Abstract
Background
The mechanism of the light-dependent movements of chloroplasts is based on actin and myosin but its details are largely unknown. The movements are activated by blue light in terrestrial angiosperms. The aim of the present study was to determine the role of myosin associated with the chloroplast surface in the light-induced chloroplast responses in Arabidopsis thaliana. The localization of myosins was investigated under blue light intensities generating avoidance and accumulation responses of chloroplasts. The localization was compared in wild type plants and in phot2 mutant lacking the avoidance response.
Results
Wild type and phot2 mutant plants were irradiated with strong (36 µEm−2s−1) and/or weak (0.8 µEm−2s−1) blue light. The leaf tissue was immunolabeled with antimyosin antibodies. Different arrangements of myosins were observed in the mesophyll depending on the fluence rate in wild type plants. In tissue irradiated with weak blue light myosins were associated with chloroplast envelopes. In contrast, in tissue irradiated with strong blue light chloroplasts were almost myosin-free. The effect did not occur in red light and in the phot2 mutant.
Conclusions
Myosin displacement is blue light specific, i.e., it is associated with the activation of a specific blue-light photoreceptor. We suggest that the reorganization of myosins is essential for chloroplast movement. Myosins appear to be the final step of the signal transduction pathway starting with phototropin2 and leading to chloroplast movements.
Key Words: Arabidopsis, blue light, chloroplast movements, myosins, phototropins
Introduction
The great majority of plant cells containing chloroplasts are able to redistribute them in response to light. In the dark, chloroplasts are more or less randomly distributed in the cell. In weak light they accumulate at the most illuminated cell walls, that is at the walls perpendicular to the light beam (accumulation response). In strong light they avoid the strongest illuminated areas and redistribute to the walls parallel to the light direction (avoidance response). These responses of chloroplasts are restricted to the illuminated cell and depend on the direction, wavelength and intensity of the incident light.1 The movements are directed by blue light and, in some species (e.g., Mougeotia, Vallisneria, Ceratodon) also co-directed by red light.2–4
Much of our understanding of blue light signaling in plants has come from the isolation of blue light-response mutants of Arabidopsis thaliana. Two groups of blue-light photoreceptors have already been identified: cryptochromes, involved mainly in regulation of the circadian clock and phototropins. Phototropins phot1 and phot2 represent a family of flavoprotein photoreceptors that mediate light-induced chloroplast movement.5,6 Studies involving phot1phot2 double mutant have shown that these two photoreceptors act redundantly in the control of the chloroplast migration towards low intensity blue light i.e., the accumulation response.6 However, the avoidance response to strong light is mediated solely by phot2, indicating that the functions of phot1 and phot2 do not overlap entirely.
The motor system for chloroplast phototranslocations is known to have two components: actin and myosin, but few details of their functioning have been revealed to date. The response of the actin cytoskeleton to light has been studied in Arabidopsis, Selaginella, Vallisneria and Adiantum.7–10 In dark-adapted cells of Vallisneria, the actin filaments were composed mainly of thin bundles, uniformly distributed. A honeycomb-like actin network formed around chloroplasts after irradiation with weak red light.9 In contrast, detailed studies of the shapes and frequencies of appearance of particular actin formations did not show any correlation between actin organization and chloroplast movement in Arabidopsis.7 Thus, the modes of actin involvement in chloroplast translocation are different in water and terrestrial angiosperms. Moreover, actin does not appear to be the main target for the blue-light signal directing chloroplast relocations in terrestrial angiosperms.
Myosins are members of the actin-activated ATPase family, which transport various cargoes along actin tracks and translocate microfilaments along immobile surfaces e.g., the plasma membrane. They are engaged in many physiological processes in the animal and plant kingdoms. Out of 18 known classes of myosins three have been found in plants: VIII, XI and XIII. Two of them, VIII and XI, are present in the Arabidopsis genome.11–13
Myosin VIII has been revealed in the cell periphery as a component of plasmodesmata and pit-fields.14–16 It plays a role in vacuole-driven cell elongation, maturation of the cell plate, endocytosis, cytokinesis, cell-to-cell coupling, gravisensing and osmosensing.14,17–19 In contrast, myosin XI has been reported as involved in cytoplasmic streaming, organelle movement and polar auxin transport.20,21
Myosins of both classes occur on chloroplast surfaces and, most probably, they are partners for actin in the motile system of chloroplasts.19,21,22 Indication that myosins are involved in chloroplast movements comes from inhibitor studies on water plants, Vallisneria and Lemna trisulca.23,22 In these species the movement stopped after incubation with a general myosin inhibitor: N-ethylmaleimid. No evidence has been presented to date that myosins might be controlled by light absorbed by a photoreceptor.
In this work we inquired into the role of myosins associated with the chloroplast surface in the mechanism of chloroplast movements in Arabidopsis. To clarify this role, we investigated their behavior under the influence of light. The localization of myosin was investigated under blue light intensities generating avoidance and accumulation responses of chloroplasts. The localization was compared in wild type and phot2 mutant lacking the avoidance response.
Results
Localization of myosins in the spongy mesophyll in A. thaliana.
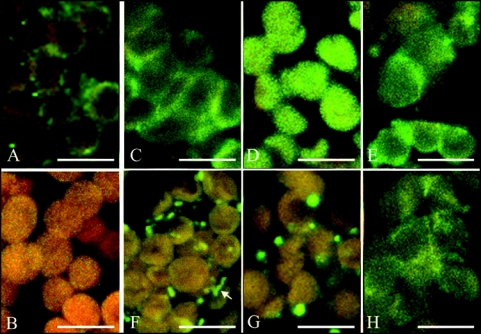
Two forms of fluorescent structures, a continuous fog-like fluorescent layer, and/or foci, were visible in the cytoplasm and on chloroplasts of A. thaliana WT and of the phot2 mutant tissue (Fig. 1). The chloroplasts were covered with a smooth fluorescent film or dotted by granules of various shapes and size. A similar image of myosin distribution was detected in the cytoplasm. Occasionally, elongated shapes were found, apparently reflecting myosins located along actin cables (Fig. 1F, marked by arrow). In a few experiments myosins were detected along plasamalemma (data not shown). Similar patterns, i.e., granules and homogeneous fluorescence, were observed on intact (class A) chloroplasts isolated from WT leaves (Suppl. Fig. 4).
Figure 1.
Confocal immunofluorescence images of Arabidopsis thaliana mesophyll, wild type (WT), phot1 and phot2 mutant plants. Myosins are labeled with anti-rabbit (smooth and skeletal) antibodies and with secondary FITC-conjugated antibodies (yellow-green color). Red-orange color comes from chloroplast autofluorescence. Left column, WT, Arrangements of myosins in the dark-adapted tissue (A); Control with secondary antibodies only (B); Wild type cells, weak blue light-irradiated (C). Wild type tissue, strong blue light-irradiated (F). Weak (D) and strong (G) blue light-irradiated phot1 mutant. Weak (E) and strong (H) blue light-irradiated phot2 mutant. Note the difference in the arrangements of myosins between strong and weak blue-irradiated WT tissue and the similarity of phot2 irrespective of light intensity. The arrow at (F) points at myosins located along an actin cable. The bars are 10 µm.
Additional images showing an overview of the immunofluorescent staining in the wild type and mutant plants are provided in the supplementary material.
Distribution of myosins in A. thaliana WT under various light conditions.
In the dark-adapted mesophyll myosins were present both in the cytoplasm and on plastids (Fig. 1A). Their distribution in the cell was fairly uniform, with more myosin located occasionally on the plastid surfaces or in the cytoplasm. Neither homogenous fluorescence nor foci dominated on the average.
This uniform distribution of myosins changed in the cells treated with blue light. In tissue irradiated with weak blue light of 0.8 µEm−2s−1 myosins were associated with chloroplast envelopes, covering almost uniformly their surfaces (Fig. 1C). In contrast, in tissue irradiated with strong blue light of 36 µEm−2s−1, chloroplasts were almost myosin-free (Fig. 1F). Moreover, more fluorescence staining was visible in the cytoplasm. Thus, myosin localization in the cell depended on the intensity of blue light (see also Suppl. Fig. 1). Furthermore, the effect of myosin redistribution was reversible: In tissue irradiated consecutively with strong blue light and weak blue light (each irradiation lasting 30 minutes) the localization of myosin was characteristic of the latter illumination, i.e., the uniform labeling of chloroplasts was recuperated (data not shown).
Red light of equal quantum flux was applied to eliminate the possibility that myosin displacement was non-specific with regard to chloroplast movement, e.g., due to the thermal effect of strong light, or to photosynthesis. In general, the red light-irradiated mesophyll resembled the dark-adapted tissue. The labeling looked similar in the strong and weak red light-irradiated tissue: the samples were indistinguishable, and antibodies were located in the cytoplasm and/or on the chloroplast surfaces independently of the red light fluence-rate (data not shown).
Myosins in mutants with abnormal chloroplast photomovements.
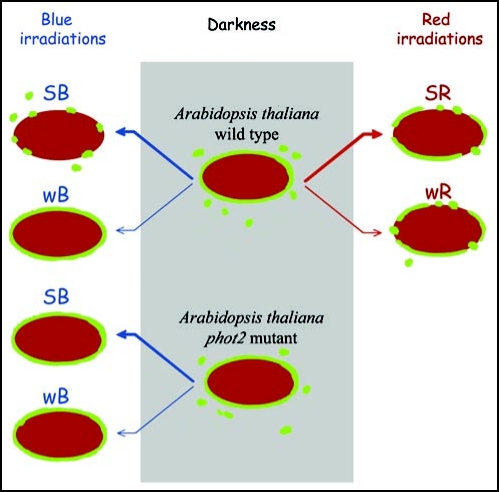
To obtain evidence that myosin rearrangements are indeed involved in the mechanism of chloroplast movement, we tested a phot2 mutant which lacks phototropin2, the photoreceptor controlling the avoidance response. The blue light-induced redistribution of myosin was clearly absent in the mutant. Myosins were preferentially localized on chloroplast surfaces in the phot2 plants irrespective of light intensity (Fig. 1E and H). Both in strong and weak blue light-irradiated tissue the localization of myosin was identical with that observed in WT tissue after weak blue light. The results obtained in WT and mutant plants are summarized in Figure 2.
Figure 2.
Schematic of myosin localization at the chloroplasts in irradiated leaves of Arabidopsis thaliana wild type and phot2 mutant. SB, strong blue light; wB, weak blue light; SR, strong red light; wR, weak red light.
In the mutant lacking phototropin1 the relocalization of myosins under blue light was the same as in WT (compare Fig. 1C and F with D and G). This conforms with a relative lack of specificity of phot1 in mediating chloroplast movements as compared with phot2 which is highly specific to the avoidance response.
Materials and Methods
Plant material.
Plants were grown in a growth chamber (Sanyo, Japan) at 23°C with a constant 85% humidity, using a commercial soil for pot plants. Illumination was provided by fluorescent lamps (FL40SS.W/37, Sanyo), with a photoperiod 10 h of 90 µEm−2s−1/14 h of darkness. The illumination was measured with a LI-250A quantum-meter (LI-COR). The experiments were carried out using mature leaves obtained from 4–6 week old plants. Whole plants were dark-adapted for at least 10 h at room temperature before the leaves were detached for tests. Arabidopsis thaliana wild-type (WT) seeds were obtained from Lehle seeds (Round Rock, TX, USA). A. thaliana phot2 and phot1 mutant seeds were donated by Dr. Anthony R. Cashmore (Plant Science Institute, Department of Biology, University of Pennsylvania, Philadelphia, PA, USA).
Light treatment.
Test irradiations were performed using a halogen lamp (100 W, 12 V; Royal Philips Electronics). Blue light was obtained with a dichroic additive filter 450 nm (Edmund Optics Inc., NJ, USA) and a heat-absorbing filter C805. Red light was obtained with a combination of RG1, a dichroic 710-nm short-pass filter (PZO, Warsaw, Poland), and a C805 filter. The broad band thus obtained had an emission plateau from 642 to 668 nm and a half-band width of 58 nm. Unless otherwise stated, the filters were from Schott (Jena, Germany). Photon fluence rates were 36 µEm−2s−1 for strong light and 0.8 µEm−2s−1 for weak light. Neutral-density filters were used to reduce fluence rates to the required levels. Leaves were irradiated for 30 min in a moisture chamber covered with a transparent foil to prevent loss of water from the samples.
Fixation and staining.
The lower epidermis was removed from the leaves. Subsequently they were cut into pieces of about 2 mm × 3 mm and infiltrated with water in a syringe before light treatment was performed. Following irradiation the samples were infiltrated in a syringe with a fixing solution. The fixing solution comprised ASB (actin-stabilization buffer; 50 mM Pipes, 10 mM EGTA and 5 mM MgSO4·7H2O) with 2% paraformaldehyde (Sigma), 1% (w/v) DMSO (Sigma), 1 tablet (in 50 ml) of a proteinase inhibitor cocktail (Roche) and 2 µM Na2-ATP (Sigma). The samples were incubated for 2 h in the fixing solution at 16°C and then washed in ASB. After rinsing, the specimens were incubated for 1 h in 1:10 times diluted primary antibodies (Smooth and Skeletal, Sigma) at 16°C and then, after rinsing in ASB, for 1 h in 1:200 diluted secondary antibodies (Anti Rabbit FITC conjugated, Sigma) at 16°C.
The cell wall constitutes a barrier to the use of immunofluorescence techniques to visualize proteins in plant cells. Digesting enzymes loosening the cell wall are commonly used to introduce antibodies into the cell. Another, perhaps less invasive but more time-consuming approach requires slicing and staining of fixed tissue. Peeling off the lower epidermis of Arabidopsis leaves turned out to produce enough damage to the cell wall of spongy mesophyll to enable the introduction of antibodies. On the other hand, this technique proved to be non-invasive for chloroplast redistribution, thus it could be used in the study of their mechanisms.
Currently, anti-plant-myosin antibodies are not commercially available. We compared the myosin labeling by animal antibodies (Smooth and Skeletal) with polyclonal anti-plant myosin antibodies which were a kind gift of Department of Plant Cell Biology, University of Bonn, Germany.17 The results obtained in weak and strong blue light were comparable, therefore we decided to continue the study on the light-induced relocation of myosins using commercial animal antibodies.
Confocal microscopy.
Confocal microscopy was performed using a BioFad MRC1024 system (Zeiss, formerly CellScience Division of Bio-Rad Laboratories, Hemel Hempstead, UK) with an inverted Nikon Diaphot 300 microscope (Nikon). The microscope was equipped with a x60 PlanApo 1.4 NA (numerical aperture) oilimmersion lens and a 100 mW argon ion air-cooled laser (ITL). Two fluorescence detection channels were used: one for autofluorescence of chlorophyll (with a 585 low-pass filter) and the second for FITC (with a 540DF30 filter). Both fluorescence emissions were excited with 488 nm. In the images the signal intensities have been adjusted for optimal presentation.
Disussion
Myosins (VIII and XI) have been recently shown in the tissues of maize and Arabidopsis, and on isolated chloroplasts by labeling with plant anti-myosin antibodies.19,21 Within the context of chloroplast movement, myosins were labeled with antibodies directed against bovine myosins in Lemna trisulca fronds.22 They have been located on chloroplast surfaces but their behavior in light has not been investigated.
Our experiments focused on the reorganization of myosins under specific light conditions causing directional chloroplast movements. In Arabidopsis, chloroplast responses to light have been characterized by Trojan and Gabryś using a photometric method.24 Blue light fluence-rates which activate opposing responses of chloroplasts (accumulation and avoidance), appear also to induce changes in myosin distribution in the cell. Moreover, this relocalization is reversible, which indicates that the light signal inducing the chloroplast responses may indeed be directed to myosins.
In a recent work we have shown that blue light did not affect the actin architecture in Arabidopsis and Nicotiana.7 The present work provides evidence that myosins are the main target for the blue-light chloroplast-orientation signal. It also confirms the existence of at least two different mechanisms of chloroplast movement, one operating in higher terrestrial plants and another one in algae and water plants.25
In multi-layered tissue it is barely possible to provide good heat dissipation. Thus, an effect observed in mesophyll irradiated with strong blue light might be due to local heating with the ensuing thermal gradients in the cell. Localization of myosins at the chloroplast surface might also be controlled by photosynthesis. In that case, blue and red light would act similarly. The results obtained with red light show however that myosin displacement is blue light specific, i.e., that it is associated with the activation of a specific blue-light photoreceptor.
The proposed role of myosins is further supported by the results obtained with Arabidopsis chloroplast movement mutants. In phot2 mutant plants, chloroplasts show the accumulation, not avoidance, response in strong blue light.5 Accordingly, in the absence of the strong light-mediating photoreceptor, myosins are found on chloroplast surfaces, as under weak blue light. This suggests the involvement of phot2 in the myosin reorganization i.e., points to a new role for phototropin2. In contrast to the results obtained for phot2, the phot1 mutant showed an undisturbed relocalization of myosins, indistinguishable from the images obtained in wild type. This confirms that the mechanisms of accumulation and avoidance responses differ fundamentally in blue-sensitive species.25,26 More data are needed to characterize these mechanisms in detail.
Taken together, our findings shed new light on the final step in the light-signal transduction pathway, and on the function of myosins in chloroplast movements.
Acknowledgements
We wish to thank Dr. Jurek Dobrucki (Division of Cell Biophysics, Faculty of Biotechnology, Jagiellonian University) for the use of the confocal microscope. We are deeply indebted to Professors Dieter Volkmann and Frantisek Baluska form Department of Plant Cell Biology, Institute of Cell Molecular Biology, University of Bonn for their kind gift of anti-plant myosin antibodies. This work was supported by grant No. PBZ/110/P04/2004 from the State Committee for Scientific Research.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4509
Supplementary Material
References
- 1.Haupt W. Chloroplast movement: From phenomenology to molecular biology. Prog Bot. 1999;60:3–35. [Google Scholar]
- 2.Kagawa T, Suetsugu N. Photometrical analysis with photosensory domains of photoreceptors in green algae. FEBS Lett. 2007;581:368–374. doi: 10.1016/j.febslet.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 3.Izutani Y, Takagi S, Nagai R. Orientation movements of chloroplasts in Vallisneria epidermal cells: Different effects of light at low-and high-fluence rate. Photochem Photobiol. 1990;51:105–111. [Google Scholar]
- 4.Meske V, Ruppert V, Hartmann E. Structural basis for the red light induced repolarization of tip growth in caulonema cells of Ceratodon purpureus. Protoplasma. 1996;192:189–198. [Google Scholar]
- 5.Jarillo JA, Gabryś H, Capel J, Alonso JM, Ecke JR, Cashmore AR. Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature. 2001;410:952–954. doi: 10.1038/35073622. [DOI] [PubMed] [Google Scholar]
- 6.Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K. Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA. 2001;98:6969–6974. doi: 10.1073/pnas.101137598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krzeszowiec W, Rajwa B, Dobrucki J, Gabryś H. Actin cytoskeleton in Arabidopsis thaliana under blue and red light. Biol Cell. 2007;99:251–260. doi: 10.1042/BC20060077. [DOI] [PubMed] [Google Scholar]
- 8.Cox G, Hawes CR, van der Lubbel L, Juniper BE. High-voltage electron microscopy of whole, critical-point dried plant cells. II. Cytoskeletal structures and plastid motility in Selaginella. Protoplasma. 1987;140:173–186. [Google Scholar]
- 9.Dong XJ, Ryu JH, Takagi S, Nagai R. Dynamic changes in the organization of microfilaments associated with the photocontrolled motility of chloroplasts in epidermal cells of Vallisneria. Protopasma. 1996;195:18–24. [Google Scholar]
- 10.Kadota A, Wada M. Photoinduction of formation of circular structures by microfilaments on chloroplasts during intracellular orientation in protonemal cells of fern Adiantum capillus-veneris. Protoplasma. 1992;167:97–107. [Google Scholar]
- 11.Berg JS, Powell BC, Cheney RE. A millennial myosin census. Mol Biol Cell. 2001;12:780–794. doi: 10.1091/mbc.12.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy SN, Day IS. Analysis of the myosins encoded in the recently completed Arabidopsis thaliana genome sequence. Genome Biol. 2001;7 doi: 10.1186/gb-2001-2-7-research0024. 0024.1-.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bezanilla M, Horton AC, Sevener HC, Quatrano RS. Phylogenetic analysis of new plant myosin sequences. J Mol Evol. 2003;57:229–239. doi: 10.1007/s00239-003-2469-7. [DOI] [PubMed] [Google Scholar]
- 14.Baluska F, Barlow PW, Volkmann D. Actin and myosin VIII in developing root cells. In: Staiger CJ, Baluska F, Volkmann FD, Barlow PW, editors. Actin: A Dynamic Framework for Multiple Plant Cell Functions. Dordrecht: Kluwer Academic Publishers; 2000. pp. 457–476. [Google Scholar]
- 15.Reichelt S, Kendrick-Jones J. Myosins. In: Staiger CJ, Baluska F, Volkmann FD, Barlow PW, editors. Actin: A Dynamic Framework for Multiple Plant Cell Functions. Dordrecht: Kluwer Academic Publishers; 2000. pp. 29–44. [Google Scholar]
- 16.Overall RL, White RG, Blackman LM, Radford JE. Actin and Myosin in plasmodesmata. In: Staiger CJ, Baluska F, Volkmann FD, Barlow PW, editors. Actin: A Dynamic Framework for Multiple Plant Cell Functions. Dordrecht: Kluwer Academic Publishers; 2000. pp. 497–515. [Google Scholar]
- 17.Reichelt S, Knight AE, Hodge TP, Baluska F, Samaj J, Volkmann D, Kendrick-Jones J. Characterization of the unconventional myosin VIII in plant cells and its localization at the post-cytokinetic cell wall. Plant J. 1999;19:555–567. doi: 10.1046/j.1365-313x.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- 18.Volkmann D, Mori T, Tirlapur UK, Koenig K, Fujiwara T, Kendrick-Jones J, Baluska F. Unconventional myosins of the plant-specific class VIII: Endocytosis, cytokinesis, plasmodesmata/pit-fields, and cell-to-cell coupling. Cell Biol Inter. 2003;27:289–291. doi: 10.1016/s1065-6995(02)00330-x. [DOI] [PubMed] [Google Scholar]
- 19.Wojtaszek P, Anielska-Mazur A, Gabryś H, Baluska F, Volkmann D. Recruitment of myosin VIII towards plastid surfaces is root cap-specific and provides the evidence for actomyosin involvement in root osmosensing. Funct Plant Biol. 2005;32:721–736. doi: 10.1071/FP05004. [DOI] [PubMed] [Google Scholar]
- 20.Holweg C, Nick P. Arabidopsis myosin XI mutant is defective in organelle movement and polar auxin transport. Proc Natl Acad Sci USA. 2004;101:10488–10493. doi: 10.1073/pnas.0403155101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Wang Z, Pesacreta TC. A subclass of myosin XI is associated with mitochondria, plastids, and the molecular chaperone TCP-1a in maize. Cell Motil Cytosk. 2004;57:218–232. doi: 10.1002/cm.10168. [DOI] [PubMed] [Google Scholar]
- 22.Malec P, Rinaldi RA, Gabryś H. Light-induced chloroplast movements in Lemna trisulca: Identification of the motile system. Plant Sci. 1996;120:127–137. [Google Scholar]
- 23.Liebe S, Menzel D. Actomyosin-based motility of endoplasmic reticulum and chloroplasts in Vallisneria mesophyll cells. Biol Cell. 1995;85:207–222. doi: 10.1016/0248-4900(96)85282-8. [DOI] [PubMed] [Google Scholar]
- 24.Trojan A, Gabryś H. Chloroplast distribution in Arabidopsis thaliana (L.) depends on light conditions during growth. Plant Physiol. 1996;111:419–425. doi: 10.1104/pp.111.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabryś H. Blue light-induced orientation movements of chloroplasts - Recent progress. Acta Phys Plant. 2004;26:473–478. [Google Scholar]
- 26.Grabalska M, Malec P. Blue light-induced chloroplast phototranslocations in Lemna trisulca L. (Duckweed) are controlled by two separable cellular mechanisms as suggested by different senstivity to wortmannin. Photochem Photobiol. 2004;79:343–348. doi: 10.1562/le-03-16.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.