Abstract
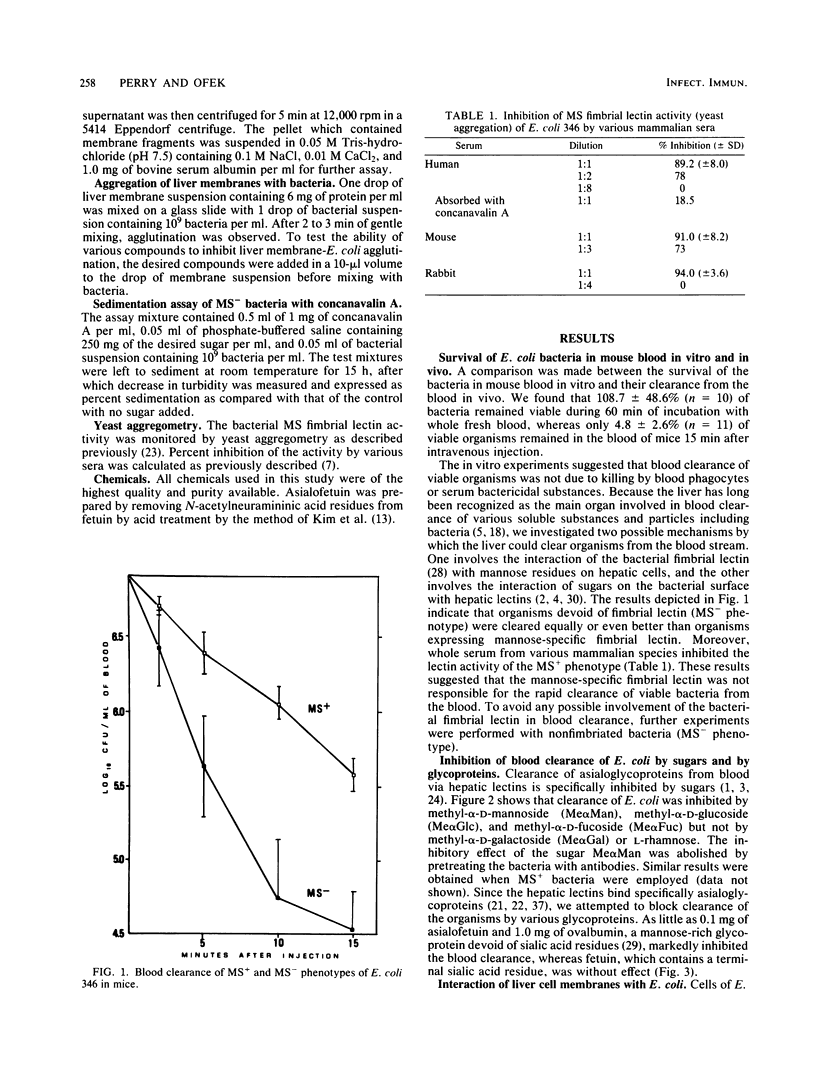
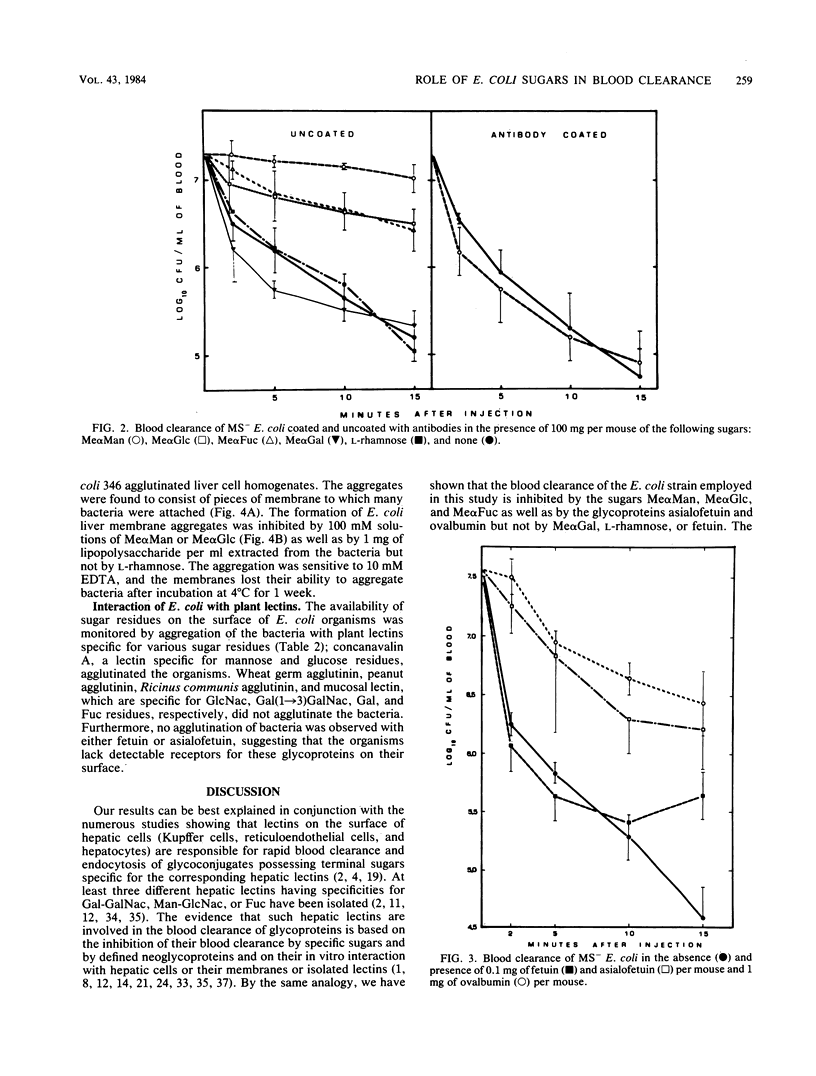
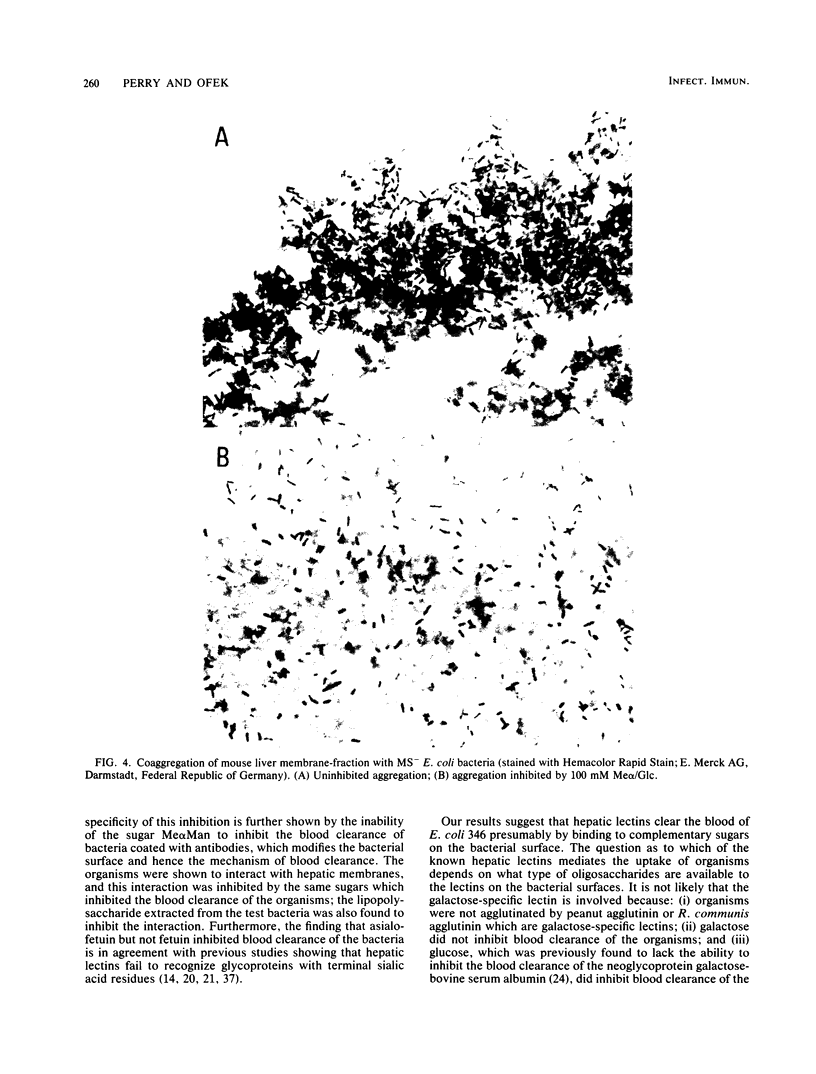
The effects of sugars and glycoproteins that are known to bind to lectins of liver tissue on the clearance of cells of Escherichia coli from mouse blood was investigated. The administration of 100 mg per mouse of methyl-alpha-D-mannoside, methyl-alpha-D-glucoside, or methyl-alpha-D-fucoside, but not of methyl-alpha-D-galactoside or L-rhamnose, markedly inhibited the blood clearance of cells of E. coli 346. Clearance was similarly inhibited by 0.1 and 1.0 mg per mouse of asialofetuin or ovalbumin, respectively, whereas fetuin had no effect. The inhibitory effects of the sugars on blood clearance was abolished by pretreating the E. coli cells with antibodies against whole organisms. All of these effects were equal for fimbriated and nonfimbriated phenotypes of E. coli 346. Homogenates of mouse liver tissue coaggregated with nonfimbriated cells of E. coli. The aggregation was blocked by 100 mM solutions of methyl-alpha-D-mannoside, or methyl-alpha-D-glucoside, 1 mg of bacterial lipopolysaccharide per ml, or 10 mM EDTA but not by L-rhamnose. These results suggest that the mannose-N-acetylglucosamine hepatic lectin recognizes specific sugars on the surface of E. coli and may be centrally involved in the nonimmune clearance of nonfimbriated E. coli from the blood of the infected host.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achord D. T., Brot F. E., Sly W. S. Inhibition of the rat clearance system for agalacto-orosomucoid by yeast mannans and by mannose. Biochem Biophys Res Commun. 1977 Jul 11;77(1):409–415. doi: 10.1016/s0006-291x(77)80213-1. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- BENACERRAF B., SEBESTYEN M. M., SCHLOSSMAN S. A quantitative study of the kinetics of blood clearance of P32-labelled Escherichia coli and Staphylococci by the reticuloendothelial system. J Exp Med. 1959 Jul 1;110(1):27–48. doi: 10.1084/jem.110.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenziger J. U., Maynard Y. Human hepatic lectin. Physiochemical properties and specificity. J Biol Chem. 1980 May 25;255(10):4607–4613. [PubMed] [Google Scholar]
- Barondes S. H. Lectins: their multiple endogenous cellular functions. Annu Rev Biochem. 1981;50:207–231. doi: 10.1146/annurev.bi.50.070181.001231. [DOI] [PubMed] [Google Scholar]
- Hill R. L., Pizzo S. V., Imber M., Lehrman M., Prieels J. P., Glasgow L. R., Guthrow C. E., Paulson J. C. Receptors on hepatocytes that bind ligands containing fucosyl alpha 1,3 N-acetylglucosamine linkages. Birth Defects Orig Artic Ser. 1980;16(1):85–91. [PubMed] [Google Scholar]
- Izhar M., Nuchamowitz Y., Mirelman D. Adherence of Shigella flexneri to guinea pig intestinal cells is mediated by a mucosal adhesion. Infect Immun. 1982 Mar;35(3):1110–1118. doi: 10.1128/iai.35.3.1110-1118.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn L. D. Nonspecific and cooperative binding of lectins to microorganisms. Physiol Chem Phys. 1982;14(1):3–7. [PubMed] [Google Scholar]
- Kawasaki T., Ashwell G. Carbohydrate structure of glycopeptides isolated from an hepatic membrane-binding protein specific for asialoglycoproteins. J Biol Chem. 1976 Sep 10;251(17):5292–5299. [PubMed] [Google Scholar]
- Kawasaki T., Etoh R., Yamashina I. Isolation and characterization of a mannan-binding protein from rabbit liver. Biochem Biophys Res Commun. 1978 Apr 14;81(3):1018–1024. doi: 10.1016/0006-291x(78)91452-3. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Perdomo J., Nordberg J. Glycoprortein biosynthesis in small intestinal mucosa. I. A study of glycosyltransferases in microsomal subfractions. J Biol Chem. 1971 Sep 10;246(17):5466–5476. [PubMed] [Google Scholar]
- Kolb H., Vogt D., Herbertz L., Corfield A., Schauer R., Schlepper-Schäfer J. The galactose-specific lectins on rat hepatocytes and Kupffer cells have identical binding characteristics. Hoppe Seylers Z Physiol Chem. 1980 Nov;361(11):1747–1750. [PubMed] [Google Scholar]
- Leunk R. D., Moon R. J. Association of type 1 pili with the ability of livers to clear Salmonella typhimurium. Infect Immun. 1982 Jun;36(3):1168–1174. doi: 10.1128/iai.36.3.1168-1174.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard Y., Baenziger J. U. Characterization of a mannose and N-acetylglucosamine-specific lectin present in rat hepatocytes. J Biol Chem. 1982 Apr 10;257(7):3788–3794. [PubMed] [Google Scholar]
- Morell A. G., Gregoriadis G., Scheinberg I. H., Hickman J., Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971 Mar 10;246(5):1461–1467. [PubMed] [Google Scholar]
- Morell A. G., Irvine R. A., Sternlieb I., Scheinberg I. H., Ashwell G. Physical and chemical studies on ceruloplasmin. V. Metabolic studies on sialic acid-free ceruloplasmin in vivo. J Biol Chem. 1968 Jan 10;243(1):155–159. [PubMed] [Google Scholar]
- Morell A. G., Scheinberg I. H. Solubilization of hepatic binding sites for asialo-glycoproteins. Biochem Biophys Res Commun. 1972 Aug 21;48(4):808–815. doi: 10.1016/0006-291x(72)90679-1. [DOI] [PubMed] [Google Scholar]
- Perry A., Ofek I., Silverblatt F. J. Enhancement of mannose-mediated stimulation of human granulocytes by type 1 fimbriae aggregated with antibodies on Escherichia coli surfaces. Infect Immun. 1983 Mar;39(3):1334–1345. doi: 10.1128/iai.39.3.1334-1345.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo S. V., Lehrman M. A., Imber M. J., Guthrow C. E. The clearance of glycoproteins in diabetic mice. Biochem Biophys Res Commun. 1981 Jul 30;101(2):704–708. doi: 10.1016/0006-291x(81)91315-2. [DOI] [PubMed] [Google Scholar]
- Prieels J. P., Pizzo S. V., Glasgow L. R., Paulson J. C., Hill R. L. Hepatic receptor that specifically binds oligosaccharides containing fucosyl alpha1 leads to 3 N-acetylglucosamine linkages. Proc Natl Acad Sci U S A. 1978 May;75(5):2215–2219. doi: 10.1073/pnas.75.5.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. K. A modified method for the isolation of the plasma membrane from rat liver. Biochim Biophys Acta. 1970 Jan 6;196(1):1–9. doi: 10.1016/0005-2736(70)90159-8. [DOI] [PubMed] [Google Scholar]
- Schaefer R. L., Keller K. F., Doyle R. J. Lectins in diagnostic microbiology: use of wheat germ agglutinin for laboratory identification of Neisseria gonorrhoeae. J Clin Microbiol. 1979 Nov;10(5):669–672. doi: 10.1128/jcm.10.5.669-672.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Eshdat Y., Silverblatt F. J., Ofek I. Bacterial adherence to cell surface sugars. Ciba Found Symp. 1981;80:119–141. doi: 10.1002/9780470720639.ch9. [DOI] [PubMed] [Google Scholar]
- Simpson D. L., Thorne D. R., Loh H. H. Lectins: endogenous carbohydrate-binding proteins from vertebrate tissues: functional role in recognition processes? Life Sci. 1978 Mar;22(9):727–748. doi: 10.1016/0024-3205(78)90242-4. [DOI] [PubMed] [Google Scholar]
- Stahl P., Gordon S. Expression of a mannosyl-fucosyl receptor for endocytosis on cultured primary macrophages and their hybrids. J Cell Biol. 1982 Apr;93(1):49–56. doi: 10.1083/jcb.93.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert R. J., Morell A. G., Scheinberg I. H. The existence of a second route for the transfer of certain glycoproteins from the circulation into the liver. Biochem Biophys Res Commun. 1976 Feb 9;68(3):988–993. doi: 10.1016/0006-291x(76)91243-2. [DOI] [PubMed] [Google Scholar]
- Summerfield J. A., Vergalla J., Jones E. A. Modulation of a glycoprotein recognition system on rat hepatic endothelial cells by glucose and diabetes mellitus. J Clin Invest. 1982 Jun;69(6):1337–1347. doi: 10.1172/JCI110573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T., Pricer W. E., Jr, Ashwell G. Subcellular membrane topology and turnover of a rat hepatic binding protein specific for asialoglycoproteins. J Biol Chem. 1979 Feb 25;254(4):1038–1043. [PubMed] [Google Scholar]
- Townsend R., Stahl P. Isolation and characterization of a mannose/N-acetylglucosamine/fucose-binding protein from rat liver. Biochem J. 1981 Jan 15;194(1):209–214. doi: 10.1042/bj1940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lenten L., Ashwell G. The binding of desialylated glycoproteins by plasma membranes of rat liver. Development of a quantitative inhibition assay. J Biol Chem. 1972 Jul 25;247(14):4633–4640. [PubMed] [Google Scholar]
- Weir D. M., Blackwell C. C. Interaction of bacteria with the immune system. J Clin Lab Immunol. 1983 Jan;10(1):1–12. [PubMed] [Google Scholar]
- van den Bosch J. F., de Graaff J., MacLaren D. M. Virulence of Escherichia coli in experimental hematogenous pyelonephritis in mice. Infect Immun. 1979 Jul;25(1):68–74. doi: 10.1128/iai.25.1.68-74.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



