Abstract
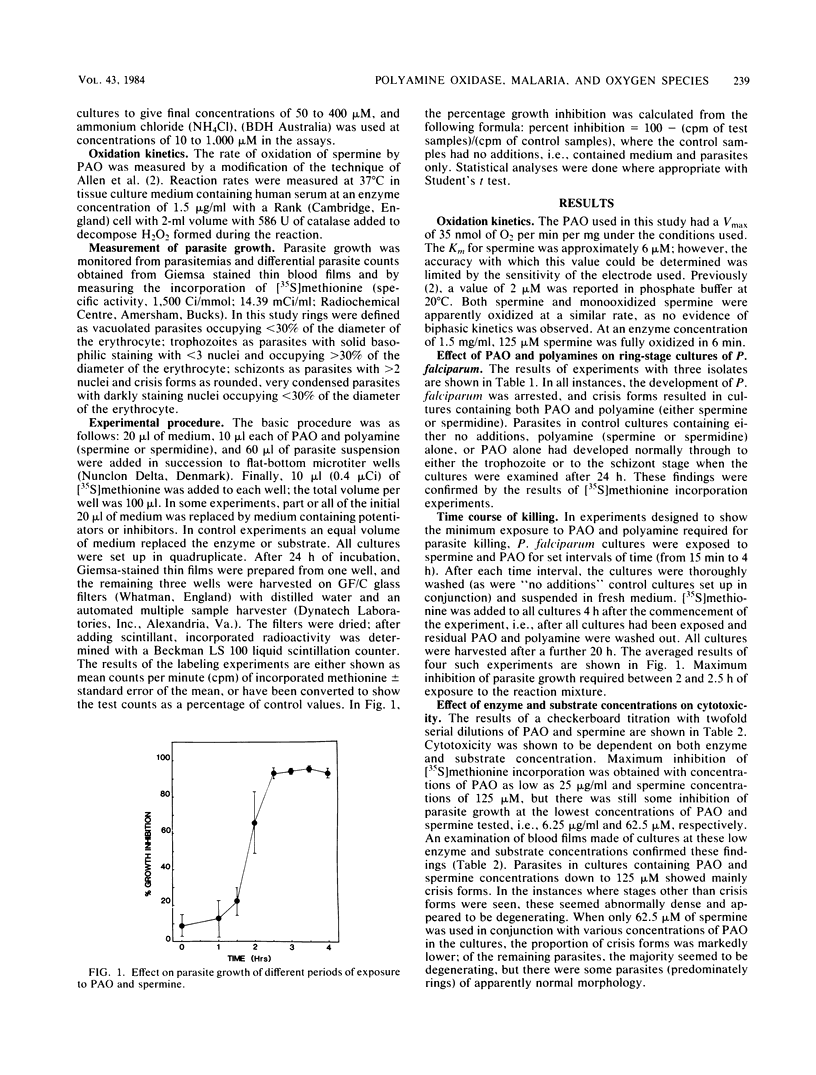
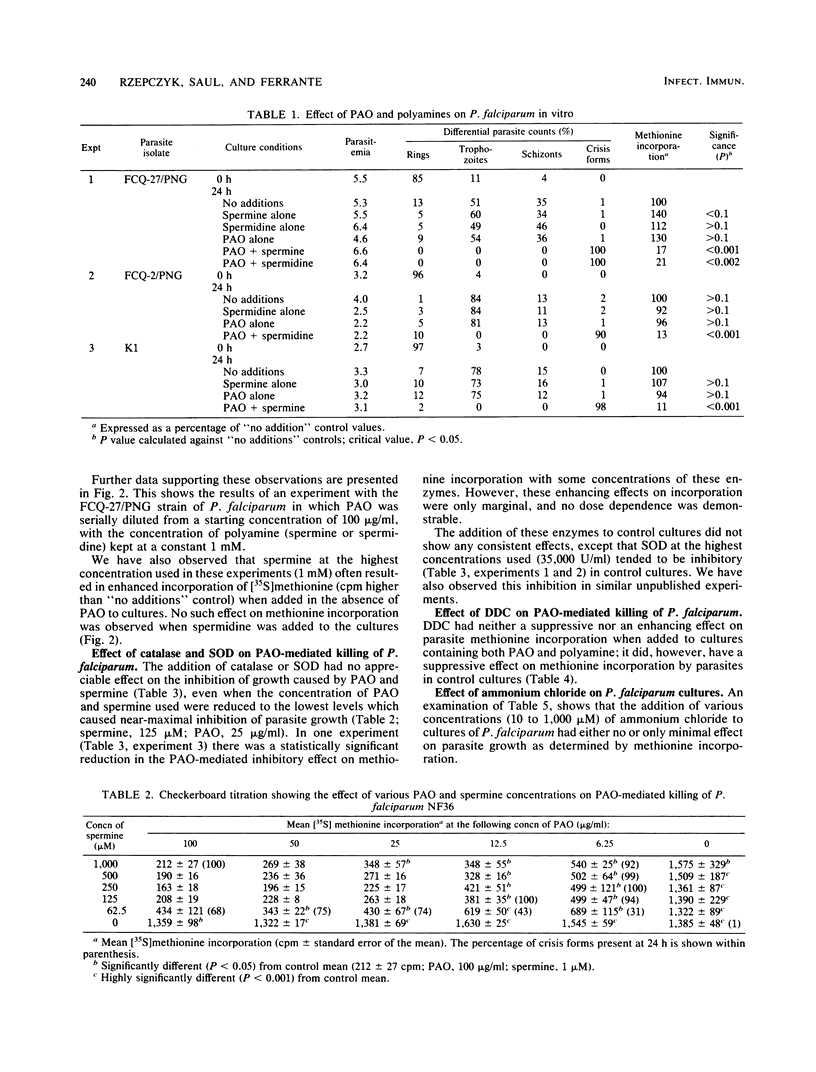
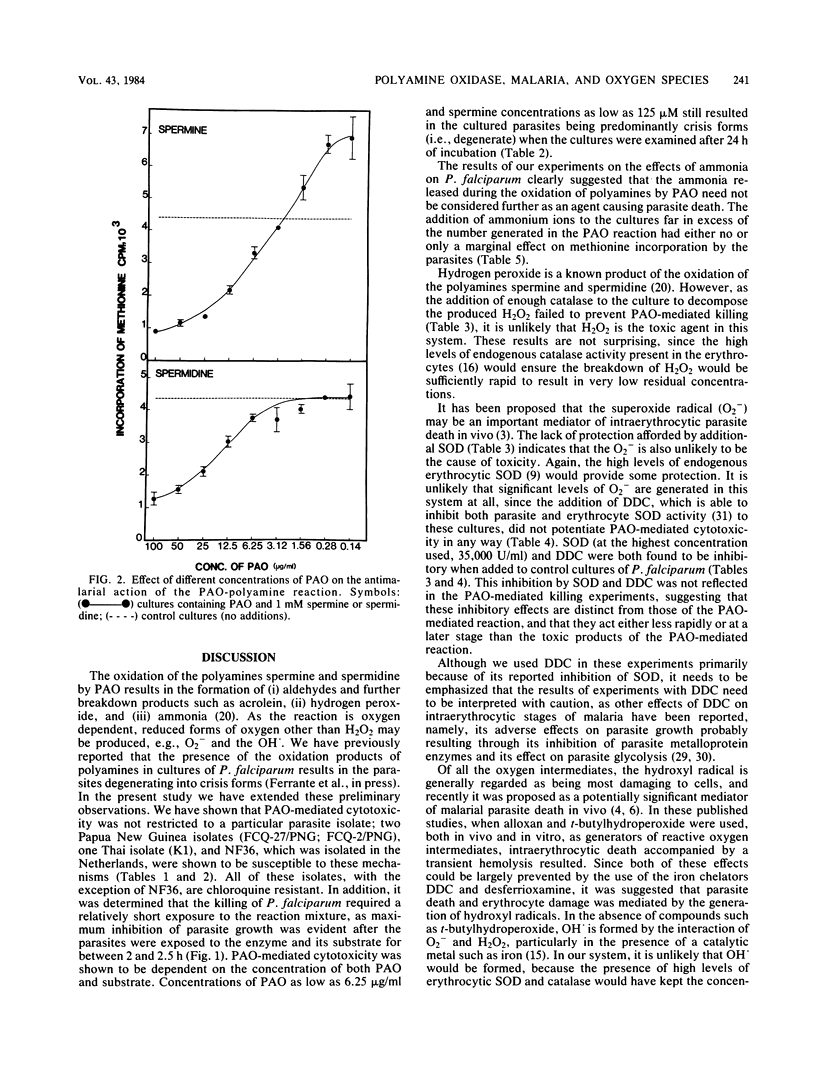
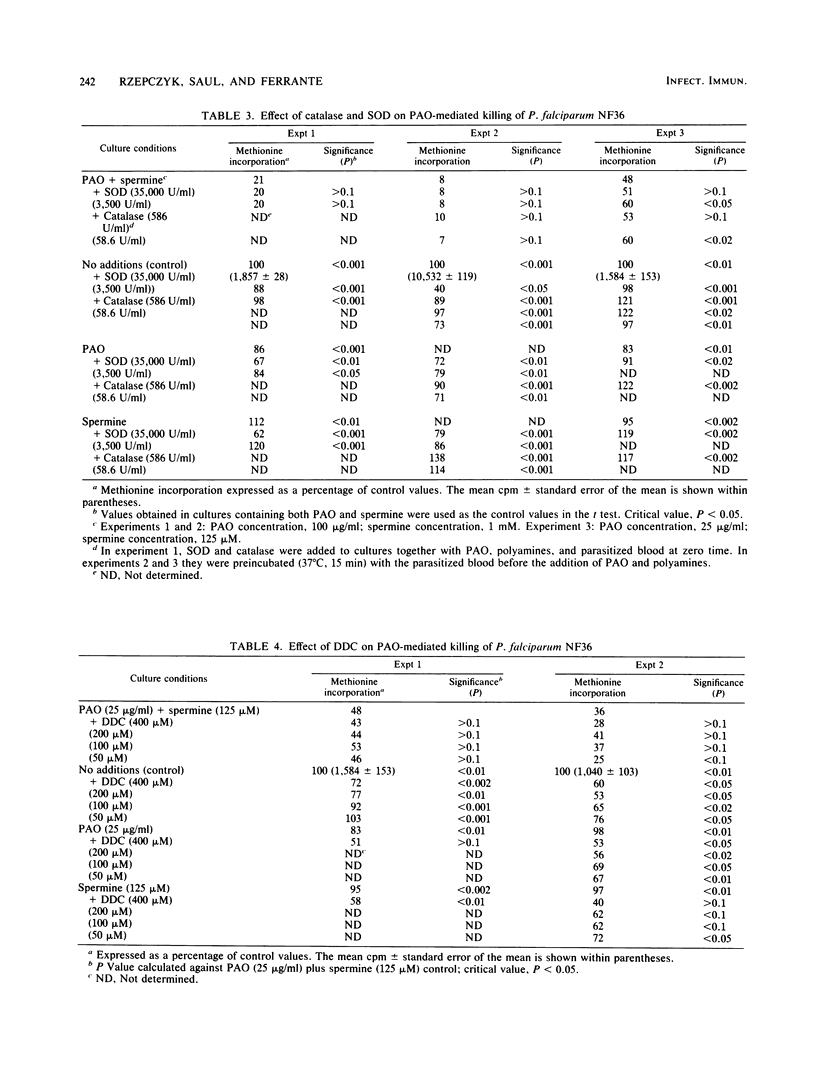
The polyamines spermine and spermidine, in the presence of polyamine oxidase, were shown to be cytotoxic in vitro to various isolates of Plasmodium falciparum. Neither polyamines nor polyamine oxidase alone was cytotoxic. This cytotoxicity was manifested by the degeneration of the parasites into crisis forms and by the inhibition of methionine incorporation by the parasites. Only 2 to 2.5 h of exposure to the reaction mixture (polyamine oxidase, 100 micrograms/ml; spermine, 1 mM) resulted in parasite death. It was shown that ammonia, hydrogen peroxide, and associated reactive oxygen intermediates produced during the oxidation of polyamines were not the cause of the parasite death observed in this system. This suggested that aldehydes or further breakdown products of these, e.g., acrolein (or both), need to be considered as the effector substances of the polyamine oxidase-mediated killing of P. falciparum.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALARCON R. A. ISOLATION OF ACROLEIN FROM INCUBATED MIXTURES OF SPERMINE WITH CALF SERUM AND ITS EFFECTS ON MAMMALIAN CELLS. Arch Biochem Biophys. 1964 Jul 20;106:240–242. doi: 10.1016/0003-9861(64)90183-3. [DOI] [PubMed] [Google Scholar]
- Allen J. C., Smith C. J., Hussain J. I., Thomas J. M., Gaugas J. M. Inhibition of lymphocyte proliferation by polyamines requires ruminant-plasma polyamine oxidase. Eur J Biochem. 1979 Dec;102(1):153–158. doi: 10.1111/j.1432-1033.1979.tb06275.x. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Eugui E. M. A radical interpretation of immunity to malaria parasites. Lancet. 1982 Dec 25;2(8313):1431–1433. doi: 10.1016/s0140-6736(82)91330-7. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cowden W. B., Butcher G. A. Free oxygen radical generators as antimalarial drugs. Lancet. 1983 Jan 29;1(8318):234–234. doi: 10.1016/s0140-6736(83)92603-x. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cox F. E., Allison A. C. Protection of mice against Babesia spp. and Plasmodium spp. with killed Corynebacterium parvum. Parasitology. 1977 Feb;74(1):9–18. doi: 10.1017/s003118200004748x. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H. Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect Immun. 1983 Jan;39(1):1–6. doi: 10.1128/iai.39.1.1-6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Richmond J. E., Wills E. J., Allison A. C. Intra-erythrocytic death of the parasite in mice recovering from infection with Babesia microti. Parasitology. 1977 Oct;75(2):189–196. doi: 10.1017/s0031182000062338. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concetti A., Massei P., Rotilio G., Brunori M., Rachmilewitz E. A. Superoxide dismutase in red blood cells: method of assay and enzyme content in normal subjects and in patients with beta-thalassemia (major and intermedia). J Lab Clin Med. 1976 Jun;87(6):1057–1064. [PubMed] [Google Scholar]
- Ferrante A., Allison A. C., Hirumi H. Polyamine oxidase-mediated killing of African trypanosomes. Parasite Immunol. 1982 Sep;4(5):349–354. doi: 10.1111/j.1365-3024.1982.tb00446.x. [DOI] [PubMed] [Google Scholar]
- Forman H. J., York J. L., Fisher A. B. Mechanism for the potentiation of oxygen toxicity by disulfiram. J Pharmacol Exp Ther. 1980 Mar;212(3):452–455. [PubMed] [Google Scholar]
- Fridovich I. Oxygen: boon and bane. Am Sci. 1975 Jan-Feb;63(1):54–59. [PubMed] [Google Scholar]
- Fukami H., Tomida I., Morino T., Yamada H., Oki T., Kawasaki H., Ogata K. Phagocidal action of oxidized spermine and its analogues. Biochem Biophys Res Commun. 1967 Jul 10;28(1):19–24. doi: 10.1016/0006-291x(67)90399-3. [DOI] [PubMed] [Google Scholar]
- Grun J. L., Weidanz W. P. Immunity to Plasmodium chabaudi adami in the B-cell-deficient mouse. Nature. 1981 Mar 12;290(5802):143–145. doi: 10.1038/290143a0. [DOI] [PubMed] [Google Scholar]
- Hartz J. W., Funakoshi S., Deutsch H. F. The levels of superoxide dismutase and catalase in human tissues as determined immunochemically. Clin Chim Acta. 1973 Jun 28;46(2):125–132. doi: 10.1016/0009-8981(73)90019-3. [DOI] [PubMed] [Google Scholar]
- Kimes B. W., Morris D. R. Inhibition of nucleic acid and protein synthesis in Escherichia coli by oxidized polyamines and acrolein. Biochim Biophys Acta. 1971 Jan 1;228(1):235–244. doi: 10.1016/0005-2787(71)90563-6. [DOI] [PubMed] [Google Scholar]
- Kimes B. W., Morris D. R. Preparation and stability of oxidized polyamines. Biochim Biophys Acta. 1971 Jan 1;228(1):223–234. doi: 10.1016/0005-2787(71)90562-4. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Myler P., Saul A., Mangan T., Kidson C. An automated assay of merozoite invasion of erythrocytes using highly synchronized Plasmodium falciparum cultures. Aust J Exp Biol Med Sci. 1982 Feb;60(Pt 1):83–89. doi: 10.1038/icb.1982.6. [DOI] [PubMed] [Google Scholar]
- Rzepczyk C. M., Clark I. A. Demonstration of a lipopolysaccharide-induced cytostatic effect on malarial parasites. Infect Immun. 1981 Aug;33(2):343–347. doi: 10.1128/iai.33.2.343-347.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzepczyk C. M., Clark I. A. Failure of bacterial lipopolysaccharide to elicit a cytostatic effect on Plasmodium vinckei petteri in C3H/HeJ mice. Infect Immun. 1982 Jan;35(1):58–63. doi: 10.1128/iai.35.1.58-63.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel L. W., Adler A. Antimalarial activity of selected aromatic chelators. Mol Pharmacol. 1980 Sep;18(2):320–325. [PubMed] [Google Scholar]
- Scheibel L. W., Adler A., Trager W. Tetraethylthiuram disulfide (Antabuse) inhibits the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5303–5307. doi: 10.1073/pnas.76.10.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthipark U., Krungkrai J., Jearnpipatkul A., Yuthavong Y., Panijpan B. Superoxide dismutase (SOD) in mouse red blood cells infected with Plasmodium berghei. J Parasitol. 1982 Apr;68(2):337–339. [PubMed] [Google Scholar]
- TABOR H., TABOR C. W. SPERMIDINE, SPERMINE, AND RELATED AMINES. Pharmacol Rev. 1964 Sep;16:245–300. [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]


