Abstract
Chitosan, a deacetylated chitin derivative, behaves like a general elicitor, inducing a non-host resistance and priming a systemic acquired immunity. The defence responses elicited by chitosan include rising of cytosolic H+ and Ca2+, activation of MAP-kinases, callose apposition, oxidative burst, hypersensitive response (HR), synthesis of abscissic acid (ABA), jasmonate, phytoalexins and pathogenesis related (PR) proteins. Putative receptors for chitosan are a chitosan-binding protein, recently isolated, and possibly the chitin elicitor-binding protein (CEBiP). Nevertheless, it must be pointed out that biological activity of chitosan, besides the plant model, strictly depends on its physicochemical properties (deacetylation degree, molecular weight and viscosity), and that there is a threshold for chitosan concentration able to switch the induction of a cell death programme into necrotic cell death (cytotoxicity).
Key words: chitosan, induced resistance, MAMP, PAMP, PCD, PRR, SAR
Recognition of microbe-associated molecular patterns (MAMPs), by pattern recognition receptors (PRRs), represents the major trait of innate immunity common to plants and animals. In plant immunity, MAMPs, more commonly known as general elicitors, include lipopolysaccharides (LPS), peptidoglycans, flagellin and fungal cell wall fragments (chitin/chitosan oligomers), phospholipids, oxylipins, fatty acids, sterols, proteins, double stranded RNA and methylated DNA, able to elicit a host defence response by binding to specific PRRs. In this view, chitosan, a deacetylated chitin derivative, behaves like a general elicitor, inducing a non-host resistance, by a PRR-mediated recognition, and priming a systemic acquired immunity (or systemic acquired resistance, SAR).1 The defence responses elicited by chitosan include: raising of cytosolic Ca2+, activation of MAP-kinases, callose apposition, oxidative burst, hypersensitive response (HR), synthesis of abscissic acid (ABA), jasmonate, phytoalexins and pathogenesis related proteins (PR) (Fig. 1 and Table 1).2–20
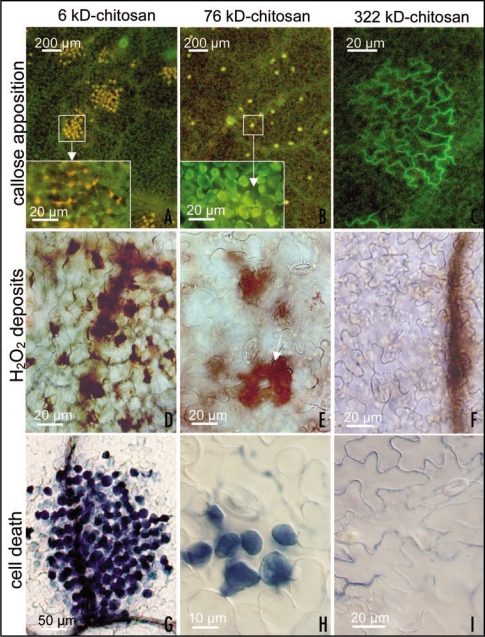
Figure 1.
Different responses induced on Phaseolus vulgaris leaves by treatment with solutions of chitosan with 85% deacetylation degree and different molecular weights; all solutions have been prepared at 0.15% w/v in 0.05 M acetic acid and adjusted at pH 5.6. Callose detection with aniline blue (A–C) 12 h after treatment shows that 76 kD-chitosan elicits the formation of a network of small bright yellow fluorescent spots (B) due to callose apposition between the plasmalemma and the cell wall of some mesophyll cells (see the enlargement in the inset), while 6 kD-chitosan induces lesions involving numerous cells fluorescing in yellow-orange, possibly as consequence of the overlap of phenolics autofluorescence and callose fluorescence (see the enlargement in the inset). In 322 kD chitosan-treated leaves numerous green-fluorescent patches (C), due to chitosan deposits, are present on the leaf epidermis along cell walls, but rarely callose apposition is present. Detection of H2O2 deposits (as brownish precipitates in D–F) with 3-3′-diaminobenzydine (DAB), 24 h after 6 kD-chitosan treatment, indicates that the lesions in (A) are constituted by necrotizing cells as a consequence of extensive H2O2 deposition (D). At the same time, leaves treated with 76 kD-chitosan show moderate H2O2 deposits limited to the same mesophyll cells involved in callose apposition, and often localized around the substomatal cavity into which chitosan can permeate (E, arrow). No H2O2 deposition is present in 322 kD-chitosan treated plants (F). Evans blue staining to detect dead cells (stained in blue) 24 h after treatment (G–I) shows that the lesions in (A) have already evolved in extensive necrotic cell death, while only some of the cells with callose deposition visible in (B) had turned to programmed cell death (H) (as previously shown with other techniques). No dead cells are present in leaves treated with 322 kD-chitosan (I).
Table 1.
Defence responses elicited by chitosan
| Plant responses | Ref. |
| Calcium transient | 3,11 |
| Plasma membrane H+-ATPase inhibition | 21 |
| MAP-kinase activation | 12,15 |
| Callose apposition | 4,16,18 |
| Reactive oxygen species | 12,13,14,20 |
| Hypersensitive response/ | |
| Programmed cell death | 11,14,20 |
| Abscisic acid | 19 |
| Jasmonate | 6 |
| Phytoalexins | 5,10,17 |
| Pathogenesis related proteins | 2,8,9,13,15 |
| Systemic acquired resistance | 14,18 |
Recently, in their work entitled ‘Early events induced by chitosan on plant cells’, Amborabé and colleagues21 provided a novel and original insight on the early processes elicited by chitosan in plant. They showed that the effect of chitosan on the plasma membrane H+-ATPase activity occurred at least 30 min after treatment, i.e., earlier than other events triggered by chitosan and mentioned above (callose, oxidative burst, HR, phytoalexins, PR proteins). However, the references provided by the authors are somewhat incomplete and, according to our opinion, they did not consider some important topics related to chitosan-induced resistance in plant.
In their discussion, they hypothesized on the presence of a putative receptor for chitosan, without taking into account the work of Chen and Xu22 on the isolation of a chitosan-binding protein, possibly a receptor. They also did not consider that chitosan induces the expression of a receptor-like kinase (RLKs) gene15 and the activation of MAP-kinase pathway in different plant species.12–15 Moreover, a plasma membrane receptor for chitin has been identified in rice cells, both at gene and protein level.23 The mature chitin elicitor-binding protein (CEBiP) structurally differs from the two major classes of PRRs, the receptor-like proteins (RLPs) and the RLKs, both groups containing extracellular leucine-reach reapeats (LRRs). CEBiP has a transmembrane domain at the C terminus, but lacks of LRRs and intracellular kinase domains normally present in RLKs, like the receptor FLAGELLIN SENSITIVE 2 (FLS2). Two lysine motifs (LysM) are present in the extracellular portion of CEBiP, involved in chitin perception. It is supposed that receptors with extracellular LysM motifs are responsible for chitin sensing, such as Nod-factor receptor kinases (NFR1 and NFR2), involved in the symbiotic signaling between leguminous plants and arbuscular mycorrhiza fungi or rhizobial bacteria in root nodule formation.23,24 Therefore, it is possible that chitosan recognition also occurs by a putative chitosan-binding protein with extracellular LysM domains, the latter playing a key role in chitin recognition. Interestingly, knockdown of CEBiP gene by RNA interference resulted in the suppression of the chitin-induced defence response, whereas treatment with LPS did not affect ROS generation in CEBiP-RNAi cell lines.23
Amborabé and colleagues,21 while discussing the chitosan-induced cytotoxicity, did not consider that this elicitor, depending both on its concentration and its physiochemical properties (deacetylation degree, molecular weight and viscosity),5,16,25 can activate a HR, i.e., a programmed cell death (PCD) phenomenon at the onset of the SAR. In other words, it exists a threshold concentration, for each chitosan type, able to switch PCD into necrotic cell death (cytotoxicity), that should be evaluated for each considered plant model.11,14,20 In this view, it is of fundamental importance, when using chitosan as elicitor, to assess and report its physiochemical properties, as well as to consider that the type of acid solvent may be determinant for the biological activity.14
Finally, as well as for other elicitors, the concentration and physicochemical properties of chitosan employed in field experiments on plant induced resistance are decisive in determining the induction of priming (the capacity for augmented defence expression in plant after pathogen challenge) or the activation of plant direct defences, the latter a less effective defence strategy and more costly in term of plant fitness.26,27
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7408
References
- 1.Iriti M, Faoro F. Review of innate and specific immunity in plants and animals. Mycopathologia. 2007;164:57–64. doi: 10.1007/s11046-007-9026-7. [DOI] [PubMed] [Google Scholar]
- 2.Walker-Simmons M, Jin D, West CA, Hadwiger L, Ryan CA. Comparison of proteinase inhibitor-inducing activities and phytoalexin elicitor activities of a pure fungal endopolygalacturonase, pectic fragments and chitosans. Plant Physiol. 1984;76:833–836. doi: 10.1104/pp.76.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kauss H. Callose biosynthesis as a Ca++-regulated process and possible relations to the induction of other metabolic changes. J Cell Sci. 1985;2:89–103. doi: 10.1242/jcs.1985.supplement_2.5. [DOI] [PubMed] [Google Scholar]
- 4.Conrath U, Domard A, Kauss H. Chitosan-elicited synthesis of callose and of coumarin derivatives in parsley cell suspension cultures. Plant Cell Rep. 1989;8:152–155. doi: 10.1007/BF00716829. [DOI] [PubMed] [Google Scholar]
- 5.Hadwiger LA, Ogawa T, Kuyama H. Chitosan polymer sizes effective in inducing phytoalexin accumulation and fungal suppression are verified with synthesized oligomers. Mol Plant Microbe Interact. 1994;7:531–533. doi: 10.1094/mpmi-7-0531. [DOI] [PubMed] [Google Scholar]
- 6.Doares SH, Syrovets T, Weiler EW, Ryan CA. Oligogalacturonides and chitosan activate plant defence genes through the octadecanoic pathway. PNAS. 1995;92:4095–4098. doi: 10.1073/pnas.92.10.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadwiger LA. Host-parasite interactions: elicitation of defence responses in plants with chitosan. EXS. 1999;87:185–200. doi: 10.1007/978-3-0348-8757-1_13. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal GK, Rakwal R, Tamogami S, Yonekura M, Kubo A, Saji H. Chitosan activates defence/stress response(s) in the leaves of Oryza sativa seedlings. Plant Physiol Biochem. 2002;40:1061–1069. [Google Scholar]
- 9.Romanazzi G, Nigro F, Ippolito A, Di Venere D, Salerno M. Effects of pre-and post-harvest chitosan treatments to control storage grey mold of table grapes. J Food Sci. 2002;67:1862–1867. [Google Scholar]
- 10.Khan W, Prithiviraj B, Smith DL. Chitosan and chitin oligomers increase phenylalanine ammonia-lyase and tyrosine ammonia-lyase activities in soybean leaves. J Plant Physiol. 2003;160:859–863. doi: 10.1078/0176-1617-00905. [DOI] [PubMed] [Google Scholar]
- 11.Zuppini A, Baldan B, Millioni R, Favaron F, Navazio L, Mariani P. Chitosan induces Ca2+ mediated programmed cell death in soybean cells. New Phytol. 2003;161:557–568. doi: 10.1046/j.1469-8137.2003.00969.x. [DOI] [PubMed] [Google Scholar]
- 12.Hu X, Neill SJ, Fang J, Cai W, Tang Z. Mitogen-activated protein kinases mediate the oxidative burst and saponin synthesis induced by chitosan in cell cultures of Panax ginseng. Sci China C life Sci. 2004;47:303–312. doi: 10.1360/03yc0074. [DOI] [PubMed] [Google Scholar]
- 13.Lin W, Hu X, Zhang W, Rogers WJ, Cai W. Hydrogen peroxide mediates defence responses induced by chitosans of different molecular weights in rice. J Plant Physiol. 2005;162:937–944. doi: 10.1016/j.jplph.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Iriti M, Sironi M, Gomarasca S, Casazza AP, Soave C, Faoro F. Cell death-mediated antiviral effect of chitosan in tabacco. Plant Physiol Biochem. 2006;44:893–900. doi: 10.1016/j.plaphy.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Lizama-Uc G, Estrada-Mota IA, Caamal-Chan MG, Souza-Perera R, Oropeza-Salìn C, Islas-Flores I, Zuñiga-Aguillar JJ. Chitosan activates a MAP-kinase pathway and modifies abundance of defence-related transcripts in calli of Cocus nucifera L. Physiol Mol Plant Pathol. 2007;70:130–141. [Google Scholar]
- 16.Faoro F, Iriti M. Callose synthesis as a tool to screen chitosan efficacy in inducing plant resistance to pathogens. Caryologia. 2007;60:121–124. [Google Scholar]
- 17.Chakraborty M, Karun A, Mitra A. Accumulation of phenilpropanoid derivatives in chitosan-induced cell suspension culture of Cocos nucifera. J Plant Physiol. 2008 doi: 10.1016/j.jplph2008.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Faoro F, Maffi D, Cantu D, Iriti M. Chemical-induced resistance against powdery mildew in barley: the effects of chitosan and benzothiadiazole. Biocontrol. 2008;53:387–401. [Google Scholar]
- 19.Iriti M, Faoro F. Abscisic acid mediates the chitosan-induced resistance in plant against viral disease. Plant Physiol Biochem. 2008;46:1106–1111. doi: 10.1016/j.plaphy.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Li S, Zhao X, Du Y, Lin B. Oligochitosan induces cell death and hydrogen peroxide accumulation in tobacco suspension cells. Pestic Biochem Physiol. 2008;90:106–113. [Google Scholar]
- 21.Amborabé B-E, Bonmort J, Fleurat-Lessard P, Roblin G. Early events induced by chitosan on plant cells. J Exp Bot. 2008;59:2317–2324. doi: 10.1093/jxb/ern096. [DOI] [PubMed] [Google Scholar]
- 22.Chen H-P, Xu LL. Isolation and characterization of a novel chitosan-binding protein from non-heading Chinese cabbage leaves. J Integ Plant Biol. 2005;47:452–456. [Google Scholar]
- 23.Kaku H, Nishizawa Y, Ishii-Minami N, Akimoto-Tomiyama C, Dohmae N, Takio K, Minami E, Shibuya N. Plant cells recognize chitin fragments for defence signalling through a plasma membrane receptos. Proc Natl Acad Sci USA. 2006;103:11086–11091. doi: 10.1073/pnas.0508882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knogge W, Scheel D. LysM receptors recognize friend and foe. Proc Natl Acad Sci USA. 2006;103:10829–10830. doi: 10.1073/pnas.0604601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabrera JC, Messiaen J, Cambier P, Van Cutsem P. Size, acetylation and concentration of chitooligosaccharide elicitors determine the switch from defence involving PAL activation to cell death and water peroxide production in arabidopsis cell suspensions. Physiol Plant. 2006;127:44–56. [Google Scholar]
- 26.Iriti M, Faoro F. Does benzothiadiazole-induced resistance increase fitness cost in bean? J Plant Pathol. 2003;85:265–270. [Google Scholar]
- 27.Van Hulten M, Pelser M, van Loon LC, Pieterse CMJ, Ton J. Costs and benefits of priming for defence in Arabidopsis. Proc Natl Acad Sci USA. 2006;103:5602–5607. doi: 10.1073/pnas.0510213103. [DOI] [PMC free article] [PubMed] [Google Scholar]