brain tumor proteins. Ann N Y Acad Sci. 1969;159:490–6. [Google Scholar]
- 73.Siris JH. Concerning the immunological specificity of glioblastoma multiforme. Bull Neurol NY. 1936;4:597–601. [Google Scholar]
- 74.Wickremesinghe HR, Yates PO. Immunological properties of neoplastic neural tissues. Br J Cancer. 1971;25:711–20. doi: 10.1038/bjc.1971.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Waksman BH, et al. A study of the chemical nature of components of bovine white matter effective in producing allergic encephalomyelitis in the rabbit. J Exp Med. 1954;100:451–71. doi: 10.1084/jem.100.5.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tuohy VK, et al. A synthetic peptide from myelin proteolipid protein induces experimental allergic encephalomyelitis. J Immunol. 1988;141:1126–30. [PubMed] [Google Scholar]
- 77.Linington C, et al. T cells specific for the myelin oligodendrocyte glycoprotein mediate an unusual autoimmune inflammatory response in the central nervous system. Eur J Immunol. 1993;23:1364–72. doi: 10.1002/eji.1830230627. [DOI] [PubMed] [Google Scholar]
- 78.Wekerle H, et al. Animal models. Ann Neurol. 1994;36 Suppl:S47–53. doi: 10.1002/ana.410360714. [DOI] [PubMed] [Google Scholar]
- 79.Bigner DD, Pitts OM, Wikstrand CJ. Induction of lethal experimental allergic encephalomyelitis in nonhuman primates and guinea pigs with human glioblastoma multiforme tissue. J Neurosurg. 1981;55:32–42. doi: 10.3171/jns.1981.55.1.0032. [DOI] [PubMed] [Google Scholar]
- 80.Stuart G, Krikorian K. A fatal neuro-paralytic accident of anti-rabies treatment. Lancet. 1930;1:1123–5. [Google Scholar]
- 81.Stuart G, Krikorian K. The neuro-paralytic accidents of anti-rabies treatment. Ann Trop Med. 1928;22:327–77. [Google Scholar]
- 82.Remlinger P. Accidents paralytiques au cours du traitement antirabique. Ann Inst Pasteur (Paris) 1905;19:625–46. [PubMed] [Google Scholar]
- 83.Remlinger P. Contribution a l'etude de la toxine rabique (faits experimentaux et clinique) C R Soc Biol. 1904;56:348–50. [Google Scholar]
- 84.Pasteur L. Methode pour prevenir la rage apres morsure. C R Acad Sci. 1885;101:765–74. [Google Scholar]
- 85.Ommaya AK. Immunotherapy of gliomas: a review. Adv Neurol. 1976;15:337–59. [PubMed] [Google Scholar]
- 86.Mahaley MS, Jr, et al. J Neurosurg. 1983;59:201–7. doi: 10.3171/jns.1983.59.2.0201. [DOI] [PubMed] [Google Scholar]
- 87.Albright L, Seab JA, Ommaya AK. Intracerebral delayed hypersensitivity reactions in glioblastoma multiforme patients. Cancer. 1977;39:1331–6. doi: 10.1002/1097-0142(197703)39:3<1331::aid-cncr2820390348>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 88.Bullard DE, et al. A preliminary study utilizing viable HLA mismatched cultured glioma cells as adjuvant therapy for patients with malignant gliomas. Br J Cancer. 1985;51:283–9. doi: 10.1038/bjc.1985.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wikstrand CJ, Bigner DD. Hyperimmunization of non-human primates with BCG-CW and cultured human glioma-derived cells. Production of reactive antisera and absence of EAE induction. J Neuroimmunol. 1981;1:249–60. doi: 10.1016/0165-5728(81)90029-1. [DOI] [PubMed] [Google Scholar]
- 90.Bloom HJ, et al. Glioblastoma multiforme: a controlled trial to assess the value of specific active immunotherapy in patients treated by radical surgery and radiotherapy. Br J Cancer. 1973;27:253–67. doi: 10.1038/bjc.1973.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trouillas P. Immunology and immunotherapy of cerebral tumors. Current status. Rev Neurol (Paris) 1973;128:23–38. [PubMed] [Google Scholar]
- 92.Gilboa E. The promise of cancer vaccines. Nat Rev Cancer. 2004;4:401–11. doi: 10.1038/nrc1359. [DOI] [PubMed] [Google Scholar]
- 93.Gilboa E. The makings of a tumor rejection antigen. Immunity. 1999;11:263–70. doi: 10.1016/s1074-7613(00)80101-6. [DOI] [PubMed] [Google Scholar]
- 94.Lennerz V, et al. The response of autologous T cells to a human melanoma is dominated by mutated neoantigens. Proc Natl Acad Sci U S A. 2005;102:16013–8. doi: 10.1073/pnas.0500090102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–9. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 96.Loeb LA. A mutator phenotype in cancer. Cancer Res. 2001;61:3230–9. [PubMed] [Google Scholar]
- 97.Libermann TA, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313:144–7. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- 98.Bigner SH, et al. Characterization of the epidermal growth factor receptor in human glioma cell lines and xenografts. Cancer Res. 1990;50:8017–22. [PubMed] [Google Scholar]
- 99.Chu CT, et al. Receptor dimerization is not a factor in the signalling activity of a transforming variant epidermal growth factor receptor (EGFRvIII) Biochem J. 1997;324:855–61. doi: 10.1042/bj3240855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Batra SK, et al. Epidermal growth factor ligand-independent, unregulated, cell-transforming potential of a naturally occurring human mutant EGFRvIII gene. Cell Growth Differ. 1995;6:1251–9. [PubMed] [Google Scholar]
- 101.Nishikawa R, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci U S A. 1994;91:7727–31. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang HS, et al. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem. 1997;272:2927–35. doi: 10.1074/jbc.272.5.2927. [DOI] [PubMed] [Google Scholar]
- 103.Moscatello DK, et al. Transformation and altered signal transduction by a naturally occurring mutant EGF receptor. Oncogene. 1996;13:85–96. [PubMed] [Google Scholar]
- 104.Pedersen MW, et al. Expression of a naturally occurring constitutively active variant of the epidermal growth factor receptor in mouse fibroblasts increases motility. Int J Cancer. 2004;108:643–53. doi: 10.1002/ijc.11566. [DOI] [PubMed] [Google Scholar]
- 105.Boockvar JA, et al. Constitutive EGFR signaling confers a motile phenotype to neural stem cells. Mol Cell Neurosci. 2003;24:1116–30. doi: 10.1016/j.mcn.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 106.Lammering G, et al. Inhibition of the type III epidermal growth factor receptor variant mutant receptor by dominant-negative EGFR-CD533 enhances malignant glioma cell radiosensitivity. Clin Cancer Res. 2004;10:6732–43. doi: 10.1158/1078-0432.CCR-04-0393. [DOI] [PubMed] [Google Scholar]
- 107.Nagane M, et al. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56:5079–86. [PubMed] [Google Scholar]
- 108.Montgomery RB, et al. Expression of oncogenic epidermal growth factor receptor family kinases induces paclitaxel resistance and alters beta-tubulin isotype expression. J Biol Chem. 2000;275:17358–63. doi: 10.1074/jbc.M000966200. [DOI] [PubMed] [Google Scholar]
- 109.Lammering G, et al. Radiation-induced activation of a common variant of EGFR confers enhanced radioresistance. Radiother Oncol. 2004;72:267–73. doi: 10.1016/j.radonc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 110.Li B, et al. Mutant epidermal growth factor receptor displays increased signaling through the phosphatidylinositol-3 kinase/AKT pathway and promotes radioresistance in cells of astrocytic origin. Oncogene. 2004;23:4594–602. doi: 10.1038/sj.onc.1207602. [DOI] [PubMed] [Google Scholar]
- 111.Lammering G, et al. EGFRvIII-mediated radioresistance through a strong cytoprotective response. Oncogene. 2003;22:5545–53. doi: 10.1038/sj.onc.1206788. [DOI] [PubMed] [Google Scholar]
- 112.Sok JC, et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res. 2006;12:5064–73. doi: 10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 113.Weinstein IB. Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science. 2002;297:63–4. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 114.Mellinghoff IK, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–24. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 115.Wikstrand CJ, et al. Monoclonal antibodies against EGFRvIII are tumor specific and react with breast and lung carcinomas and malignant gliomas. Cancer Res. 1995;55:3140–8. [PubMed] [Google Scholar]
- 116.Frederick L, et al. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60:1383–7. [PubMed] [Google Scholar]
- 117.Wong AJ, et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci U S A. 1992;89:2965–9. doi: 10.1073/pnas.89.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sugawa N, et al. Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proc Natl Acad Sci U S A. 1990;87:8602–6. doi: 10.1073/pnas.87.21.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ekstrand AJ, et al. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991;51:2164–72. [PubMed] [Google Scholar]
- 120.Aldape KD, et al. Immunohistochemical detection of EGFRvIII in high malignancy grade astrocytomas and evaluation of prognostic significance. J Neuropathol Exp Neurol. 2004;63:700–7. doi: 10.1093/jnen/63.7.700. [DOI] [PubMed] [Google Scholar]
- 121.Heimberger AB, et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;11:1462–6. doi: 10.1158/1078-0432.CCR-04-1737. [DOI] [PubMed] [Google Scholar]
- 122.Cunningham MP, et al. Coexpression, prognostic significance and predictive value of EGFR, EGFRvIII and phosphorylated EGFR in colorectal cancer. Int J Oncol. 2005;27:317–25. [PubMed] [Google Scholar]
- 123.Garcia de Palazzo IE, et al. Expression of mutated epidermal growth factor receptor by non- small cell lung carcinomas. Cancer Res. 1993;53:3217–20. [PubMed] [Google Scholar]
- 124.Moscatello DK, et al. Frequent expression of a mutant epidermal growth factor receptor in multiple human tumors. Cancer Res. 1995;55:5536–9. [PubMed] [Google Scholar]
- 125.Okamoto I, et al. Expression of constitutively activated EGFRvIII in non-small cell lung cancer. Cancer Sci. 2003;94:50–6. doi: 10.1111/j.1349-7006.2003.tb01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Luo X, Gong X, Tang CK. Suppression of EGFRvIII-mediated proliferation and tumorigenesis of breast cancer cells by ribozyme. Int J Cancer. 2003;104:716–21. doi: 10.1002/ijc.11007. [DOI] [PubMed] [Google Scholar]
- 127.Ge H, Gong X, Tang CK. Evidence of high incidence of EGFRvIII expression and coexpression with EGFR in human invasive breast cancer by laser capture microdissection and immunohistochemical analysis. Int J Cancer. 2002;98:357–61. doi: 10.1002/ijc.10224. [DOI] [PubMed] [Google Scholar]
- 128.Olapade-Olaopa EO, et al. Evidence for the differential expression of a variant EGF receptor protein in human prostate cancer. Br J Cancer. 2000;82:186–94. doi: 10.1054/bjoc.1999.0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pelloski CE, et al. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J Clin Oncol. 2007;25:2288–94. doi: 10.1200/JCO.2006.08.0705. [DOI] [PubMed] [Google Scholar]
- 130.Humphrey PA, et al. Anti-synthetic peptide antibody reacting at the fusion junction of deletion-mutant epidermal growth factor receptors in human glioblastoma. Proc Natl Acad Sci U S A. 1990;87:4207–11. doi: 10.1073/pnas.87.11.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sampson JH, et al. Unarmed, tumor-specific, monoclonal antibody effectively treats brain tumors. Proc Natl Acad Sci U S A. 2000;97:7503–8. doi: 10.1073/pnas.130166597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Reist CJ, et al. Tumor-specific anti-epidermal growth factor receptor variant III monoclonal antibodies: use of the tyramine-cellobiose radioiodination method enhances cellular retention and uptake in tumor xenografts. Cancer Res. 1995;55:4375–82. [PubMed] [Google Scholar]
- 133.Wikstrand CJ, et al. Investigation of a synthetic peptide as immunogen for a variant epidermal growth factor receptor associated with gliomas. J Neuroimmunol. 1993;46:165–73. doi: 10.1016/0165-5728(93)90246-u. [DOI] [PubMed] [Google Scholar]
- 134.Perera RM, et al. Treatment of human tumor xenografts with monoclonal antibody 806 in combination with a prototypical epidermal growth factor receptor-specific antibody generates enhanced antitumor activity. Clin Cancer Res. 2005;11:6390–9. doi: 10.1158/1078-0432.CCR-04-2653. [DOI] [PubMed] [Google Scholar]
- 135.Jungbluth AA, et al. A monoclonal antibody recognizing human cancers with amplification/overexpression of the human epidermal growth factor receptor. Proc Natl Acad Sci U S A. 2003;100:639–44. doi: 10.1073/pnas.232686499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Johns TG, et al. Novel monoclonal antibody specific for the de2–7 epidermal growth factor receptor (EGFR) that also recognizes the EGFR expressed in cells containing amplification of the EGFR gene. Int J Cancer. 2002;98:398–408. doi: 10.1002/ijc.10189. [DOI] [PubMed] [Google Scholar]
- 137.Luwor RB, et al. Monoclonal antibody 806 inhibits the growth of tumor xenografts expressing either the de2-7 or amplified epidermal growth factor receptor (EGFR) but not wild-type EGFR. Cancer Res. 2001;61:5355–61. [PubMed] [Google Scholar]
- 138.Mishima K, et al. Growth suppression of intracranial xenografted glioblastomas overexpressing mutant epidermal growth factor receptors by systemic administration of monoclonal antibody (mAb) 806, a novel monoclonal antibody directed to the receptor. [erratum appears in Cancer Res 2001 Oct 15;61(20):7703–5] Cancer Res. 2001;61:5349–54. [PubMed] [Google Scholar]
- 139.Heimberger AB, et al. Epidermal growth factor receptor vIII peptide vaccination is efficacious against established intracerebral tumors. Clin Cancer Res. 2003;9:4247–54. [PubMed] [Google Scholar]
- 140.Heimberger AB, et al. Dendritic cells pulsed with a tumor-specific peptide induce long-lasting immunity and are effective against murine intracerebral melanoma. Neurosurgery. 2002;50:158–66. doi: 10.1097/00006123-200201000-00024. [DOI] [PubMed] [Google Scholar]
- 141.Wu AH, et al. Identification of EGFRvIII-derived CTL epitopes restricted by HLA A0201 for dendritic cell based immunotherapy of gliomas. J Neurooncol. 2006;76:23–30. doi: 10.1007/s11060-005-3280-7. [DOI] [PubMed] [Google Scholar]
- 142.Lorimer IA, et al. Immunotoxins that target an oncogenic mutant epidermal growth factor receptor expressed in human tumors. Clin Cancer Res. 1995;1:859–64. [PubMed] [Google Scholar]
- 143.Archer GE, et al. Regional treatment of epidermal growth factor receptor vIII-expressing neoplastic meningitis with a single-chain immunotoxin, MR-1. Clin Cancer Res. 1999;5:2646–52. [PubMed] [Google Scholar]
- 144.Kuan CT, et al. Increased binding affinity enhances targeting of glioma xenografts by EGFRvIII-specific scFv. Int J Cancer. 2000;88:962–9. doi: 10.1002/1097-0215(20001215)88:6<962::aid-ijc20>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 145.Ochiai H, et al. EGFRvIII-targeted immunotoxin induces antitumor immunity that is inhibited in the absence of CD4+ and Cd8+ T cells. Cancer Immun Immunother. 2007 doi: 10.1007/s00262-007-0363-7. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Reist CJ, et al. In vitro and in vivo behavior of radiolabeled chimeric anti- EGFRvIII monoclonal antibody: comparison with its murine parent. Nucl Med Biol. 1997;24:639–47. doi: 10.1016/s0969-8051(97)00080-2. [DOI] [PubMed] [Google Scholar]
- 147.Reist CJ, et al. Improved targeting of an anti-epidermal growth factor receptor variant III monoclonal antibody in tumor xenografts after labeling using N-succinimidyl 5-iodo-3-pyridinecarboxylate. Cancer Res. 1997;57:1510–5. [PubMed] [Google Scholar]
- 148.Reist CJ, et al. Astatine-211 labeling of internalizing anti-EGFRvIII monoclonal antibody using N-succinimidyl 5-[211At]astato-3-pyridinecarboxylate. Nucl Med Biol. 1999;26:405–11. doi: 10.1016/s0969-8051(98)00120-6. [DOI] [PubMed] [Google Scholar]
- 149.Purev E, et al. Immune responses of breast cancer patients to mutated epidermal growth factor receptor (EGF-RvIII, Delta EGF-R, and de2-7 EGF-R) J Immunol. 2004;173:6472–80. doi: 10.4049/jimmunol.173.10.6472. [DOI] [PubMed] [Google Scholar]
- 150.Sampson JH, et al. EGFRvIII-specific peptide-pulsed DCs are safe and induce immunologic, histologic, and radiographic responses in patients with malignant gliomas. 2007 In preparation. [Google Scholar]
- 151.Schmittling R, et al. Detection of EGRFvIII-specific Antibody Responses in Patients with Malignant Glioma. 2007 In preparation. [Google Scholar]
- 152.Vaccination-Group E. Phase II Trial of Epidermal Growth Factor Receptor Variant III Peptide Vaccination in Patients with Newly-diagnosed Glioblastoma Multiforme. 2007 Submitted. [Google Scholar]
- 153.Heimberger AB, et al. An Epidermal Growth Factor Receptor Variant III Peptide Vaccination Appears Promising in Newly Diagnosed GBM Patients; Preliminary Results of a Randomized Phase II Clinical Trial in American Association of Neurological Surgeons; San Francisco, CA. 2006. [Google Scholar]
- 154.Heimberger AB, et al. Tumor-specific peptide vaccination in newly-diagnosed patients with GBM. American Society of Clinical Oncology Annual Meeting; Atlanta, GA. 2006. [Google Scholar]
- 155.Heimberger A, et al. Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: Case study. Neuro-oncology. 2007 doi: 10.1215/15228517-2007-046. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Westphal M, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-oncology. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Heimberger AB, et al. Tumor-specific peptide vaccination in newly-diagnosed patients with GBM. Journal of Clinical Oncology. 2006:24. [Google Scholar]
- 158.Simmons ML, et al. Analysis of complex relationships between age, p53, epidermal growth factor receptor, and survival in glioblastoma patients. Cancer Res. 2001;61:1122–8. [PubMed] [Google Scholar]
- 159.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bigner DD. Biology of gliomas: potential clinical implications of glioma cellular heterogeneity. Neurosurgery. 1981;9:320–6. [PubMed] [Google Scholar]
- 161.Bigner DD, et al. How heterogeneous are gliomas? Cancer Treat Rep. 1981;65 Suppl 2:45–9. [PubMed] [Google Scholar]
- 162.Wikstrand CJ, Bigner SH, Bigner DD. Demonstration of complex antigenic heterogeneity in a human glioma cell line and eight derived clones by specific monoclonal antibodies. Cancer Res. 1983;43:3327–34. [PubMed] [Google Scholar]
- 163.Wikstrand CJ, et al. Antigenic heterogeneity of human anaplastic gliomas and glioma- derived cell lines defined by monoclonal antibodies. J Neuropathol Exp Neurol. 1985;44:229–41. doi: 10.1097/00005072-198505000-00002. [DOI] [PubMed] [Google Scholar]
- 164.Wikstrand CJ, et al. Cell surface localization and density of the tumor-associated variant of the epidermal growth factor receptor, EGFRvIII. Cancer Res. 1997;57:4130–40. [PubMed] [Google Scholar]
- 165.Thomas RK, et al. High-throughput oncogene mutation profiling in human cancer. Nat Genet. 2007;39:347–51. doi: 10.1038/ng1975. [DOI] [PubMed] [Google Scholar]
- 166.Hoshi K, et al. Rapid detection of epidermal growth factor receptor mutations in lung cancer by the SMart-Amplification Process. Clin Cancer Res. 2007;13:4974–83. doi: 10.1158/1078-0432.CCR-07-0509. [DOI] [PubMed] [Google Scholar]
- 167.Kuan CT, et al. Glycoprotein nonmetastatic melanoma protein B, a potential molecular therapeutic target in patients with glioblastoma multiforme. Clin Cancer Res. 2006;12:1970–82. doi: 10.1158/1078-0432.CCR-05-2797. [DOI] [PubMed] [Google Scholar]
- 168.Schietinger A, et al. A mutant chaperone converts a wild-type protein into a tumor-specific antigen. Science. 2006;314:304–8. doi: 10.1126/science.1129200. [DOI] [PubMed] [Google Scholar]
- 169.Kobayashi S, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 170.de Vries IJM, et al. Immunomonitoring tumor-specific T cells in delayed-type hypersensitivity skin biopsies after dendritic cell vaccination correlates with clinical outcome. J Clin Oncol. 2005;23:5779–87. doi: 10.1200/JCO.2005.06.478. [DOI] [PubMed] [Google Scholar]
- 171.Keilholz U, Martus P, Scheibenbogen C. Immune Monitoring of T-Cell Responses in Cancer Vaccine Development. Clin Cancer Res. 2006;12:2346s–52s. doi: 10.1158/1078-0432.CCR-05-2540. [DOI] [PubMed] [Google Scholar]
- 172.Betts MR, et al. Characterization of functional and phenotypic changes in anti-Gag vaccine-induced T cell responses and their role in protection after HIV-1 infection. Proc Natl Acad Sci U S A. 2005;102:4512–7. doi: 10.1073/pnas.0408773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Betts MR, et al. Antigen-specific T-cell-mediated immunity after HIV-1 infection: implications for vaccine control of HIV development. Expert Rev Vaccines. 2006;5:505–16. doi: 10.1586/14760584.5.4.505. [DOI] [PubMed] [Google Scholar]
- 174.Betts MR, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med. 2004;10:294–8. doi: 10.1038/nm999. [DOI] [PubMed] [Google Scholar]
- 176.Yu P, et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. 2004;5:141–9. doi: 10.1038/ni1029. [DOI] [PubMed] [Google Scholar]
- 177.Spiotto MT, Schreiber H. Rapid destruction of the tumor microenvironment by CTLs recognizing cancer-specific antigens cross-presented by stromal cells. Cancer Immun. 2005;5:8. [PubMed] [Google Scholar]
- 178.Zhang B, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dudley ME, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005 Apr 1;23:2346–57. doi: 10.1200/JCO.2005.00.240. 005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Morgan RA, et al. Cancer Regression in Patients After Transfer of Genetically Engineered Lymphocytes. Science. 2006 doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Piccart-Gebhart MJ, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 182.Slamon DJ, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 183.DeMattos RB, et al. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:8850–5. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nicoll JAR, et al. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–52. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 185.Bard F, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–9. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 186.Schenk D, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–7. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 187.Bacskai BJ, et al. Imaging of amyloid-beta deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat Med. 2001;7:369–72. doi: 10.1038/85525. [DOI] [PubMed] [Google Scholar]