Abstract
Plant growth and development are coordinalely controlled by several internal factors and environmental signals. To sense these environmental signals, the higher plants have evolved a complex signaling network, which may also cross talk with each other. Plants can respond to the signals as individual cells and as whole organisms. Various receptors including phytochromes, G-proteins coupled receptors (GPCR), kinase and hormone receptors play important role in signal transduction but very few have been characterized in plant system. The heterotrimeric G-proteins mediate the coupling of signal transduction from activated GPCR to appropriate downstream effectors and thereby play an important role in signaling. In this review we have focused on some of the recent work on G-proteins and two of the effectors, PLC and PLD, which have been shown to interact with Gα subunit and also discussed their role in abiotic stress tolerance.
Key words: abiotic stress, G-protein couple receptor, heterotrimeric G-protein, phospholipases, plant receptors, signal transduction
Introduction
Plants perceive a wide range of external and internal signals, which are used to regulate various responses in development and adaptation.1 The exogenous signals include light, temperature, mechanical perturbations like wind, humidity, CO2, edaphic factors like soil water and nutrients, and gravity while the endogenous signals include growth regulators, developmental regulators, and metabolites.2 Exposure to primary stimuli including light, hormones and pathogen derived elicitors can cause membrane depolarizations of the plant cells within 10–30 seconds, which is linked to early Ca2+ influx and anion efflux through ion channels.3 Being themselves static, plants have to have very efficient systems to respond to the environment that fluctuates throughout the day. It seems that the signals are mostly perceived at the level of membrane and therefore transmembrane events are the likely routes for signal generation and transduction. In plants, the best-characterized plasma membrane-based receptors are of two kinds: (i) transmembrane receptor enzymes (usually kinase), (ii) G-protein-coupled receptors (GPCRs). Over the last few years, a number of receptors have been identified in plants, which has helped in understanding the signal transduction network in plants. Presently in plants, the G-proteins are reported to be involved in processes such as ion channel and abscisic acid signaling4 and modulation of cell proliferation445 in Arabidopsis. However, a wide range of processes-including seed germination, shoot and root growth, and stomatal regulation are altered in Arabidopsis and rice plants with mutations in G-protein components. Their role has been also shown in GA-pathways and in some developmental responses. Recently, role of Gα in pathogenicity6 and biotic stresses.7,8 has also been indicated. In this review we have dealt with signal coupling through GPCR, G-proteins and phospholipases especially on their role under abiotic stress environmental conditions.
G-Proteins
By late 1970's through the work of Ross, Gilman and Rodbell in animal system, it was known that the production of cAMP by the action of epinephrine was mediated by GTP binding proteins called G-proteins. In the decade of 80's such proteins were purified, characterized and the genes encoding these proteins were cloned. There are two kinds of G-proteins, monomeric and heterotrimeric.9 In this article we will briefly comment on the latter class, which as mediators are involved in the transmission of external signals via receptor molecules to effector molecule. The name of heterotrimeric G-proteins comes from the fact that they form a complex of three different proteins: G-alpha (Gα), G-beta (Gβ) and G-gamma (Gγ).10,11 The Gα in its inactive state is bound to GDP. The Gα has molecular mass of 35–46 kDa and is post-translationally modified by myristoylation/palmitoylation, which enhances its association with membranes. Structurally, Gα is highly diverse and contains five conserved domains, G1 to G5, which are involved in GTP binding and hydrolysis. In plant Gα, the p-loop for NTP binding, the DxxGQ motif for GTP hydrolysis and the NKxD motif for guanine recognition are conserved (see ref. 12). The C-terminal part interacts with seven transmembrane receptor and downstream effectors. The N-terminal part interacts with the Gβ-Gγ dimer and also contains palmitoylation and myristoylation sites. The molecular mass of Gβ subunit is 35–36 kDa and it belongs to the WD40 family of proteins. Four to 16 copies of WD (tyrosine-aspartic acid pair) repeats are present in a single protein with more or less constant distance of 40 (∼40–60) residues, hence called WD40. The WD repeats are involved in protein-protein interaction. The Gγ subunit is more diverse in structure and has a molecular mass of 6–10 kDa and undergoes isoprenylation at C-terminus region at CAAX motif. Each Gγ subunit carries a lipid chain at C-terminus, which anchors the Gβ-Gγ dimer and the inactive trimer to the plasma membrane.10,11
On activation, as mentioned above Gα exchanges GDP with GTP and following dissociation activates downstream proteins. Infact, Gα subunit cycle between an inactive GDP bound and an active GTP-bound form. The activated Gα subunit (Gα-GTP) leads to the dissociation of Gβ-Gγ dimer from Gα. Both these moieties interact with various downstream effector molecules and initiate unique intracellular signaling responses. Termination of the active state is achieved by the inherent GTPase activity of the Gα form. After the signal propagation, the GTP of Gα-GTP is hydrolyzed to GDP and Gα becomes inactive (Gα-GDP), which leads to its reassociation with Gβ-Gγ dimer to form the inactive heterotrimeric complex.10
In plants the G-proteins cascade is studied to some extent in Arabidopsis and rice and much of it is still not revealed. The work on plant G-proteins has been recently (reviewed by Temple and Jones (2007) ref. 12). The genomes of diploid angiosperms, such as that of the model species Arabidopsis thaliana, contains only single canonical Gα gene, GPA1,13 one Gβ gene, AGB1,14 and two Gγ genes, AGG1 and AGG2.15,16 Two Gα-subunits (PGA1 and PGA2) are reported from pea.17 It has been shown using domain mutation analysis that myristolyation or acetylation alone is not the only requirement to direct GPA1 to membranes.18 Like in animal systems, the two Gγ subunits in Arabiopsis and also rice Gγ have CAAX-box at the C-terminal. One of the rice Gγ has been found to have higher molecular weight of around 17 kDa as compared to most of them of around 11 kDa.19 In mammalian genome G-protein subunits exist as multigene families e.g., alpha has 20, beta 5 and gamma 11 genes. However, in plant genomes so far only one or two genes for each subunit have been found which suggest that G-proteins may be involved in some more restricted, specific signal transduction events.
In plants the Gα was found to remain in association with GTP. It was found that the intrinsic GTPase activity was affected by another protein, RGS (Regulator of G-protein Signaling), a 7-transmembrane protein.20 The homologs of this protein have not been reported, though in Medicago data base a plant RGS protein without transmembrane has been reported. Recently, RGS proteins have also been shown to be involved in abscisic acid signaling.21
The involvement of G-proteins in various signal transduction pathways was initially implied pharmacologically using various agonists and antagonists. Involvement of G-proteins in light signaling pathways has also been shown. Our own studies had also indicated an involvement of G-proteins in light mediated nitrate reductase gene expression and also in development.22,23 A Gα cloned from carrot seedlings showed regulation in response to high temperature and high salinity.24 Recently, we have cloned all the three subunits of G-proteins from pea and reported the first direct evidence that Gα is involved in salinity and heat stress tolerance while Gβ is involved in only heat stress tolerance.25
G-Protein-Coupled Receptors (GPCRs)
The GPCR are important as they serve as the gateway for signal transduction induced by ligand binding. In animal system the GPCRs exist as a superfamily of integral membrane protein receptors that contain seven transmembrane α-helical regions, which bind to a vast variety of ligands and are involved in various signaling pathways.26 Although the human genome contains about 1000 GPCRs but only single gene (GCR1) encoding a putative GPCR has been identified in Arabidopsis thaliana, which was reported to be cell cycle regulated27 and also involved in ABA signaling in guard cells.28 Using a different bioinformatics tool it was shown that there might be as many as 394 divergent GPCR candidates in Arabidopsis.29 Based on sequence homology and functional similarity the animal GPCRs can be grouped into several families such as Family A (rhodopsin-like), Family B (secretin-like), Family C (metabotropic/ pheromone), Family D (Fungal pheromone), Family E (cAMP receptors) and Family F (Frizzled/Smoothened).30 Recently, through computational studies of Family A and Family B of GPCRs Vohra et al. (2007)31 reported that the plant GCR1 (GPCR) contained homology with Family A, Family B and Family E of GPCRs. The extracellular loops of the GPCR can be glycosylated and contained two highly conserved cysteine residues, which build disulfide bonds to stabilize the receptor structure. GPCR is also known as a guanine nucleotide-exchange factor (GEF) that promotes the exchange of GDP/GTP associated with Gα subunit.9 The activation of GPCRs though ligand interaction stimulates its GEF activity. The activated GPCRs interact with their cognate G-protein, inducing GDP release with subsequent GTP binding to the Gα subunit.9,12 The exchange of GDP for GTP resulted in activation of the Gα subunit (Gα-GTP), which leads to the dissociation of Gβ/Gγ dimer from Gα. Both these moieties interact with various downstream effector molecules and initiate unique intracellular signaling responses. After the signal propagation, the GTP of Gα-GTP is hydrolyzed to GDP and Gα becomes inactive (Gα-GDP), which leads to its reassociation with the Gβ/Gγ dimer to form the inactive heterotrimeric complex Gα subunit.9,12,30,31
Earlier, the GCR2 from Arabidopsis thaliana was reported to be a plant GPCR for the ABA. The ABA is perceived by several different types of receptors in plant cells. At the cell surface, the ABA signal is proposed to be perceived by GCR2, which mediates ABA responses in seed germination, early seedling development and stomatal movement. GCR2 was also proposed to contain seven-transmembrane (7TM) domains. However, recently, Gao et al. (2007)32 found through genetic characterization that there is no role for the reported ABA receptor, GCR2, in ABA control of seed germination and early seedling development in Arabidopsis. Also by using multiple robust transmembrane prediction systems, GCR2 was predicted not to be a 7TM protein, a structural hallmark of GPCRs.32 These authors also reported that loss-of-function mutations in GCR2-LIKE 1 (GCL1) did not confer ABA insensitivity.32 Another putative receptor is GCR1 for which no ligand has been identified yet.33 A novel GPCR containing a lipid kinase domain has recently been identified in Dictyostelium that regulates cell density sensing.34 Recently, we have reported the isolation of a pea GPCR gene and the characterization of the encoded GPCR protein and found that this protein interacted with all the subunits of the G-proteins.25
GPCRs have also been reported to control many cellular processes by regulating phospholipid signaling pathways.35 Usually, in response to the stress stimuli, these receptors help to stimulate the phospholipase C (PLC) activity which hydrolyse the membrane phosphoinositide PIP2 to DAG and the second messengers IP3. Many receptors also stimulate phospholipase D (PLD), leading to the generation of the versatile lipid, phosphatidic acid (PA). Usually, the different PLC and PLD isoforms take differential positions in receptor signaling and are regulated by small GTPases of the Ras, Rho and ARF families. The PIP2 also has signaling capacity by itself and can affect the activity and subcellular localization of PLD and several other proteins. Recently, Oude Weernink et al. (2007)35 presented an overview of how these signaling pathways are governed by GPCRs.
Signal Coupling
Receptor mediated signal transduction in animal systems is either carried forward via the mechanism of protein-protein interaction or via activation of other proteins whose activity leads to the generation of second messengers.2,36,37 For example in animal systems, the receptor tyrosine kinase signaling leads to the phosphorylation of the intracellular domain of the receptor at the tyrosine residues to which a specific class of proteins having SH2, SH3 domains bind which inturn bind other proteins like Ras (a small G-protein) that carries forward the signaling via activation of other kinases. Alternatively, other proteins like JAK (janus kinase) bind to the phosphorylated intracellular domain of receptor kinase and inturn phosphorylate another protein, STAT (signal transducer and activator of transcription). The phosphorylated STAT dimerizes and enters the nucleus to activate transcription. Such a scenario is known to operate for serine/threonine receptor kinases. However, as we mentioned earlier this mode of signal operation has not been reported and well studied in plants. The alternative mode is to activate ionic channels in the membrane, for example to regulate calcium uptake and release, or to activate enzymes like phospholipase C or D which through their enzymatic actions can release second messengers or activate heterotrimeric G-proteins, which can effect the activity of enzymes like adenylcyclase for the production of cAMP. In plants, in majority of cases, this alternate mode of action, in addition to 2-component signaling and the movement of receptors directly in the nucleus, seems to be operative.38 In this section a brief account of the nature and role of G-proteins, PLD and PLC in stress tolerance are presented.
Phospholipases as G-Protein Effectors
It has been shown that G-protein coupling could lead to the release of second messengers via effectors, which are either ion channels or enzymes. In plants, the presence of the well-known effector molecules of animal systems like adenyl cyclase, PLCβ, GPCR kinases etc have not been established. There is however some evidence that G-protein coupling may be mediated via their interaction with phospholipases. We present below a brief account of the information available on PLA2, PLC and PLD in plant systems as putative candidates for G-protein effectors.
The lipid derived second messengers are generated by the hydrolysis of phospholipids by the action of specific phospholipases, these are designated as A, C or D (PLA, PLC, PLD) depending on the site of cleavage on the phospholipid backbone. They play an important role in plant grwth and development including embryo maturation, seed germination, auxin-stimulated cell division and organ senescence, and also in response to environmental stress including abiotic and biotic stresses.39
In addition PLB is present which removes two fatty acids from phospholipids and thus has both PLA and lysoPLA activities. A few reports do exist on the presence of PLB in plants but there are also reports of several proteins that possess PLB like activites, such as the purified PLA2 from broad bean40 and ricin chain from castor bean.41 The catalytic properties of these phospholipases are often controlled by their interaction with other proteins like G-proteins, by phosphorylation, interaction with lipids and also activation by calcium.42 Recently, the role of sphingolipids in plants has also been suggested. However its role, other than in ABA responses need to be further investigated.51
PLC.
The hydrolysis of phosphoinositide bisphosphate (PIP2) by specific PLC is one of the earliest key events by which more than 100 extracellular signaling molecules are known to regulate functions of their target cells in animal systems.39 The products of the PLC reaction are the two second intracellular messengers, IP3 and DAG. The IP3 induces the release of Ca2+ from internal stores and DAG activates protein kinase C. PLCs have been classified into three subfamilies, designated γ (gamma), β (beta) and δ (delta) on the basis of size and amino acid sequences.39 Members of the beta subfamily are activated by heterotrimeric G-proteins and members of the gamma subfamily are activated by protein tyrosine kinase-linked receptors. The mechanism of activation of the delta subfamily members still not well known. Despite differences in their regulation, PLCs of all three subfamilies have similar catalytic properties. They are specific for phosphoinositides, hydrolysing PI, PIP, and PIP2 but not the 3-phosphate-containing phosphoinositides.43,44 They are dependent on Ca2+ for activity and their substrate specificities are controlled by Ca2+ levels.
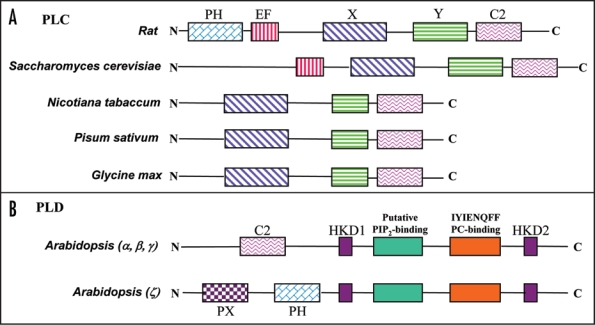
There are different families of PLC in animal system; PLC beta, PLC gamma and PLC delta. All of these have pleckstrin homology PH) domain, EF hand domain, X and Y catalytic domain and C2 domain (protein kinase C-conserved 2 domain). In PLC gamma there are SH2 and SH3 domains between X and Y region. The beta form has an extended C-terminal region. The delta is the shortest form In plants the first cDNA clone encoding delta phosphoinositol-specific PLC (PI-PLC) was isolated from soybean and was localized in cytoplasm and plasma membranes.45 It was found that there are different isoforms in plants and three isoforms of PI-PLC were cloned from potato leaves.46 The isolation and characterization of PI-PLC from tobacco and pea was done and shown to be regulated by light in a tissue specific manner.47 Cloning of (PI-PLC) cDNA from a number of plant species revealed that they have only the delta form of PLC. When compared, to other PLC's plant PLCs show both X and Y domains, and the C2 domain, however PH domain is absent in the plant PI-PLC delta (Fig. 1A). The X and the Y domain are the catalytic regions, which are separated by variable length of amino acids (in different organisms), however they come together in a 3-D structure The C2 domain in plant PLC delta was also shown to bind calcium.47 Recently, Echevarría-Machado et al (2007)48 reported a partial purification of membrane-associated PLC. They found that there are at least two forms (57 and 67 kDa in sizes) of membrane associated PLC in transformed roots of Catharanthus roseus, which are differentially regulated during transformed root growth.48
Figure 1.
Domain organization of different PI-specific PLC and PLD. (A) Comparison of the domain structures of plant PLCs with the protein from other sources. It shows that plant PLC delta (the only form reported in plants) does not have PH and EF hand domains. (B) The structures of Arabidopsis PLD (α, β, γ and ζs).
The involvement of PLC in stress signaling has been indicated in a number of studies. The genes encoding PI-PLC were found to be induced to a significant extent under environmental stresses. A role of PI-PLC in the generation of ABA-induced oscillations in [Ca2+]cyt has been suggested. PI-PLC also plays an important role in the gibberellin-induced expression of α-amylase molecules closely related to the germination processes of rice and in secondary ABA responses. Liu et al., (2006a and b)49,50 reported that PIP2-PLC plays a key role in free salicylic acid and ABA-associated thermotolerance resulting from heat acclimation. Since calcium acts as a molecular switch to trigger downstream signaling events and the proline accumulation occurs in various plants in response to environmental stresses (especially drought), hence the calcium signaling via PI-PLC was reported to be essential for proline accumulation upon ionic but not nonionic hyperosmotic stresses in Arabidopsis.51 These results also demonstrated the specific involvement of lipid signaling pathway to discriminate between ionic and nonionic stresses.
Role of PLC in Avr-induced disease resistance has also been implicated. In this case PLC/diacylglycerol kinase (PLC/DGK) mediated production of PA was found to be involved in disease resistance signaling.52 Recently, Chen et al (2007)53 have shown that the PLC/diacylglycerol kinase (PLC/DGK)-mediated signalling plays an important role in benzothiadiazole-induced oxidative burst, hyperensitive response, and activation of defence response in rice. The involvement of PLC/DAG in PA formation was also shown under aluminium stress in Coffea arabica suspension cells.54 Dowd et al. (2006)55 and Helling et al (2006)56 reported that Petunia phospholipase C1 is also involved in pollen tube growth.
PLD.
PLD catalyzes the hydrolysis of phospholipids, at their terminal phosphodiester bond to phosphatidic acid (PA) and a free polar head group such as choline or inositol. The activation of PLD in the cell generates signaling messengers and is involved in a wide range of cellular processes, including stress and defense response, meiosis, phytochrome action, membrane metabolism and vesicular trafficking.4,57 The activity of PLD is highly regulated and its cellular regulation is often coordinated with the networks of other cellular signaling machinery.
The PA is a second messenger molecule, which is involved in many fundamental cellular processes. PLD and its product PA both are involved in a number of signalling pathways regulating cell proliferation, membrane vesicle trafficking and defence responses in eukaryotic cells. Potocký et al. (2003)58 had reported that PLD and PA have also involved in the process of polarised plant cell expansion as represented by pollen tube growth.
Animal PLD activity is known to be stimulated by a large number of cell surface receptors and is elaborately regulated by intracellular factors, including protein kinase C isoforms, small GTPases of the ARF, Rho and Ras families and, particularly, by the phosphoinositide, PIP2. The PIP2 acts as substrate for the generation of second messengers by PLC and also recruit and/or activate a variety of actin regulatory proteins, ion channels and other signaling proteins, including PLD, by direct interaction. The synthesis of PIP2 by phosphoinositide 5-kinase (PIP5K) isoforms is known to be regulated by small GTPases and PA. Recently, Oude Weernink et al (2007)59 have described the regulation of PLD by membrane receptors and also suggested that the close encounter of PLD and PIP5K isoforms with small GTPases permits the execution of specific cellular functions. PLD has been identified and purified from many plants.
In higher plants, the PLD encoding genes constitute a large gene family. The cDNA for PLD has been isolated from many plant species, such as castor bean, Arabidopsis, cabbage, tobacco, rice and maize.39 There are at least 12 members of the PLD family in Arabidopsis thaliana15,60 and 17 PLD members found in different chromosomes have been identified in rice.57 All PLDs cloned from plants require Ca2+ for activity. Each of the C2-PLDs is inferred to have a Ca2+/phospho-lipid-binding C2 domain of ∼130 amino acid residues near its N-terminal. Two animal-like Arabidopsis PLDs (PLDζ1 and ζ2) do not have C2 domain, but are equipped with Phox hology (PX) domain (Fig. 1B).
The PLD in plants have been shown to have different domains (Fig. 1B). The most conserved domain is the HKD motif (HxKxxxxD/E) that is present two times. They are away from each other and form the active site. Between these motifs are the PIP2-binding site and a PC-binding site (IYIENQFF motif ). The N-terminus has the C2 domain for calcium binding which is unique to plant PLDs. In some PLD (PLDζ), one finds the presence of PX (conserved pro-rich motif) and also PH domain (Fig. 1B) (see ref. 61). The PA generated by PLD can function as a second messenger or can also be further metabolized by phosphatidate phosphatase (PP) to form DAG, which is an activator of PKC in signal transduction pathways. Though there are some reports on the presence of PKC type activity in plants, the genes encoding this and its role in signaling has not yet been established.62 It seems therefore that PA may be playing a major role for signal transduction in plant systems. Role of PLD has been studied under different stress conditions (see refs. 63–65). It was found that under phosphate limitation stress presence of some forms of PLD were essential for root growth.66 The activation of PLD in wounding of leaves and also a key participation in organ senescence-associated cellular changes in higher plants has been shown.4 Transgenic studies have shown that the suppressed expression of rice PLDβ1 results in reduced sensitivity to exogenous ABA during seed germination.57
By studying its expression under drought stress and also comparing the transcript of this gene in sensitive and tolerant cultivars, it was suggested that PLD may be involved in drought sensitivity and tolerance responses.67 Rajashekar et al (2006)68 have reported that freezing tolerance in Arabidopsis can be induced by suppression of PLDα1. Recently, grape berry PLD has been cloned and found to be involved in the heat response in post-harvest grape berry.69 Early PLDα-dependent transcripts were identified in Arabidopsis that were exposed to water stress which further emphasized role of PLD in drought stress condtions.70
PLDzeta2 from Arabidopsis thaliana has been isolated and reported that it regulates vesicle trafficking and is required for auxin response.71 Devaiah et al. (2007)72 reported that seed quality and viability is enhanced by suppressing PLDα1 in Arabidopsis, suggesting its role in seed deterioration and aging. Therefore, a high level of PLDα1 is detrimental to seed quality, and attenuation of PLDα1 expression has the potential to improve oil stability, seed quality and seed longevity.
Interactions Between G-Proteins, PLD and PLC
The mechanism by which G-proteins pass on the message to downstream elements is not very clear in plant systems. Attempts have been made to identify proteins that are coupled to heterotrimeric G-proteins. In a review by Assmann (2002)73 it was mentioned that phospholipases might be regulated by G-proteins. Although from the study on Brassica napus it was inferred that there is no direct interaction of PLDα with G-protein in in vitro conditions,74 yet at present one of the well characterized effector of Gα is PLD. It was suggested long back that G-protein activation also stimulated PLD signaling.75 It was shown that activation of PLD by ABA was dependent on GTP and inhibitor (like pertussis toxin), that effect G-proteins, blocked PLD activation. These results indicated a role of G-proteins in stimulation of PLD activity.76 In fact, Lein and Saalbach (2001)77 provided the first indication for a direct regulation of PLDα1 by a heterotrimeric G-protein alpha-subunit in tobacco plant. In one of the detailed studies by Zhao and Wang (2004)78 it was found that PLDα1 in fact interacts with GTPα subunit. Using mutated protein and immunoprecipitation techniques, it was found that DRY motif on PLDα1 was the site of interaction and it preferred GDP state of Gα. In fact the GTP state was found to be inhibitory for interaction. It was also found that PLDα1 interaction with Gα stimulates intrinsic GTPase activity of Gα (see refs. 64). The physiological significance of this interaction has been recently indiacted in stress conditions. It is well known that under drought stress plants tend to accumulate ABA, which controls water loss by regulating stomata opening and closing. Under ABA stress, PA and PLDα1 seem to interact with Gα subunit to mediate ABA inhibition of stomatal opening.79
In an earlier study it was found that regulation of DNA synthesis was mediated by the activation of GPCR, leading to Gα activation, which in turn affects PLC activity80 however, in this study no direct interaction was shown. Recently, we have reported that pea Gα and PLCδ proteins interact with each others, moreover, this interaction was further mapped to the carboxy-terminal domain (C2 domain) of pea PLCδ.25 However, in the animal system, the interaction between Gαq and PLCβ has been reported and has been mapped to its carboxyl terminus.81 We also found that pea PLCδ protein stimulates the GTPase activity of pea Gα. This clearly suggested that PLCδ is one of the effector molecules of the Gα subunit. In the animal system, it has been reported that PLCβ has the ability to activate intrinsic GTPase activity of Gαq. In plant, only the PLCδ isoform has been cloned, which stimulates Gα GTPase activity and thereby suggests a cross-talk between them. Whether this interaction has any effect on the activity of PLC still needs to be investigated.25
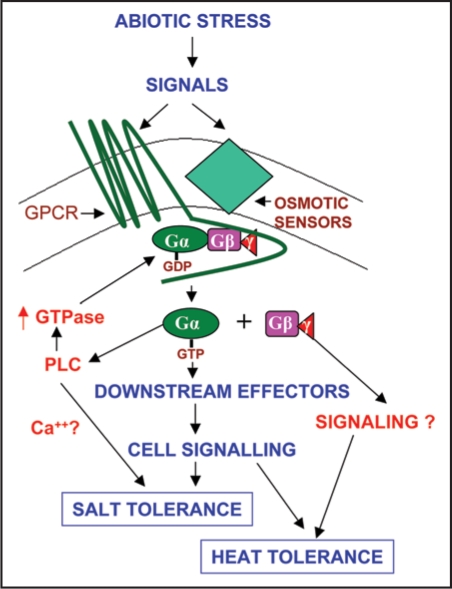
Besides signaling being transduced via Gα, our studies with development of transgenic plants overexpressing the Gα or Gβ subunits and their differential response to salinity and heat stress indicated that Gα mediated pathway is responsible for conferring salinity and high temperature stress whereas the pathways triggered by Gβ lead to heat tolerance.25 On the basis of these results, we propose a model (Fig. 2) depicting the role of G proteins in providing abiotic stress tolerance. Abiotic stress generates signals that are perceived by either GPCR or osmotic sensors present in the cell membrane and this leads to activation of G protein (i.e., dissociation of Gα and Gβγ dimer) and hence regulating downstream effectors. It is possible that Gα mediated signaling involves activation/ modulation of some down stream effectors conferring salinity and heat tolerance.25 One of the effectors molecules is PLC, and this pathway may lead to an increase in calcium in addition to activation of other pathways (Fig. 2). The burst of calcium increase can lead to the activation of downstream calcium-dependent pathways. In fact the role of calcium in salinity stress tolerance has been elucidated via a number of mechanisms. It is possible that PLC could be involved in salinity tolerance. Thus it seems that cross talk between Gα and PLC can be an important step in transducing signals leading to salinity tolerance and also in regulating Gα mediated pathways The PLC can turn the signal off by activating the GTPase activity of Gα. The other possibility could be the production of PA via PLC-DAGK or via PLD which in turn can activate downstream components leading to stress tolerance as has been shown in other systems. Overall, the discovery of the cross-talk between Gα with PLC and PLD should make an important study for better understanding of G-proteins/PLC/PLDmediated stress signaling pathways in plants.
Figure 2.
Schematic representation of possible role of heterotrimeric G-proteins in providing salinity tolerance to plants. On basis of interactions of Gα and PLC and the role of Gα in salinity stress tolerance, the possible mechanism of salinity tolerance may operate through PLC, whereas, mechanism of heat tolerance still remains to discover.
In addition to interaction with PLC and PLD, there are reports that G-proteins may interact with other proteins also. Using yeast-two-hybrid analysis, the Arabidopsis Gα was found to interact with cupin-domain protein designated pirin (PRN1) in both GDP and GTP state.82 A proteomic profile of rice dwarf mutant, which lack Gα subunit, revealed down regulation of seven seed embryo proteins. One of these, receptor for activated C kinase (RACK) also increased in lines where Gα was constitutively overexpressed. These studies showed that RAKC is regulated by Gα.83 One of the protein involved in blue light mediated synthesis of phenylpyruvate and phenylalanine, prephenate dehydratase protein (PD1) was found to have strong interaction with GPA1 of Arabidopsis. The activated GPA1 by GPCR was found to activate PD1 thus suggesting involvement of these three components in signal transduction in light regulation of phenylalanine and subsequent metabolites derived from it.84
Cross Talk of Phospholipases with Reactive Oxygen Species (ROS)
Though not directly related to activation via G-proteins, it has been shown that cold induced activation of PLC and PLD leads to expression of different genes. This was shown by blocking PLC/PLD activation by inhibitors like U73112 and ethanol, respectively.85 In many cases it has been shown that in response to stress overproduction of ROS commences. These are detoxified by antioxidants and also by antioxidant enzymes. Hence there may be a relationship of ROS signaling to lipid signaling. It was shown earlier that depletion of PLDα in Arabidopsis decreased PA production as expected but there was also decrease in superoxide production. Addition of PA enhanced synthesis of superoxide, which suggested an important role of PLD in superoxide generation.86 Infact it has been suggested that the mechanism by which PLDδ positively contributes to stress tolerance is through signaling mehanisms that induce resistance to damage that is caused by ROS.87,88 It seems that different PLDs function differently with respect to ROS signaling. PLDδ is activated by H2O2 whereas PLDα1 and PA are involved in the production of ROS (see ref. 64 and references therein). Recently, salt stress mediated increase in ROS was found to be mediated via phospholipid-regulated signaling pathways.89 In this role of PI-kinases was shown but there was no mention for the involvement of PLC.
One of the second messenger related to stress responses is NO. It was found that NO is required for the production of lipid second messenger, PA, via activation of PLC and DAG in tomato cell cultures treated with xylanase, a fungal elicitor. PA was required for xylanase induced ROS production.90 These results indicated that PLC/diacylglycerol kinase-derived PA represents a novel downstream component of NO signaling cascade during plant defense. This shows cross talk between two different signaling pathways during plant defense.
Conclusions and Perspectives
Plants are constantly exposed with many biotic and abiotic environmental stresses, which cause considerable losses in crop yields worldwide, while the demand for food and energy is on the rise.91,92 Recently, Walley et al. (2007)93 beautifully described that mechanical wounding stress also induces both the biotic and abiotic stress responses via a novel cis-element. The mechanisms by which plant cells perceive the signals and transduce these into the cells are the central focus of plant bioligists. In general, the plant do not follow exact same signaling cascades described in nonplant systems, but contain several unique components and unique unexpected cross-talk among signaling components. With the publication of the complete sequence of Arabidopsis genome and its analysis, it seems that plants encode a very large number of proteins that may be involved in signal processing. This may be because of the fact that plants are sessile and therefore have to very effectively perceive the environmental variables for their growth, development and adaptation. And also since their survival strategy is of a defensive nature and not an offensive one. To meet these demands they have on one hand retained some of the components of bacterial signal pathway, as for example the two-component receptors for hormones, and on the other hand have some components of higher eukaryotic animal systems.
Since Gα, PLC and PLD are involved in stress tolerance, it seems that cross talk between these can be an important step in transducing signals leading to stress tolerance and also in regulating Gα mediated pathways The downstream elements of this pathway that are finally regualting gene expression leading to the production of specific metabolites required for tolerance have not been elucidated. Also the mechnism by which the Gα signaling is turned off is not clear. Our preliminary work suggests that the PLC can turn the signal off by activating the GTPase activity of Gα therby regulating the function of G-protein. The recent results on the role of Gβ in stress tolerance need further work to find out its downstream interacting partners. The oucome of this research will lead to a better understanding of stress signaling pathways and will also be usefull for its application in agricultural biotechnology.
Acknowledgements
The authors thank Dr. Renu Tuteja for critical reading and corrections on the review article and Mr. Hung Dang Quang and Dr. Shikha Misra for their help in preparation of the illustrations. This work was partially supported by the grants from the Department of Science and Technology and Department of Biotechnology, Government of India. We apologize if some references could not be cited due to space constraint.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/5303
References
- 1.Gilroy S, Trewavas A. Signal processing and Transduction in plant cell: The end of the beginning. Nature Rev Mol Cell Biol. 2001;2:307–314. doi: 10.1038/35067109. [DOI] [PubMed] [Google Scholar]
- 2.Mahajan S, Tuteja N. Cold, salinity and drought stresses: An overview. Arch Biochem Biophys. 2005;444:139–158. doi: 10.1016/j.abb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Dangl JL, Preuss D, Schroeder JI. Talking through walls: Signaling in plant development. Cell. 1995;83:1071–1077. doi: 10.1016/0092-8674(95)90134-5. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Ullah H, Jones AM, Assmann SM. G protein regulation of ion channel and abscisic acid signaling in Arabidopsis guard cells. Science. 2001;15:2070–2072. doi: 10.1126/science.1059046. [DOI] [PubMed] [Google Scholar]
- 5.Ullah H, Chen J, Young J, Im K, Sussman M, Jones AM. Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science. 2001;292:2066–2069. doi: 10.1126/science.1059040. [DOI] [PubMed] [Google Scholar]
- 6.Latijnhouwers M, Ligterink W, Vleeshouwers VG, van West P, Govers F. A G-alpha subunit controls zoospore motility and virulence in the potato late blight pathogen Phytpphthora infestans. Mol Microbiol. 2004;51:925–936. doi: 10.1046/j.1365-2958.2003.03893.x. [DOI] [PubMed] [Google Scholar]
- 7.Warpeha KM, Lateef SS, Lapik Y, Anderson M, Lee BS, Kaufman LS. G-protein-coupled receptor 1, G-protein Galpha-subunit 1, and prephenate dehydratase 1 are required for blue light-induced production of phenylalanine in etiolated Arabidopsis. Plant Physiol. 2006;140:844–855. doi: 10.1104/pp.105.071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trusov Y, Rookes JE, Chakravorty D, Armour D, Schenk PM, Botella JR. Heterotrimeric G proteins facilitate Arabidopsis resistance to pathogens and are involved in jasmonate signaling. Plant Physiology. 2006;140:210–220. doi: 10.1104/pp.105.069625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamm H. The many faces of G-protein signaling. J Biol Chem. 1966;273:669–672. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- 10.Jones AM, Assmann SM. Plants: The latest model system for G-protein research. EMBO report. 2004;5:572–578. doi: 10.1038/sj.embor.7400174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma H. Plant G-proteins: The different faces of GPA1. Currr Biol. 2001;11:R869–R871. doi: 10.1016/s0960-9822(01)00519-x. [DOI] [PubMed] [Google Scholar]
- 12.Temple BRS, Jones AM. The plant heterotrimeric G-protein complex. Ann Rev Pl Biol. 2007;58:249–266. doi: 10.1146/annurev.arplant.58.032806.103827. [DOI] [PubMed] [Google Scholar]
- 13.Ma H, Yanofsky MF, Meyerowitz EM. Molecular cloning and characterization of GPA1, a G protein α subunit gene from Arabidopsis thaliana. Proc Natl Acad Sci USA. 1990;87:3821–3825. doi: 10.1073/pnas.87.10.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss CA, Garnaat CW, Mukai K, Huang H, Ma H. Isolation of cDNA encoding guanine nucleotide-binding protein β-subunit homologues from maize (ZGB1) and Arabidopsis (AGB1) Proc Natl Acad Sci USA. 1994;91:9554–9558. doi: 10.1073/pnas.91.20.9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arabidopsis Genome Initiative, author. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- 16.Mason MG, Botella JR. Isolation of a novel G-protein γ-subunit from Arabidopsis thaliana and its interaction with Gβ. Biochim Biophys Acta. 2001;1520:147–153. doi: 10.1016/s0167-4781(01)00262-7. [DOI] [PubMed] [Google Scholar]
- 17.Marsh JF, Kaufman LS. Cloning and characterization of PGA1 and PGA2: Two G protein α-subunits from pea that promote growth in the yeast Saccharomyces cerevisiae. Plant J. 1999;19:237–247. doi: 10.1046/j.1365-313x.1999.00516.x. [DOI] [PubMed] [Google Scholar]
- 18.Adjobo-Hermans MJW, Goedhart J, Gadella TWJ. Plant G-protein heterotrimers require dual lipidation motifs of Ga and Gv and do not dissociate upon activation. J Cell Sci. 2006;119:5087–5097. doi: 10.1242/jcs.03284. [DOI] [PubMed] [Google Scholar]
- 19.Kato C, Mizutani T, Tamaki H, Kumagai H, Kamiya T, Hirobe A, Fujisawa Y, Kato H, Iwasaki Y. Characterzation of heterotrimeric G-protein complexes in rice plasma membrane. Plan J. 2004;38:320–331. doi: 10.1111/j.1365-313X.2004.02046.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen JG, Willard FS, Huang J, Liang J, Chasse S, Jones AM, Siderovsky DP. A seven-transmembrane RGS protein that modulates plant cell proliferation. Science. 2003;301:1728–1731. doi: 10.1126/science.1087790. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Ji F, Xie H, Liang J, Zhang J. The regulator of G-protein signaling proteins involved in sugar and abscisic acid signaling in Arabidopsis seed germination. Plant Physiol. 2006;140:302–310. doi: 10.1104/pp.105.069872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanan N, Sopory SK. A role of G-proteins and calcium in light regulated primary leaf formation in Sorghum bicolor. J Expt Bot. 1998;49:1695–1703. [Google Scholar]
- 23.RaghuRam N, Chandok MR, Sopory SK. Light regulation of nitrate reductase gene expression in maize involves G-protein. Mol Cell Biochem Res Commun. 1999;2:86–90. doi: 10.1006/mcbr.1999.0154. [DOI] [PubMed] [Google Scholar]
- 24.Asakura Y, Kurosaki F. Cloning and expression of Dcga encoding alpha subunit of GTP binding protein in carrot seedlings. Biol Pharm Bull. 2007;30:1800–1804. doi: 10.1248/bpb.30.1800. [DOI] [PubMed] [Google Scholar]
- 25.Misra S, Wu Y, Venkataraman G, Sopory S, Tuteja N. Heterotrimeric G-protein complex and G-protein-coupled receptor from a legume (Pisum sativum): Role in salinity and heat stress and cross-talk with Phospholipase C. Plant J. 2007;51:656–669. doi: 10.1111/j.1365-313X.2007.03169.x. [DOI] [PubMed] [Google Scholar]
- 26.Plakidou-Dymock S, Dymock D, Hooley R. A higher plant seven-transmembrane receptor that influences sensitivity to cytokinins. Curr Biol. 1997;8:315–324. doi: 10.1016/s0960-9822(98)70131-9. [DOI] [PubMed] [Google Scholar]
- 27.Colucci G, Apone F, Alyeshmerni N, Chalmers D, Chrispeels MJ. GCR1, the putative Arabidopsis G protein-coupled receptor gene is cell cycle-regulated, and its overexpression abolishes seed dormancy and shortens time to flowering. Proc Natl Acad Sci USA. 2002;96:7575–7580. doi: 10.1073/pnas.072087699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandey S, Assmann SA. The Arabidopsis putative G protein-coupled receptor GCR1 interacts with the G protein α subunit GPA1 and regulates abscisic acid signaling. Plant Cell. 2004;16:1616–1632. doi: 10.1105/tpc.020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moriyama EN, Strope PK, Opiya SO, Chen Z, Jones AM. Mining the Arabidopsis thaliana genome for highly-divergent seven transmembrane receptors. Genome Biol. 2006;7:R96. doi: 10.1186/gb-2006-7-10-r96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolakowski LF., Jr “GCRDb: A G-protein-coupled receptor database”. Receptors Channels. 1994;2:1–7. [PubMed] [Google Scholar]
- 31.Vohra S, Chintapalli SV, Illingworth CJ, Reeves PJ, Mullineaux PM, Clark HS, Dean MK, Upton GJ, Reynolds CA. Computational studies of family A and family B GPCRs. Biochem Soc Trans. 2007;35:749–754. doi: 10.1042/BST0350749. [DOI] [PubMed] [Google Scholar]
- 32.Gao Y, Zeng Q, Guo J, Cheng J, Ellis BE, Chen JG. Genetic characterization reveals no role for the reported ABA receptor, GCR2, in ABA control of seed germination and early seedling development in Arabidopsis. Plant J. 2007;52:1001–1013. doi: 10.1111/j.1365-313X.2007.03291.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Yue Y, Li B, Nie Y, Li W, Wu WH, Ma L. A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid. Science. 2007;315:712–716. doi: 10.1126/science.1135882. [DOI] [PubMed] [Google Scholar]
- 34.Bakthavatsalam D, Brazill D, Gomer RH, Eichinger L, Rivero F, Noegel AA. A G protein-coupled receptor with a lipid kinase domain is involved in cell-density sensing. Curr Biol. 2007;17:892–897. doi: 10.1016/j.cub.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 35.Oude Weernink PA, Han L, Jakobs KH, Schmidt M. Dynamic phospholipid signaling by G protein-coupled receptors. Biochim Biophys Acta. 2007;1768:888–900. doi: 10.1016/j.bbamem.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Redhead CR, Palme K. The genes of plant signal transduction. Crit Rev Plant Sci. 1996;15:425–454. [Google Scholar]
- 37.Trewavas AJ, Malho R. Signal perception and Transduction: The origin of the phenotype. Plant Cell. 1997;9:1181–1195. doi: 10.1105/tpc.9.7.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urao T, Yamaguchi-Shinozaki K, Shinozaki K. Two component systems in plant signal transduction. Trends in Plant Sci. 2000;5:67–74. doi: 10.1016/s1360-1385(99)01542-3. [DOI] [PubMed] [Google Scholar]
- 39.Chapman KD. Phospholipase activity during plant growth and development and in response to environmental stress. Trends Plant Sci. 1998;3:419–426. [Google Scholar]
- 40.Jung KM, Kim DK. Purification and characterization of a membrane-associated 48-kilodalton phospholipase A(2) in leaves of broad bean. Plant Physiol. 2000;123:1057–1067. doi: 10.1104/pp.123.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darby SM, Miller ML, Allen RO. Forensic determination of ricin and the alkaloid marker ricinine from castor bean extracts. J Forensic Sci. 2001;46:1033–1042. [PubMed] [Google Scholar]
- 42.Meijer Harold JG, Munnik T. Phospholipid-based signaling in plants. Annu Rev Plant Biology. 2003;54:265–306. doi: 10.1146/annurev.arplant.54.031902.134748. [DOI] [PubMed] [Google Scholar]
- 43.Cote GG, Crian RC. Biochemistry of phosphoinositides. Annu Rev plant Physiol Plant Mol Biol. 1993;44:333–356. [Google Scholar]
- 44.Den Hartog M, Musgrave A, Munnik T. Nod factor-induced phosphatidic acid and diaclglycerol pyrophosphate formation: A role for phospholipase C and C in root hair deformation. Plant J. 2001;25:55–66. doi: 10.1046/j.1365-313x.2001.00931.x. [DOI] [PubMed] [Google Scholar]
- 45.Shi J, Gonzales RA, Bhattacharya MK. Characterization of a plasma membrane-associated phosphoinositide-specific phospholipase C from soybean. Plant J. 1995;8:190–381. doi: 10.1046/j.1365-313x.1995.08030381.x. [DOI] [PubMed] [Google Scholar]
- 46.Kopka J, Pical C, Gray JE, Muller-Rober B. Molecular and enzymatic characterization of three phosphoinositide -specific phospholipase C isoforms from potato. Plant Physiol. 1998;116:239–250. doi: 10.1104/pp.116.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venkataraman G, Goswami M, Tuteja N, Reddy MK, Sopory SK. Isolation and characterization of a phospholipase C delta isoform from tobacco and pea that is regulated by light in a tissue specific manner. Mol Gen Genetic. 2003;270:378–386. doi: 10.1007/s00438-003-0925-0. [DOI] [PubMed] [Google Scholar]
- 48.Echevarría-Machado I, Martínez-Estévez M, Muñoz-Sánchez JA, Loyola-Vargas VM, Hernández-Sotomayor SM, De Los Santos-Briones C. Membrane-associated phosphoinositides-specific phospholipase C forms from Catharanthus roseus transformed roots. Mol Biotechnol. 2007;35:297–309. doi: 10.1007/BF02686015. [DOI] [PubMed] [Google Scholar]
- 49.Liu HT, Liu YY, Pan QH, Yang HR, Zhan JC, Huang WD. Novel interrelationship between salicylic acid, abscisic acid, and PIP2-specific phospholipase C in heat acclimation-induced thermotolerance in pea leaves. J Exp Bot. 2006a;57:3337–3347. doi: 10.1093/jxb/erl098. [DOI] [PubMed] [Google Scholar]
- 50.Liu HT, Huang WD, Pan QH, Weng FH, Zhan JC, Liu Y, Wan SW, Liu YY. Contributions of PIP2 specific phospholipase C and free salicylic acid to heat accumulation induced thermotolerance in pea. J Pl Physiol. 2006b;163:405–416. doi: 10.1016/j.jplph.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 51.Parre E, Ghars MA, Leprince AS, Thiery L, Lefebvre D, Bordenave M, Richard L, Mazars C, Abdelly C, Savouré A. Calcium signaling via phospholipase C is essential for proline accumulation upon ionic but not nonionic hyperosmotic stresses in Arabidopsis. Plant Physiol. 2007;144:503–512. doi: 10.1104/pp.106.095281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andersson MX, Kourtchenko O, Dangl JL, Mackey D, Elerstrom M. Phopspholipase-dependent signaling during the AvrRpm1 and AvrRpt2—Induced disease resistance response in Arabidopsis thaliana. Plant J. 2006;47:947–949. doi: 10.1111/j.1365-313X.2006.02844.x. [DOI] [PubMed] [Google Scholar]
- 53.Chen J, Zhang W, Song F, Zheng Z. Phospholipase C/diacylglycerol kinase-mediated signalling is required for benzothiadiazole-induced oxidative burst and hypersensitive cell death in rice suspension-cultured cells. Protoplasma. 2007;230:13–21. doi: 10.1007/s00709-006-0195-x. [DOI] [PubMed] [Google Scholar]
- 54.Ramos-Diaz A, Brito-Argaez L, Munnik T, Hernandez-Sotomayor SM. Aluminum inhibits phosphatidic acid formation by blocking phospholipase C pathway. Planta. 2007;225:393–401. doi: 10.1007/s00425-006-0348-3. [DOI] [PubMed] [Google Scholar]
- 55.Dowd PE, Coursol S, Skirpan AL, Kao TH, Gilroy S. Petunia phospholipase c1 is involved in pollen tube growth. Plant Cell. 2006;18:1438–1453. doi: 10.1105/tpc.106.041582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Helling D, Possart A, Cottier S, Klahre U, Kost B. Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. Plant Cell. 2006;18:3519–3534. doi: 10.1105/tpc.106.047373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li G, Lin F, Xue HW. Genome-wide analysis of the phospholipase D family in Oryza sativa and functional characterization of PLDbeta1 in seed germination. Cell Res. 2007 doi: 10.1038/cr.2007.77. [DOI] [PubMed] [Google Scholar]
- 58.Potocký M, Eliás M, Profotová B, Novotná Z, Valentová O, Zárský V. Phosphatidic acid produced by phospholipase D is required for tobacco pollen tube growth. Planta. 2003;217:122–130. doi: 10.1007/s00425-002-0965-4. [DOI] [PubMed] [Google Scholar]
- 59.Oude Weernink PA, López de Jesús M, Schmidt M. Phospholipase D signaling: Orchestration by PIP2 and small GTPases. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:399–411. doi: 10.1007/s00210-007-0131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eliás M, Potocký M, Cvrcková F, Zárský V. Molecular diversity of phospholipase D in angiosperms. BMC Genomics. 2002;3:2. doi: 10.1186/1471-2164-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qin C, Wang X. The Arabidopsis phospholipase D family. Characterization of a calcium-independent and phosphatidylcholine-selective PLD1 with distinct regulatory domains. Plant Physiol. 2002;128:1057–1068. doi: 10.1104/pp.010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chandok MR, Sopory SK. ZmcPKC 70, a protein kinase c type enzyme from maize. Biochemical characterization regulation by phorbol 12 myristate 13 acetate and its possible involvement in nitrate reductase gene expression. J Biol Chem. 1998;273:19235–19242. doi: 10.1074/jbc.273.30.19235. [DOI] [PubMed] [Google Scholar]
- 63.Wang X. Phospholipase D in hormonal and stress signaling. Curr Opin Pl Biol. 2002;5:408–414. doi: 10.1016/s1369-5266(02)00283-2. [DOI] [PubMed] [Google Scholar]
- 64.Wang X. Regulatory functions of phospholipase D and phophatidic acid in plant growth, development and stress responses. Plant Physiol. 2005;139:566–573. doi: 10.1104/pp.105.068809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bergmann BO, Munnik T. The role of phospholipase D in plant stress responses. Curr Opin Plant Biol. 2006;9:512–522. doi: 10.1016/j.pbi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 66.Li M, Qin C, Weiti R, Wang X. Double knockouts of phospholipases D1 and D2 in Arabidopsis affect roots elongation during phosphate -limited growth but do not affect root hair patterning. Pl Physiol. 2006;140:761–770. doi: 10.1104/pp.105.070995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo BZ, Xu G, Cao YG, Holbrook CC, Lynch RE. Identification and characterization of phospholipase D and its association with drought susceptibilities in peanut (Arachis hypogaea) Planta. 2006;223:512–520. doi: 10.1007/s00425-005-0112-0. [DOI] [PubMed] [Google Scholar]
- 68.Rajashekar CB, Zhou HE, Zhang Y, Li W, Wang X. Suppression of phospholipase Dalpha1 induces freezing tolerance in Arabidopsis: Response of cold-responsive genes and osmolyte accumulation. J Plant Physiol. 2006;163:916–926. doi: 10.1016/j.jplph.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 69.Wan SB, Wang W, Wen PF, Chen JY, Kong WF, Pan QH, Zhan JC, Tian L, Liu HT, Huang WD. Cloning of phospholipase D from grape berry and its expression under heat acclimation. J Biochem Mol Biol. 2007;40:595–603. doi: 10.5483/bmbrep.2007.40.4.595. [DOI] [PubMed] [Google Scholar]
- 70.Mane SP, Vasquez-Robinet C, Siosson AA, Heath LS, Grene R. Early PLD alpha -mediated events in response to progressive drought stress in Arabidopsis: A transcriptome analysis. J Exp Bot. 2007;58:152–241. doi: 10.1093/jxb/erl262. [DOI] [PubMed] [Google Scholar]
- 71.Li G, Xue HW. Arabidopsis PLDzeta2 regulates vesicle trafficking and is required for auxin response. Plant Cell. 2007;19:281–295. doi: 10.1105/tpc.106.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Devaiah SP, Pan X, Hong Y, Roth M, Welti R, Wang X. Enhancing seed quality and viability by suppressing phospholipase D in Arabidopsis. Plant J. 2007;50:950–957. doi: 10.1111/j.1365-313X.2007.03103.x. [DOI] [PubMed] [Google Scholar]
- 73.Assmann S. Hetrotrimeric and unconventional GTP binding proteins in plant cell signaling. Plant Cell. 2002;14:S355–S373. doi: 10.1105/tpc.001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Novotna Z, martinec J, Profotova B, Zdarova S, Kader JC, Valentova O. In vitro distriution and characterization of membrane associated PLD and PI-PLC in Brassica napus. J Exp Bot. 2003;54:691–698. doi: 10.1093/jxb/erg070. [DOI] [PubMed] [Google Scholar]
- 75.Munnik T, Arisz SA, Vrije TD, Musgrave A. G-Protein activation stimulates phospholipase D signaling in plants. Plant Cell. 1995;7:2197–2210. doi: 10.1105/tpc.7.12.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ritchie S, Gilroy S. Abscisic acid stimulation of phospholipase D in the barley aleurone is G-protein mediated and localized to the plasma membrane. Plant Physiol. 2000;124:693–702. doi: 10.1104/pp.124.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lein W, Saalbach G. Cloning and direct G-protein regulation of phospholipase D from tobacco. Biochim Biophys Acta. 2001;1530:172–183. doi: 10.1016/s1388-1981(00)00182-7. [DOI] [PubMed] [Google Scholar]
- 78.Zhao J, Wang X. Arabidopsis phospholipase Dα1 interacts with the hetertimeric G-protein A- subunit through a motif analogous to the DRY motif in G-protein coupled receptors. J Biol Chem. 2004;279:1794–1800. doi: 10.1074/jbc.M309529200. [DOI] [PubMed] [Google Scholar]
- 79.Mishra G, Zhang W, Deng F, Zhao J, Wang X. A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science. 2006;312:264–266. doi: 10.1126/science.1123769. [DOI] [PubMed] [Google Scholar]
- 80.Apone F, Alyeshmerni N, Wiens K, Chalmers D, chrispeels MJ, Colucci G. The G-protein coupled receptor GCR1 regulates DNA synthesis through activation of phosphatidylinositol-specific phospholipase C. Plant Physiol. 2003;133:571–579. doi: 10.1104/pp.103.026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim HY, Cote GG, Crain RC. Inositol 1,4,5-triphosphate may mediate closure of K+ channels by light and darkness in Samanea saman motor cells. Planta. 1996;198:279–287. doi: 10.1007/BF00206254. [DOI] [PubMed] [Google Scholar]
- 82.Lapik VR, Kauffman LS. The Arabidopsis cupin domain protein AtPirin1 interacts with G protein a-subunit GPA1 and regulates seed germination and early seedling development. Plant Cell. 2003;15:1578–1590. doi: 10.1105/tpc.011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Komatsu S, Abassi F, kobori E, Fujisawa Y, Katao H, Iwasaki Y. Proteomic analysis of rice embryo: An approach for investigating Ga proein regulated proteins. Proteomics. 2005;5:3932–3941. doi: 10.1002/pmic.200401237. [DOI] [PubMed] [Google Scholar]
- 84.Warpeha KM, Lateef SS, Lapik Y, Anderson M, Lee BS, Kaufman LS. G-protein coupled receptor 1, G-protein Galpha subunit 1, and prephenalate dehydratase 1 are required for blue light induced production of phenylalanine in etiolated Arabidopsis. Plant Physiol. 2006;140:844–855. doi: 10.1104/pp.105.071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vergnolle C, Vaultier MN, Tacconat L, Renou JP, Kader JC, Zachowsk M, Ruellend E. The cold induced early activation of phospholipases C and D determines the response of two distinct clusters of genes in Arabidopsis cell suspensions. Plant Physiol. 2005;139:1217–1233. doi: 10.1104/pp.105.068171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sang Y, Cui D, Wang X. Phospholipase D and phosphatidic acid-mediated generation of superoxide in Arabidopsis. Plant Physiol. 2001;126:1449–1458. doi: 10.1104/pp.126.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li W, Li M, Zhang W, Welti R, Wang X. The plasma membrane bound phospholipase D delta enhances freezing tolerance in Arabidopsis thaliana. Nature Biotechnol. 2004;22:427–433. doi: 10.1038/nbt949. [DOI] [PubMed] [Google Scholar]
- 88.Zhang W, Yu L, Zhang Y, Wang X. Phospholipase D in the signaling networks of plant response to abscisic acid and reactive oxygen species. Biochem Biophys Acta. 2005;1736:1–9. doi: 10.1016/j.bbalip.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 89.Lesham Y, Levine A. Induction of phosphatidylinositol 3-kinase -mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. Plant J. 2007;51:185–197. doi: 10.1111/j.1365-313X.2007.03134.x. [DOI] [PubMed] [Google Scholar]
- 90.Laxalt AM, Raho N, Have AT, Lamattina L. Nitric oxide is critical for inducing phosphatidic acid accumulation in xylanase-elicited tomato cells. J Biol Chem. 2007;29:21160–21168. doi: 10.1074/jbc.M701212200. [DOI] [PubMed] [Google Scholar]
- 91.Tuteja N. Mechanisms of high salinity tolerance in plants. Methods Enzymol. 2007;428:419–438. doi: 10.1016/S0076-6879(07)28024-3. [DOI] [PubMed] [Google Scholar]
- 92.Tuteja N. Abscisic acid and abiotic stress signalling. Plant Signaling Behavior. 2007;2:135–138. doi: 10.4161/psb.2.3.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walley JW, Coughlan S, Hudson ME, Covington MF, Kaspi R, et al. Mechanical stress induces biotic and abiotic stress responses via a novel cis-element. PloS Genet. 2007 doi: 10.1131/journal.pgen.0030172. [DOI] [PMC free article] [PubMed] [Google Scholar]