Abstract
We previously demonstrated that alcohol-fed adolescent rats exhibit reductions in lumbar spine bone mineral density and vertebral body height, suggesting that chronic alcohol consumption has negative consequences for skeletal development during adolescence. Binge alcohol consumption is common in adolescents and young adults, yet little is known about its consequences on skeletal integrity or the attainment of peak bone mass. We used a previously validated binge alcohol exposure model to test the hypothesis that binge alcohol treatment of adolescent rats would be associated with distinct temporal and site-specific bone loss profiles, with incomplete recovery from bone loss following a period of alcohol abstinence. Seventy-two male adolescent Sprague-Dawley rats were assigned to one of 6 treatment groups (n = 12/group) receiving binge alcohol (3g/kg) or saline ip, 3 consecutive days (acute binge), 4 consecutive weekly (3-day) binge cycles (chronic binge), or 4 weekly binge cycles followed by a 30-day abstinence period without alcohol or saline injections (chronic binge with abstinence). Cancellous BMD was determined by pQCT and compressive strength determined by biomechanical testing. Serum testosterone and osteocalcin levels were measured by ELISA. Tibial cancellous BMD was significantly reduced by 25% (p < 0.05) after both acute and chronic binge alcohol treatment and vertebral cancellous BMD was significantly reduced by 15% (p<0.05) after chronic binge exposure. Vertebral compressive strength was also significantly decreased by 31% (p<0.05) after chronic binge alcohol treatment. Tibial cancellous BMD returned to control levels after the 30-day alcohol abstinence period, but vertebral cancellous BMD remained 15% below control values (p <0.05) 30 days after termination of binge alcohol exposures. Serum osteocalcin levels were significantly decreased following acute binge alcohol exposure (p<0.05). These results show that binge alcohol exposure can produce both short and long-term skeletal damage in the adolescent rat. This data may have relevance to peak bone mass attainment and future risk of skeletal disease in adolescents and young adults who engage in repeated binge drinking episodes.
Keywords: Binge alcohol, adolescent rat, peak bone mass, osteoporosis, fracture
Introduction
Since bone mass is lost throughout adult life as part of the aging process, a major risk factor for the development of osteoporosis is the failure to obtain a normal peak bone mass during early adulthood (Schettler et al., 2004). Multiple studies have shown that alcohol abuse is an important risk factor for osteopenia (for a review, see Chakkalakal et al., 2005). Excessive alcohol consumption continues to be a major public health problem in the United States with dangerous drinking practices such as binge drinking common in the adolescent and young adult populations (Windle, 2003). Binge drinking exposes young people to a variety of risks; from a bone health perspective this behavior is especially troubling because it tends to occur during the period when peak bone mass is attained (Abrams, 2003). Despite this connection, little is known about how binge alcohol consumption damages the adolescent skeleton and affects the attainment of peak bone mass. Approximately 25% of young men and 20% of women ages 18–30 report engaging in at least one binge-drinking episode (defined as 6 or more drinks per occasion) each month according to a recent survey (Grossberg et al., 2004). A subset of young adults with a history of binge drinking behavior report engaging in binge drinking episodes five or more times per month, suggesting that multiple binge drinking episodes may be common in the young adult population (Grossberg et al., 2004). This data is in agreement with another study of young adult drinking behavior which suggests that problem drinking behaviors beginning during adolescence (ages 17–20) tend to continue into the early adult years (ages 30–31) (McCarthy et al., 2004) encompassing the most critical developmental periods for accruement of bone mass (Abrams, 2003).
Despite the evidence that a significant proportion of the adolescent and young adult populations tend to consume alcohol in a binge pattern, most studies of the effects of alcohol exposure on adolescent skeletal development in rodents have used chronic alcohol feeding models. These studies have shown that alcohol inhibits bone growth, decreases BMD and negatively impacts cancellous bone architecture in adolescent rats (Sampson et al., 1996, 1997, Hogan et al., 1997). Biomechanical properties of bone from growing rats are also negatively affected by chronic alcohol exposure (Hogan et al., 1997). These studies also suggest that compared to adult bone, more cortical bone than cancellous bone is lost in adolescent rats exposed to alcohol, reflective of the fact that bone growth rather than bone remodeling is the primary skeletal activity in the developing skeleton.
An important question regarding the effects of alcohol on adolescent bone is whether the developing skeleton can overcome damage caused by alcohol, or does alcohol consumption cause lasting deficits in bone mass or strength? A prior investigation demonstrated that a period of recovery following chronic alcohol consumption by adolescent rats normalizes most bone metabolism parameters affected by alcohol, but cancellous bone area (BA/TA) remained significantly decreased after a 60-day alcohol recovery period (Wezeman et al., 1999). Chronic alcohol consumption by adolescent rats also significantly lowers both vertebral bone mineral density (BMD) and vertebral body height in male and female animals compared to pair fed controls (Wezeman et al., 2003), suggesting that chronic alcohol consumption by adolescent animals negatively affects bone growth and mass, and that these deficiencies may persist well past the active drinking period. Blood alcohol levels associated with binge alcohol administration may be an important outcome determinant for the effects of patterned alcohol on the adolescent skeleton as demonstrated by a previous binge alcohol study examining the effect of moderate binge alcohol exposure (BAC: 43–106 mg/dl) in young rats. This study demonstrated an unexpected beneficial effect of moderate alcohol consumption on skeletal growth and BMD following a two-day per week alcohol binge, and no bone-related effects of 5-day per week binge alcohol administration (Sampson et al., 1999).
In the current study, we employ a model of binge alcohol exposure previously associated with aggressive bone loss in adult male and female rats (Callaci et al., 2004, 2006, Wezeman et al., 2007) for the first time in adolescent animals to address the questions raised here regarding the short and long-term effects of binge alcohol exposure on developing skeletal health. Our hypothesis was that binge alcohol treatment of adolescent rats would cause distinct temporal and site-specific decreases in normal bone mass acquisition, with incomplete recovery observed following a period of alcohol abstinence. The results presented here are part of a larger effort currently underway using adolescent animals to identify unique mechanisms responsible for short and long term bone damage caused by binge alcohol consumption prior to attainment of peak bone mass.
Materials and Methods
Binge Alcohol Model
This investigation received approval from the Loyola University Institutional Animal Care and Use Committee (IACUC). Adolescent male Sprague Dawley rats were obtained at 7 weeks of age (175–199 gram range), (Harlan, Indianapolis, IN). Prior to treatment, animals were randomly assigned to one of six treatment groups with an n of 12 animals per group, chosen based on a power analysis performed using preliminary data (not shown). For animals receiving one week of treatment the groups were: saline control (C1), and binge alcohol-treated (A1). The one-week binge alcohol group will also be referred to in the text as acute binge alcohol-treated rats. For animals receiving four weeks of treatment the treatment groups were: saline control (C4) and binge alcohol-treated (A4). The four-week binge alcohol group is also referred to in the text as chronic binge alcohol-treated rats. To test the ability of rats to recover from binge alcohol treatment effects on bone, two additional groups of rats were treated with saline or alcohol for 4 weeks as above and then held for an additional 30 days without treatment prior to euthanasia. These groups are designated as saline control-abstinent (C4-Abs) and binge alcohol-abstinent (A4-Abs). Animals were housed in pairs, with paired animals always assigned to the same treatment group. Animals were weighed prior to the initiation of treatment and twice weekly throughout the study period.
Alcohol was administered by a single daily intraperitoneal (ip) injection of a 20% (vol./vol.) ethanol/saline solution at a dose of 3 g/kg, chosen to achieve peak blood alcohol levels (BAL) of approximately 300 mg/dl (Nation et al., 1993). Control animals were given an ip injection of an equal volume of sterile isotonic saline at the time of alcohol group injections. A once daily alcohol treatment regimen was chosen to avoid alcohol withdrawal symptoms that can occur when high doses of alcohol are administered twice daily (Penland et al., 2001). Alcohol or saline injections were given starting at 9:00 AM, 3 days/week. No ip injections were given during the remaining 4 days each week. Twenty-four hours after their last saline or alcohol injection, (or 30 days later in the abstinent groups) rats were rendered unconscious by CO2 inhalation and killed by decapitation. Thus acute binge alcohol-treated animals were killed 4 days after treatment began, chronic binge treated animals on day 25 of the experiment and abstinent animals on day 55. Bone samples (tibia, lumbar vertebral segments) were removed from each animal, dissected free of all soft tissue, wrapped in saline-soaked gauze and stored at −20° C for bone mineral density and biomechanical analysis. Whole blood was also collected at the time of euthanasia, allowed to clot and spun at 250 g for 10 minutes at 4° C. Serum was collected and stored in aliquots at −80° C.
Bone Mineral Density Measurements
Cancellous and cortical bone mineral density (BMD) of the proximal tibia and 4th and 5th lumbar vertebrae from each animal were determined by quantitative computed tomography (pQCT) using a Norland Stratec bone densitometer (Orthometrix, Inc., White Plains, NY). For vertebrae, each vertebral segment was positioned uniformly on a support so that the instrument-scanning plane was perpendicular to the longitudinal axis of the vertebral body. Scout views were obtained to determine the midpoint of each vertebral body. Midpoint analysis was performed to ensure that each sample was analyzed at the exact same point to minimize variation due to density differences based on sampling area. Three consecutive measurements were performed at a resolution of 70 μm/voxel 1 mm apart from this point. Using a predetermined peel algorithm, the cancellous area of each vertebral segment was defined as 45% of the total bone cross-sectional area of each measured plane of the vertebral body, the remaining fraction was defined as cortical bone. For tibiae, three measurements were performed, beginning 1 mm from the proximal endplate and proceeding distally. The cancellous compartment was defined as 42% of the total bone cross sectional area. The instrument was set to use the threshold contour mode (soft-tissue threshold set at 220 mg/cm3) and a concentric peel algorithm. Scans were made at 50kV and 0.3mA.
Biomechanical Structural Properties of Lumbar Vertebrae
Compressive strength tests were performed on the L4 and L5 vertebral bodies from each rat using a materials testing machine (Model 5544, Instron Corp., Canton, MA). The vertebral endplates were potted in bone cement using a previously described method that results in two parallel loading surfaces necessary to perform a uniform compression test on individual rodent vertebrae (Steinke et al., 1999). The specimens were prepared so that the posterior elements of the vertebra did not contact the loading platforms. Compression testing was performed at a crosshead speed of 0.5 mm/min to eliminate any strain rate effects. A 100 kg load-cell was used to monitor the compressive load and a precision sensor was used to measure the axial deformation of the specimen. The load-deformation data was analyzed to obtain the compressive strength of the vertebrae, defined as the maximum load sustained before material failure.
Blood Alcohol Determination Assay
Blood alcohol levels were determined by colorimetric measurement of NAD reduction using an alcohol concentration determination reagent set (Pointe Scientific, Inc). Four alcohol naive 200-gram rats received a single 3g/kg dose of ethyl alcohol by ip injection 1 hour prior to euthanasia. Total trunk blood was collected, allowed to clot on ice for 20 minutes and centrifuged at 250g for 10 minutes at 4°C. Serum was incubated in colorimetric reagent at 30°C for 10 minutes and absorbance was read at 340 nm.
Serum Osteocalcin Analysis
Serum osteocalcin, a marker of bone formation activity was measured by ELISA (Biomedical Technologies Inc., Stoughton, MA). Serum samples were diluted 1:20 in the sample buffer and assayed in duplicate according to the manufacturer’s protocol. Serial two-fold dilutions of a highly purified osteocalcin standard provided in the kit were performed in sample buffer to achieve concentrations from 0.625 ng/mL to 20 ng/ml and used as a standard curve. Absorbance was measured at 450 nm. Serum levels of osteocalcin were expressed as ng/ml.
Serum Testosterone Analysis
Serum testosterone levels were determined by competitive solid-phase ELISA (Alpha Diagnostic International, San Antonio, TX) following the manufacturer’s protocol. Ten (10) μl of standards and undiluted serum samples from each animal were assayed in duplicate. Absorbance was measured at 450 nm. Serum levels of testosterone were expressed as ng/ml.
Statistical Analysis of Bone Damage Parameters
Statistical analysis was performed using the SPSS software package (SPSS Inc. Chicago, IL). Analysis of statistical significance for BMD and vertebral strength data was performed by Student’s T-Test followed by Bonferroni multiple-comparison correction procedure. Significance was noted at p ≤ 0.05. Multiple regression analyses were used to determine the relative contributions of body weight vs. treatment condition on the effects observed in the dependent variables (BMD, strength). These analyses were performed in a two-step hierarchical fashion. Information about treatment groups was carried by three dummy coded dichotomies to account for the four treatment conditions. All regression analyses were conducted in a similar fashion. First, the dependent variable was regressed upon post-treatment weight. Next, the treatment group dichotomies were added to the equation. The adjusted R2 was measured in each step and the change in this statistic was tested for significance. The change in adjusted R2 indicated the percent of variance that was explained by treatment group after accounting for the variability due to weight.
Results
General Observations
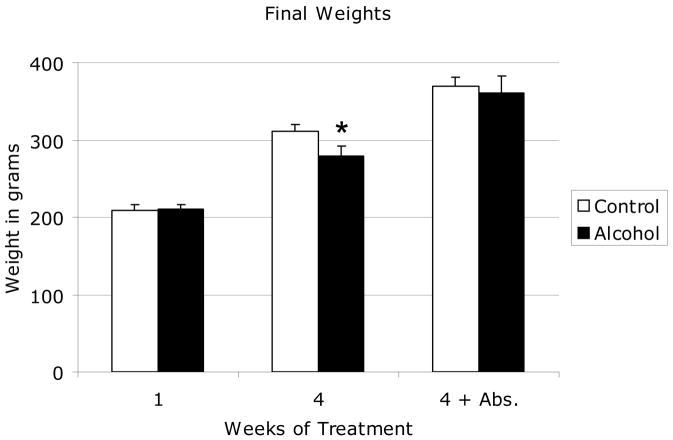
The effect of binge alcohol exposure on animal weight gain is shown in Figure 1. The average baseline weight of animals used in this study was 210 +/− 5.1 grams. All animals showed significant weight gain during the experimental period regardless of treatment. There was no significant difference in weight of animals following acute binge alcohol treatment (C1 vs. A1). Chronic binge alcohol treated rats showed a 33% increase in body weight following treatment compared to baseline weight while saline controls increased in weight by 47% during the same period. This difference in weight gain (C4 vs. A4) was significant. There was no significant difference in animal weight after 4 weeks of alcohol abstinence on day 55 of the experiment (C4-Abs vs. A4-Abs). Previous studies in our laboratory showed no significant differences in weekly food intake between control and binge alcohol-treated adult rats Callaci et al., 2006). Daily monitoring of animals revealed no evidence of alcohol withdrawal symptoms during the study period (Becker, 2000). Rats exhibited a short (approximately 1–2 hour) period of acute alcohol intoxication immediately following each ip injection. Peak blood alcohol levels, measured 1 hour after ip alcohol injection in four alcohol naïve animals not included in subsequent experiments averaged 287 mg/dl, consistent with published reports of peak BAL in rats after a 3g/kg ip alcohol administration (Nation et al., 1993). Necropsies were performed on each animal and revealed no apparent internal injuries from ip injections or obvious abnormalities from alcohol treatment.
Figure 1. Effect of Binge Alcohol Treatment on Weight Gain in Adolescent Rats.
Shown are 1—final animal weights in grams following one or four binge alcohol cycles and following a 30-day abstinence period. Data are shown as mean + standard deviation for both control and alcohol groups after 1 week, 4 weeks, and 4 weeks plus a 4 week abstinence period. * P < 0.05 control vs. alcohol at each time point, by Student’s t-test with Bonferroni multiple testing correction. N = 12 animals/group.
Because a significant effect of chronic binge alcohol treatment was observed on final post-treatment weight gain compared to control animals, we performed multiple regression analysis to determine the relative contributions of body weight vs. treatment condition (binge alcohol) on the significant effects observed in the dependent variables examined (BMD, strength). This analysis revealed that after controlling for the possible effects of weight on BMD, treatment condition accounted for an additional 10% or 20% of the variance in this parameter in tibia and vertebrae respectively and these effects were highly significant, (p =.01) Likewise, when controlling for the effects of post-treatment weight on vertebral compressive strength, treatment condition accounted for an additional 21% of the variance observed and this effect was also significant (p =.02).
Bone Mineral Density Measurements
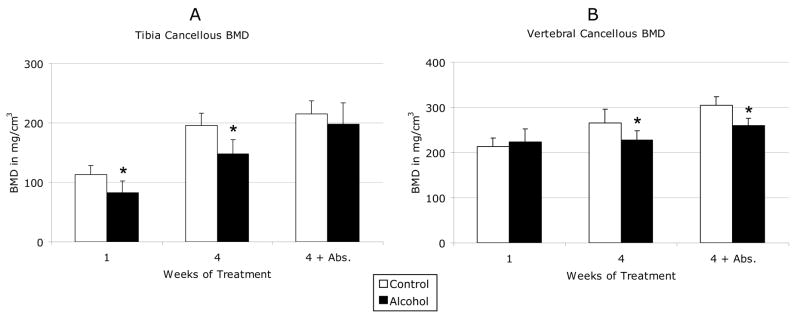
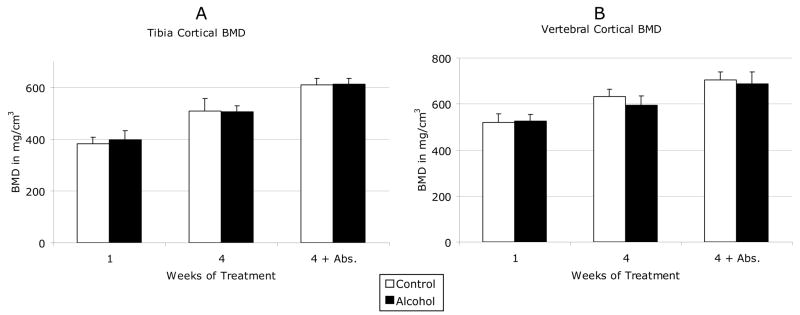
The effects of binge alcohol on cancellous and cortical BMD of proximal tibia and L4 lumbar vertebrae are shown in Figure 2. In tibia, cancellous BMD decreased by 27% after acute binge alcohol treatment (C1 vs. A1) and 25% after chronic binge treatment (C4 vs. A4) as compared to matched saline control values (Figure 2A). These differences were significant (p <0.05). Tibial cancellous BMD in chronic binge alcohol-treated rats remained 8% below matched control levels after a 4-week abstinent period, although this difference was no longer significant. In vertebrae, cancellous BMD did not decrease after acute binge alcohol but was decreased by 15% after chronic binge alcohol treatment, which was significant compared to matched control values (Figure 2B). Unlike tibia, chronic binge alcohol effects on vertebral cancellous bone persisted after the alcohol abstinence period with a continued 15% decrease in BMD compared to control (p < 0.05). No significant effects of alcohol treatment were observed on cortical BMD in tibia or vertebrae (Figure 3A/B).
Figure 2. Effects of Binge Alcohol on Cancellous Bone Mineral Density.
Shown are (a) Tibial and (b) vertebral cancellous bone mineral density in mg/cm3. Data are shown as mean + standard deviation for both control and alcohol groups after 1 week, 4 weeks, and 4 weeks plus a 4 week abstinence period. * P < 0.05 control vs. alcohol at each time point, by Student’s t-test with Bonferroni multiple testing correction. N = 12 animals/group.
Figure 3. Effects of Binge Alcohol on Cortical Bone Mineral Density.
Shown are (a) Tibial and (b) vertebral cortical bone mineral density in mg/cm3. Data are shown as mean + standard deviation for both control and alcohol groups after 1 week, 4 weeks, and 4 weeks plus a 4 week abstinence period. N = 12 animals/group.
Biomechanical Properties of Vertebral Bone
The effects of binge alcohol on L4 lumbar biomechanical compressive strength are shown in Figure 4. Compressive strength was not significantly reduced after acute binge alcohol treatment, but was reduced by 31% in animals subjected to chronic binge alcohol treatment (C4 vs. A4) (p < 0.05). However, although this alcohol-related reduction in compressive strength remained decreased by 14% lower than control levels after a 30-day alcohol abstinent period, this difference was no longer significant (C4-abs vs. A4-abs). The size of vertebral bodies as determined by cross-sectional area measurement obtained during pQCT was equivalent across treatment groups (data not shown), suggesting that the vertebral compressive strength values observed after binge alcohol treatment were the result of altered mineral and biomechanical properties of the vertebrae and were not due to treatment-related differences in vertebral body area.
Figure 4. Effects of Binge Alcohol on Lumbar Vertebral Compressive Strength.
Shown is vertebral compressive strength in kilograms. Data are shown as mean + standard deviation for both control and alcohol groups after 1 week, 4 weeks, and 4 weeks plus a 4 week abstinence period. * P < 0.05 control vs. alcohol at each time point, by Student’s t-test with Bonferroni multiple testing correction. N = 12 animals/group.
Serum Bone Remodeling and Hormone Assays
Serum osteocalcin and testosterone levels following binge alcohol treatment are shown in Table 1. We did not observe significant changes in serum testosterone levels in adolescent male rats exposed to 1 or 4 binge alcohol cycles, or after the alcohol abstinence period. We did however observe a 12 % significant reduction in serum osteocalcin levels following acute binge alcohol treatment compared to saline control animals (p < 0.05). This reduction was not observed after chronic binge alcohol treatment or following alcohol abstinence.
Table 1. Effects of Binge Alcohol on Serum Bone Metabolism Markers.
Shown are serum levels of testosterone and osteocalcin, as determined by ELISA in ng/mL. Data are shown as mean ± standard deviation for both control and alcohol groups after 1 week, 4 weeks, and 4 weeks plus a 4-week abstinence period.
| Treatment Period | ||||||
|---|---|---|---|---|---|---|
| 1 Week | 4 Weeks | 4 Weeks + Abstinence | ||||
| Assay: | Control | Alcohol | Control | Alcohol | Control | Alcohol |
| Testosterone | 3.09 ± 0.69 | 2.24 ± 1.72 | 2.73 ± 1.91 | 3.23 ± 1.15 | 3.60 ± 0.62 | 3.45 ± 1.53 |
| Osteocalcin | 213.7 ± 26.4 | 188.8 ± 20.3* | 170.5 ± 15.1 | 166.2 ± 12.5 | 156.7 ± 15.6 | 157.9 ± 13.6 |
P < 0.05 control vs. alcohol at each time point, by Student’s t-test with Bonferroni multiple testing correction. N = 12 animals/group.
Discussion
Attainment of peak bone mass generally occurs during the third decade of life (Abrams 2003) and is affected by both genetic and lifestyle factors such as diet, exercise, smoking and alcohol consumption. No further accumulation of bone mass occurs following this period, suggesting that peak bone mass level is an important outcome determinate for the probability of osteoporosis development in later decades (Schettler et al., 2004). In an attempt to gain a greater understanding of the consequences of alcohol consumption on bone mass accumulation during adolescence, this study utilized a binge alcohol exposure model previously shown to cause reproducible bone loss in adult rats (Callaci et al., 2004, 2006, Wezeman et al., 2007) in adolescent animals to test whether acute or chronic binge exposure attenuates bone mass accumulation and the development of bone strength. The ability to recover from reduced gains in mass and strength were examined in animals following an extended alcohol abstinence period.
Site-Specific Differences in Binge Alcohol-Related Effects on Bone Mass Deficits and Recovery
The effects of binge alcohol treatment on adolescent rat bone mass and strength were directly related to the duration of alcohol exposure and varied according to skeletal site. We observed a significant decrease of cancellous bone mass in the proximal tibia after acute binge exposure, and more extensive decreases following chronic binge exposure. The tibia recovered from this deficit in bone mass following binge alcohol treatment with BMD values restored to control levels after a 30-day abstinence period. Lumbar vertebrae appear more resistant to the damaging effects of binge alcohol exposure than tibia with significant reductions in lumbar BMD observed only after chronic binge exposure. Unlike the tibia, alcohol-induced damage to the lumbar spine was resistant to recovery during the abstinent period, with lumbar BMD levels remaining significantly below matched controls 30 days after the last alcohol exposure. Cortical bone appears to be resistant to binge-patterned alcohol exposure in both the adolescent animals tested in this study, and in adult rats tested previously which showed no significant changes in cortical BMD (Callaci et al., 2004). These results differ from earlier chronic alcohol exposure models, in which significant cortical bone deficits were observed (Sampson et al., 1996, 1997, Hogan et al., 1997), suggesting that the pattern and absolute amount of alcohol ingested are important factors which influence the ability of alcohol treatment to compromise cortical bone integrity.
Our observations on the effects of binge alcohol treatment on cancellous bone mass are reminiscent of studies examining the site-specific time course of bone loss in ovariectomized (OVX) rats (Wronski et al., 1988, 1989). These OVX studies demonstrated site-related differences in the appearance of osteopenia following ovariectomy with losses of approximately 50% of tibial cancellous bone mass occurring by 30–60 days post-surgery, while an equivalent amount of lumbar cancellous bone loss did not occur until 270 days post ovariectomy. These data suggest that the proximal tibia may be more susceptible to osteopenia caused by either hormonal deficiency or alcohol exposure than the lumbar spine, but our data suggests that once damaged the spine is slower to recover from loss of bone mass than is the tibia.
The effects of binge alcohol treatment on vertebral compressive strength we observed correspond to the vertebral BMD data discussed above with no effects on strength observed after one binge alcohol cycle and significant reductions in compressive strength observed after 4 binge alcohol cycles. Vertebral compressive strength was restored following the alcohol abstinence period. The remaining deficit in compressive strength exhibited by alcohol-exposed rats following alcohol abstinence (14%) was not significant. However when considered in the context of previous data, which demonstrated that chronic alcohol consumption decreases both vertebral BMD and body height in adolescent rats (Wezeman et al., 2003) our data suggests that alcohol consumption during adolescence may have structural and functional consequences for the lumbar spine that endure beyond the active drinking period.
The potential importance of our data with respect to adolescent bone health is reinforced by a recent report challenging the current paradigm that bone mass, after peaking during young adulthood, plateaus during middle age and that significant age-related bone loss does not occur until after menopause in women and during much later adulthood in men. This longitudinal population-based assessment showed that both women and men actually experience significant cancellous bone loss before age 50, which is much earlier than previously believed (Riggs et al., 2008). This new understanding of the dynamics of bone mass stability magnifies the importance of healthy bone mass accumulation during young adulthood and the avoidance of practices such as binge drinking, which may compromise attainment of peak bone mass and augment naturally occurring bone loss to a degree that significant weakening of the skeleton could occur by middle adulthood. While Riggs et al speculates as to the mechanisms responsible for this cancellous bone loss in young women and men, our data suggests that alcohol consumption during adolescence is a risk factor for cancellous bone loss in the young adult years and thus could be a contributing factor to the surprising amount of early bone loss observed by Riggs. Future studies may choose to include alcohol consumption history into such longitudinal studies of bone integrity.
The mechanisms responsible for these site-specific differences in vulnerability to bone damaging agents and recovery from damage are not well understood but may be related to differences in bone turnover rates between the two sites. Bone turnover rates are at least two-fold greater in the tibia than the spine following ovariectomy (Wronski et al., 1988, 1989). As alcohol exposure has been shown to affect both bone formation and resorption, higher rates of bone turnover in the tibia could explain both the rapidity of bone loss observed following alcohol exposure and the recovery of bone mass at this site following alcohol withdrawal. Our data does suggest a transient effect of binge alcohol exposure is to depress bone formation activity, as serum osteocalcin levels were significantly decreased following acute binge exposure but not following chronic binge treatment or after the alcohol abstinent period. Serum osteocalcin levels have not been shown to decrease in previous chronic alcohol consumption studies performed in adolescent rats (Sampson et al., 1996, Wezeman et al., 1999, Wezeman et al., 2000). Animals in both these previous studies were approximately 4 weeks of age at first alcohol exposure; our animals were approximately 7 weeks of age, so both the pattern of alcohol exposure (binge vs. chronic) and animal age (7 vs. 4 weeks) are potential mitigating factors influencing these results.
We anticipated that a significant drop in serum testosterone levels following binge exposure was plausible as previously demonstrated following chronic alcohol consumption (Wezeman et al., 1999) but did not observe this in our study. These observations suggest that alcohol consumption patterns and age at first alcohol exposure may be critical factors, which modulate the influence of alcohol on bone metabolism outcome determinants. The patterns of alcohol-induced bone loss and recovery observed in this study are currently under investigation to identify mechanisms responsible for adolescent bone damage related to binge alcohol consumption.
Effects of Treatment on Adolescent Rat Weight Gain
The binge alcohol treatment protocol utilized in this study was associated with a transient effect on weight gain in adolescent animals. This difference in weight gain between saline control and alcohol-exposed rats complicates the interpretation of bone-specific effects of alcohol during skeletal development. Factors which may contribute to weight gain differences between groups include health-related effects of ip alcohol injection, reduced food intake in alcohol-exposed animals and a direct effect of alcohol on the ability of adolescent rats to turn food nutrients ingested into body mass. While we cannot exclude the first two possibilities, weight loss was never observed in any animals during the study and no evidence of organ damage was observed during routine necropsy of alcohol-exposed animals. Although food intake levels were not measured in this study, no significant differences in weekly food intake were observed between saline-control and alcohol-treated in a previous study utilizing ip alcohol injection (Callaci et al., 2006) With respect to alcohol-related weight effects, a direct effect of alcohol exposure on weight gain in adolescent rats has been previously observed by our laboratory in adolescent animals exposed to chronic alcohol administration using the Lieber-DeCarli liquid diet (Wezeman et al., 2000, Wezeman et al., 2003). In these protocols, control animals were given liquid diet matched to the caloric intake of their alcohol-fed counterparts, eliminating the possibility of reduced caloric intake by alcohol-exposed animals but also resulted in alcohol-fed animals exhibiting a final post-treatment weight significantly lower than that of matched control animals. This previous data shows that alcohol exposure has a direct affect on adolescent rat weight gain, and suggests that a direct effect of alcohol could be responsible for, or a contributing factor in, the weight gain differences observed in the current study. Average weights of alcohol-exposed animals following the alcohol abstinent period are not different from control, arguing against any long-term effect on growth. However bone-related deficiencies persist, strengthening the argument that there is a real effect of alcohol on skeletal integrity in this model.
In conclusion, the data presented here demonstrates that binge alcohol exposure of rats during the late adolescent period results in a significant deficit in vertebral cancellous bone mass immediately following alcohol exposure that is not recovered during a prolonged alcohol abstinent period. This data has important repercussions for long-term bone health in young adults engaging in binge alcohol consumption, as it suggests that these individuals may experience persistent changes in the skeleton leading to a greater susceptibility to fracture and osteoporosis in later decades of life.
Acknowledgments
The National Institute of Health, National Institute on Alcohol Abuse and Alcoholism grant RO1 AA016138 (JJC) supported this work. This work was also supported by a gift from the Blum-Kovler Foundation. The authors gratefully acknowledge the assistance of James Sinacore, Ph.D., Assistant Professor, Loyola University of Chicago, for his assistance in statistical analysis of data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams SA. Normal acquisition and loss of bone mass. Horm Res. 2003;60(Suppl 3):717–6. doi: 10.1159/000074505. [DOI] [PubMed] [Google Scholar]
- Becker HC. Animal models of alcohol withdrawal. Alcohol Res Health. 2000;24(2):105–113. [PMC free article] [PubMed] [Google Scholar]
- Callaci JJ, Juknelis D, Patwardhan A, Sartori M, Frost N, Wezeman FH. The effects of binge alcohol exposure on bone resorption and biomechanical and structural properties are offset by concurrent bisphosphonate treatment. Alcohol Clin Exp Res. 2004;28(1):182–191. doi: 10.1097/01.ALC.0000108661.41560.BF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaci JJ, Juknelis D, Patwardhan D, Wezeman FH. Binge alcohol treatment increases vertebral bone loss following ovariectomy: compensation by intermittent parathyroid hormone. Alcohol Clin Exp Res. 2006;30(4):665–672. doi: 10.1111/j.1530-0277.2006.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkalakal DA. Alcohol-Induced Bone Loss and Deficient Bone Repair. Alcohol Clin Exp Res. 2005;29(12):2077–2090. doi: 10.1097/01.alc.0000192039.21305.55. [DOI] [PubMed] [Google Scholar]
- Grossberg PM, Brown DD, Fleming MF. Brief physician advice for high-risk drinking among young adults. Ann Fam Med. 2004;2(5):474–480. doi: 10.1370/afm.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan HA, Sampson HW, Cashier E, Ledoux N. Alcohol consumption by young actively growing rats: A study of cortical bone histomorphometry and mechanical properties. Alcohol Clin Exp Res. 1997;21(5):809–816. [PubMed] [Google Scholar]
- McCarty CA, Ebel BE, Garrison MM, DiGiuseppe DL, Christakis DA, Rivara FP. Continuity of binge and harmful drinking from late adolescence to early adulthood. Pediatrics. 2004;114(3):714–719. doi: 10.1542/peds.2003-0864-L. [DOI] [PubMed] [Google Scholar]
- Nation JR, Burkey RT, Grover CA. Lead/Ethanol interactions phamacokinetics. Alcohol. 1993;10:363–367. doi: 10.1016/0741-8329(93)90021-f. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melon LJ, III, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Amin S, Rouleau PA, Khosla S. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23(2):205–214. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson HW, Chaffin C, Lange J, DeFee B. Alcohol consumption by young actively growing rats: A histomorphometric study of cancellous bone. Alcohol Clin Exp Res. 1997;21(2):352–359. [PubMed] [Google Scholar]
- Sampson HW, Gallager S, Lange J, Chondra W, Hogan HA. Binge drinking and bone metabolism in a young actively growing rat model. Alcohol Clin Exp Res. 1999;23(7):1228–1231. doi: 10.1111/j.1530-0277.1999.tb04282.x. [DOI] [PubMed] [Google Scholar]
- Sampson HW, Perks N, Champney TH, DeFee B. Alcohol consumption inhibits bone growth and development in young actively growing rats. Alcohol Clin Exp Res. 1996;20(8):1375–1384. doi: 10.1111/j.1530-0277.1996.tb01137.x. [DOI] [PubMed] [Google Scholar]
- Schettler AE, Gustafson EM. Osteoporosis prevention starts in adolescence. J Am Acad Nurse Prac. 2004;16(7):274–282. doi: 10.1111/j.1745-7599.2004.tb00450.x. [DOI] [PubMed] [Google Scholar]
- Steinke B, Patwardhan AG, Havey R, King D. Human growth hormone transgene expression increases the biomechanical structural properties of mouse vertebrae. Spine. 1999;24:1–4. doi: 10.1097/00007632-199901010-00002. [DOI] [PubMed] [Google Scholar]
- Wezeman FH, Emanuele MA, Emanuele NV, Moskal SF, Woods M, Suri M, Steiner J, LaPaglia N. Chronic alcohol consumption during male rat adolescence impairs skeletal development through effects on osteoblast gene expression, bone mineral density and bone strength. Alcohol Clin Exp Res. 1999;23(9):1534–1542. [PubMed] [Google Scholar]
- Wezeman FH, Juknelis D, Callaci JJ. Vitamin D and ibandronate prevent cancellous bone loss associated with binge alcohol treatment in male rats. Bone. 2007;41:639–645. doi: 10.1016/j.bone.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wezeman FH, Juknelis D, Frost N, Callaci JJ. Spine mineral density and vertebral body height are altered by alcohol consumption in growing male and female rats. Alcohol. 2003;31:1–6. doi: 10.1016/j.alcohol.2003.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M. Alcohol use among adolescents and young adults. Alcohol Res Health. 2003;27(1):79–85. [PMC free article] [PubMed] [Google Scholar]
- Wronski TJ, Cintron M, Dann LM. Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcif Tissue Int. 1988;43(3):179–183. doi: 10.1007/BF02571317. [DOI] [PubMed] [Google Scholar]
- Wronski TJ, Dann LM, Horner SL. Time course of vertebral osteopenia in ovariectomized rats. Bone. 1989;10(4):295–301. doi: 10.1016/8756-3282(89)90067-7. [DOI] [PubMed] [Google Scholar]