Abstract
Light intensity is crucial for plant growth and often fluctuates on a small time scale due to altering climate conditions or sunflecks. Recently, we performed a study that looked into the growth effect of a sudden elevation of light intensity on Nicotiana tabacum seedlings.1 It was shown that an increase in light intensity leads to a pronounced increase of root-shoot-ratio as root growth reacts strongly and rapidly to an increase of light intensity. In transition experiments from low (60 µmol m−2 s−1) to high (300 µmol m−2 s−1) light intensity, root growth increased by a factor of four within four days, reaching the steady-state level measured in plants that were cultivated in high-light conditions. During the first three hours after light increase, strong fluctuations of the velocity of the root tip were observed that were putatively caused by a superposition of hydraulic and photosynthetic acclimation to the altered conditions. Experiments with externally applied sucrose and with transgenic plants having reduced capacity for sucrose synthesis indicated clearly that increasing light intensity rapidly enhanced root growth by elevating sucrose export from shoot to root.
Key Words: image processing, root growth, root-shoot-ratio, light, sucrose
Root growth is closely related to carbon import and hence to light conditions at the shoot. Carbon gain in roots is realized predominantly by import from the shoot via the phloem, while the major loss of root carbon occurs via respiration associated with growth and ion uptake.2 A number of studies have investigated differences in root growth between plants acclimated to low- or high-light environments (e.g. refs. 3–5) or between plants growing with variable external sucrose supply.6,7 A key factor directly connecting the irradiation of the shoot and the elongation of root tips is the local hexose concentration, which correlates very well with growth rates of individual roots of a given species.7,8 An increase in the sugar content of root tissue promotes growth of primary and secondary roots without affecting branching patterns or overall root architecture.9
While a large amount of data on the reaction of overall root growth to different steady-state light conditions is available, much less is known about the temporal dynamics of the acclimation process of root growth during short-term fluctuations of light intensity, which occur naturally on cloudy days, in sunflecks, in gaps of forest stands or in other heterogeneous natural growth settings. Recent studies using high-resolution, automated image processing growth monitoring methods have shown that alterations in root growth can take place within less than an hour in reaction to changes in various environmental factors such as temperature or nutrient availability.10–12 Hence, we investigated the question whether a change in shoot light environment could also induce similarly rapid reactions of root growth, using a custom-made near-infrared time-lapse imaging setup.1,10
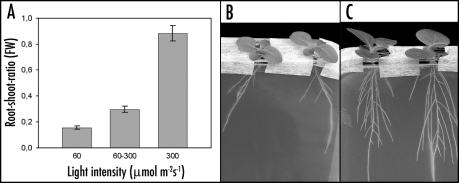
In our study, Nicotiana tabacum seedlings were cultivated on agar-filled Petri dishes that contained all essential mineral nutrients. When seedlings were exposed to high light intensity (300 µmol m−2 s−1), they showed a four times stronger root growth activity compared to plants grown at low light intensity (60 µmol m−2 s−1; 1). Shoots were also growing stronger when exposed to high light, but the response was far less pronounced than the response of roots. This led to a much higher root-shoot-ratio in plants from high light treatment compared to plants from low-light treatment (Fig. 1). The effect of light intensity on root-shoot-ratio is discussed controversely in the literature and seems to depend also on other environmental factors and on the species.13,14
Figure 1.
Root-shoot-ratio of Nicotiana tabacum seedlings from different light treatments. (A) Ratios based on fresh weight (FW) of plants that were exposed for 18 d to constant light intensities (60 or 300 µmol m−2 s−1) or were exposed to 60 µmol m−2 s−1 for 14 d and thereafter to 300 µmol m−2 s−1 (60–300) for 4 d (mean value ± SE, n = 5). (B and C) Images of typical plants (18 d after germination) cultivated on agar-filled Petri dishes with shoot outside in 60 µmol m−2 s−1(B) and 300 µmol m−2 s−1 (C), respectively. For more details of cultivation method see Nagel et al. (ref. 1).
The distribution of relative elemental growth rates along the root growth zone did not depend on the light treatment in our experiment, indicating that the relation between tissue, which is located in the meristematic zone, and tissue, which is located in the elongation zone, remains constant. This is in agreement with the findings of Muller et al.15
Another important feature of the response dynamics of roots towards increased light intensity was that strong reactions were observed immediately. During the first 30 minutes in high light, the velocity of the root tip of plants, that were acclimated to low light, decreased by 15 to 20 %, which was probably caused by a sudden increase of transpiration and a transient loss of turgor pressure. During the subsequent 2.5 hours, wild-type plants showed an oscillating increase of growth activity. Transgenic plants with decreased sucrose-6-phosphate phosphatase activity16 did not show such oscillating growth behaviour and increased growth much less in response to elevated light intensity. This result and the fact that isolated root systems, from which the shoot was clipped off, were able to retain growth activity if sucrose was present in the agar medium led to the conclusion that sucrose is the key regulatory element in root growth response to increased light.
Apart from their role as material growth substrates, carbohydrates affect growth by playing an important role as signal molecules in feedback mechanisms of gene regulation. Sucrose acts as signal molecule in source-sink relations throughout all stages of plant development17,18 and can modulate the expression of a large number of genes.19,20 Sucrose and glucose can up-regulate growth-related genes and downregulate stress-related genes21 demonstrating clearly their key function as signalling molecules for light-acclimation processes. Our results show, that root-shoot-ratio is obviously an important component of light acclimation that is regulated by sucrose.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=3447
References
- 1.Nagel KA, Schurr U, Walter A. Dynamics of root growth stimulation in Nicotiana tabacum in increasing light intensity. Plant Cell Environ. 2006;29:1936–1945. doi: 10.1111/j.1365-3040.2006.01569.x. [DOI] [PubMed] [Google Scholar]
- 2.Farrar JF, Jones DL. The control of carbon acquisition by roots. New Phytol. 2000;147:43–53. [Google Scholar]
- 3.Webb DP. Root growth in Acer saccharum marsh. Seedlings: Effects of light intensity and photoperiod on root elongation rates. Bot Gazette. 1976;137:211–217. [Google Scholar]
- 4.Vincent CD, Gregory PJ. Effects of temperature on the development and growth of winter wheat roots. I. Controlled glasshouse studies of temperature, nitrogen and irradiance. Plant Soil. 1989;119:87–97. [Google Scholar]
- 5.Aguirrezabal LAN, Deleens E, Tardieu F. Root elongation rate is accounted for by intercepted PPFD and source-sink relations in field and laboratory-grown sunflower. Plant Cell Environ. 1994;17:443–450. [Google Scholar]
- 6.Street HE, McGregor SM. The carbohydrate nutrition of tomato roots. III The effects of external sucrose concentration on the growth and anatomy of excised roots. Ann Bot. 1952;62:185–205. [Google Scholar]
- 7.Freixes S, Thibaud MC, Tardieu F, Muller B. Root elongation and branching is related to local hexose concentration in Arabidopsis thaliana seedlings. Plant Cell Environ. 2002;25:1357–1366. [Google Scholar]
- 8.Scheible WR, Lauerer M, Schulze ED, Caboche M, Stitt M. Accumulation of nitrate in the shoot acts as a signal to regulate shoot-root allocation in tobacco. Plant J. 1997;11:671–691. [Google Scholar]
- 9.Bingham IJ, Blackwood JM, Stevenson EA. Site, scale and time-course for adjustments in lateral root initiation in wheat following changes in C and N supply. Ann Bot. 1997;80:97–106. [Google Scholar]
- 10.Walter A, Spies H, Terjung S, Küsters R, Kirchgeßner N, Schurr U. Spatio-temporal dynamics of expansion growth in roots: Automatic quantification of diurnal course and temperature response by digital image sequence processing. J Ex Bot. 2002;53:689–698. doi: 10.1093/jexbot/53.369.689. [DOI] [PubMed] [Google Scholar]
- 11.Van der Weele CM, Jiang HS, Palaniappan KK, Ivanov VB, Palaniappan K, Baskin TI. A new algorithm for computational image analysis of deformable motion at high spatial and temporal resolution applied to root growth. Roughly uniform elongation in the meristem and also, after an abrupt acceleration, in the elongation zone. Plant Physiol. 2003;132:1138–1148. doi: 10.1104/pp.103.021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walter A, Feil R, Schurr U. Expansion dynamics, metabolite compostion and substance transfer of the primary root growth zone of Zea mays L. grown in different external nutrient availabilities. Plant Cell Environ. 2003;26:1451–1466. [Google Scholar]
- 13.Lambers H, Posthumus F. The effect of light intensity and relative humidity on growth rate and root respiration of Plantago lanceolota and Zea mays. J Ex Bot. 1980;31:1621–1630. [Google Scholar]
- 14.Hodge A, Paterson E, Thornton B, Millard P, Killham K. Effects of photon flux density on carbon partitioning and rhizosphere carbon flow of Lolium perenne. J Exp Bot. 1997;48:1797–1805. [Google Scholar]
- 15.Muller B, Stosser M, Tardieu F. Spatial distributions of tissue expansion and cell division rates are related to irradiance and to sugar content in the growing zone of maize roots. Plant Cell Environm. 1998;21:149–158. [Google Scholar]
- 16.Chen S, Hajirezaei M, Peisker M, Tschiersch H, Sonnewald U, Börnke F. Decreased sucrose-6-phosphate phosphatase level in transgenic tobacco inhibits photosynthesis, alters carbohydrate partitioning, and reduces growth. Planta. 2005;221:479–492. doi: 10.1007/s00425-004-1458-4. [DOI] [PubMed] [Google Scholar]
- 17.Roitsch T. Source-sink regulation by sugar and stress. Curr Opin Plant Biol. 1999;2:198–206. doi: 10.1016/S1369-5266(99)80036-3. [DOI] [PubMed] [Google Scholar]
- 18.Smeekens S. Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:49–81. doi: 10.1146/annurev.arplant.51.1.49. [DOI] [PubMed] [Google Scholar]
- 19.Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- 20.Smeekens S. Sugar regulation of gene expression in plants. Curr Opin Plant Biol. 1998;1:230–234. doi: 10.1016/s1369-5266(98)80109-x. [DOI] [PubMed] [Google Scholar]
- 21.Ho SL, Chao YC, Tong WF, Yu SM. Sugar coordinately and differentially regulates growth- and stress-related gene expression via a complex signal transduction network and multiple control mechanisms. Plant Physiol. 2001;125:877–890. doi: 10.1104/pp.125.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]