Abstract
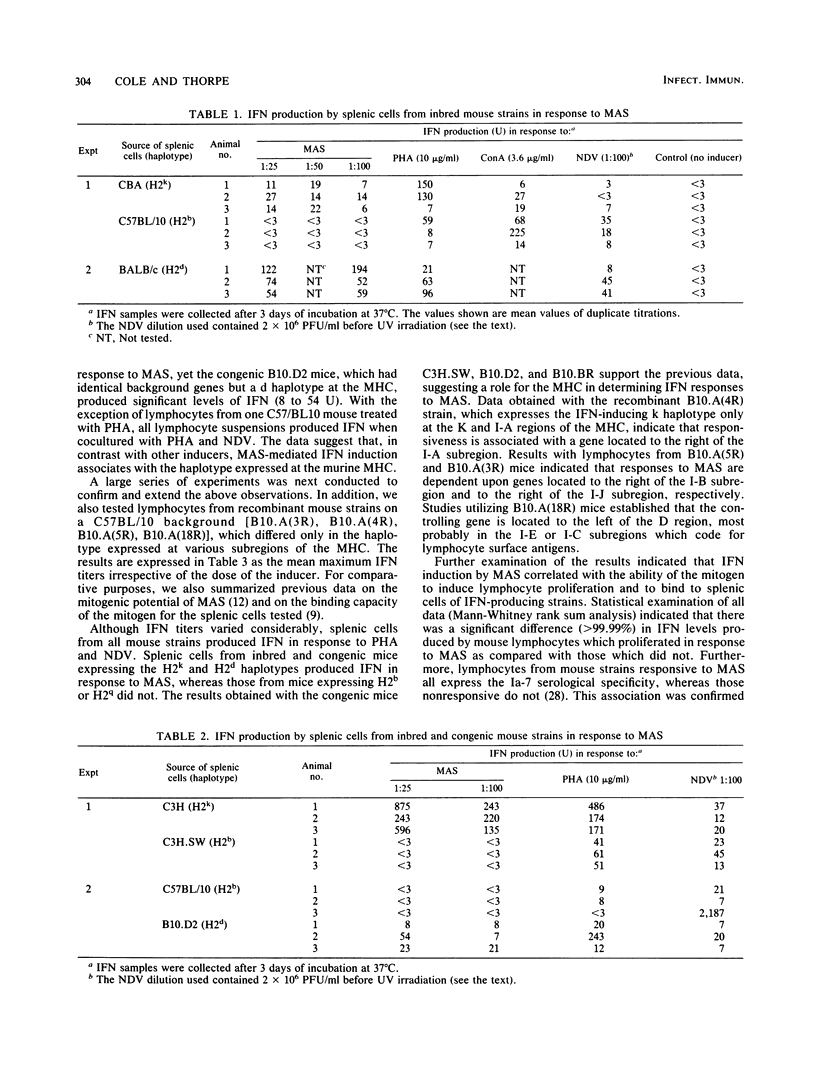
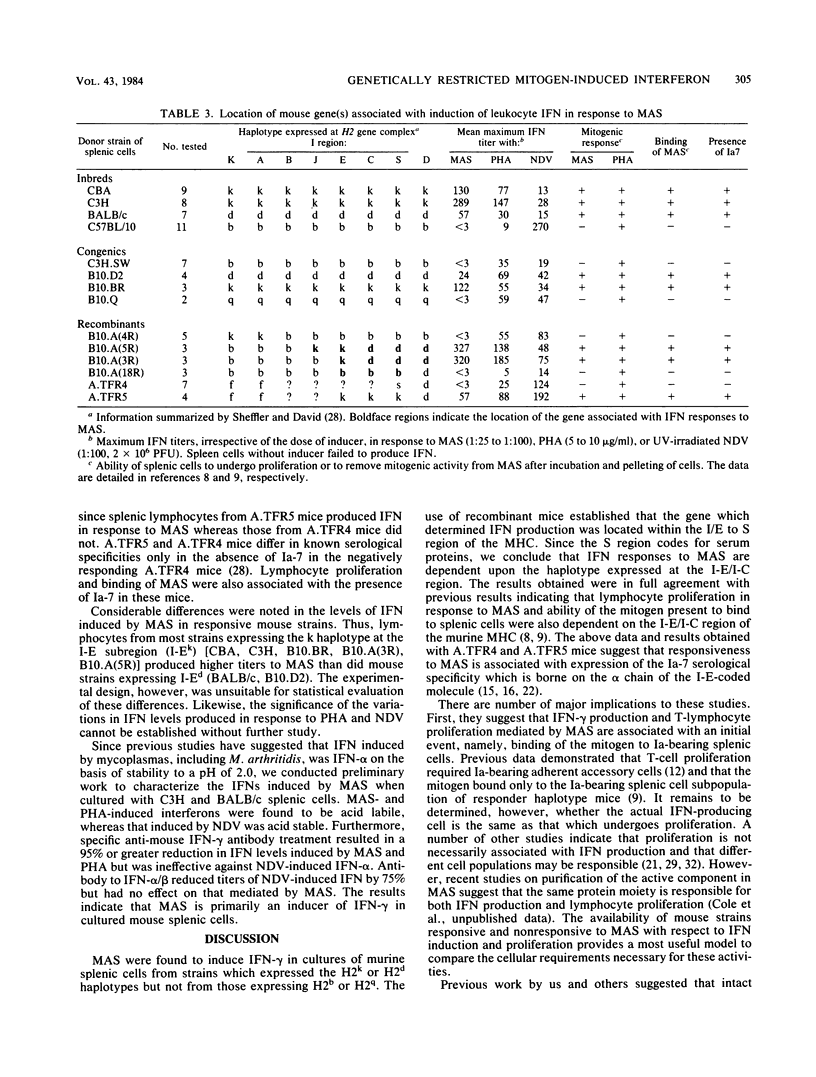
Cell-free supernatants from cultures of Mycoplasma arthritidis induced significant levels of interferon when cocultured with murine splenic cells. On the basis of physicochemical characteristics and antibody neutralization studies, the antiviral substance was identified as gamma interferon. Use of inbred and congenic mouse strains established that splenic cells from mice expressing the H2k and H2d haplotypes produced interferon in response to M. arthritidis culture supernatants, but those from mice with H2b and H2q haplotypes did not. Further studies with recombinant mouse strains established that interferon induction by the mycoplasma supernatant was associated with the haplotype expressed at the I-E/I-C subregion of the murine major histocompatibility complex. The specificity seen for interferon induction was identical with that reported earlier for induction of cytotoxic lymphocytes and for lymphocyte proliferation in response to the mitogen. All of these reactions appear to be dependent upon binding of the mitogen to specific I-E/I-C region-coded products present on splenic cell surfaces. The observations presented introduce the concept that microbial mitogens or their lymphokine products might modify immune responses and defense mechanisms of the naive host in a genetically restricted manner.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge K. E., Cole B. C., Ward J. R. Mycoplasma-dependent activation of normal lymphocytes: induction of a lymphocyte-mediated cytotoxicity for allogeneic and syngeneic mouse target cells. Infect Immun. 1977 Nov;18(2):377–385. doi: 10.1128/iai.18.2.377-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron S., Blalock J. E., Dianzani F., Fleischmann W. R., Jr, Georgiades J. A., Johnson H. M., Stanton G. J. Immune interferon: some properties and functions. Ann N Y Acad Sci. 1980;350:130–144. doi: 10.1111/j.1749-6632.1980.tb20614.x. [DOI] [PubMed] [Google Scholar]
- Beck J., Brunner H., Marcucci F., Kirchner H., Wietzerbin J. Induction of interferon by mycoplasmas in mouse spleen cell cultures. J Interferon Res. 1982;2(1):31–36. doi: 10.1089/jir.1982.2.31. [DOI] [PubMed] [Google Scholar]
- Birke C., Peter H. H., Langenberg U., Müller-Hermes W. J., Peters J. H., Heitmann J., Leibold W., Dallügge H., Krapf E., Kirchner H. Mycoplasma contamination in human tumor cell lines: effect on interferon induction and susceptibility to natural killing. J Immunol. 1981 Jul;127(1):94–98. [PubMed] [Google Scholar]
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Aldridge K. E., Ward J. R. Mycoplasma-dependent activation of normal lymphocytes: mitogenic potential of mycoplasmas for mouse lymphocytes. Infect Immun. 1977 Nov;18(2):393–399. doi: 10.1128/iai.18.2.393-399.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Daynes R. A., Ward J. R. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. I. Transformation is associated with an H-2-linked gene that maps to the I-E/I-C subregion. J Immunol. 1981 Nov;127(5):1931–1936. [PubMed] [Google Scholar]
- Cole B. C., Daynes R. A., Ward J. R. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. III. Ir gene control of lymphocyte transformation correlates with binding of the mitogen to specific Ia-bearing cells. J Immunol. 1982 Oct;129(4):1352–1359. [PubMed] [Google Scholar]
- Cole B. C., Overall J. C., Jr, Lombardi P. S., Glasgow L. A. Induction of interferon in ovine and human lymphocyte cultures by mycoplasmas. Infect Immun. 1976 Jul;14(1):88–94. doi: 10.1128/iai.14.1.88-94.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Overall J. C., Jr, Lombardi P. S., Glasgow L. A. Mycoplasma-mediated hyporeactivity to various interferon inducers. Infect Immun. 1975 Dec;12(6):1349–1354. doi: 10.1128/iai.12.6.1349-1354.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Sullivan G. J., Daynes R. A., Sayed I. A., Ward J. R. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. II. Cellular requirements for T cell transformation mediated by a soluble Mycoplasma mitogen. J Immunol. 1982 May;128(5):2013–2018. [PubMed] [Google Scholar]
- Cole B. C., Thorpe R. N. Induction of human gamma interferons by a mitogen derived form Mycoplasma arthritidis and by Phytohemagglutinin: differential inhibition with monoclonal anti-HLA.DR antibodies. J Immunol. 1983 Nov;131(5):2392–2396. [PubMed] [Google Scholar]
- Cook R. G., Vitetta E. S., Uhr J. W., Capra J. D. Structural studies on the murine Ia alloantigens--III. Tryptic peptide comparisons of allelic products of the I-E/C sub-region. Mol Immunol. 1979 Jan;16(1):29–35. doi: 10.1016/0161-5890(79)90024-5. [DOI] [PubMed] [Google Scholar]
- Cook R. G., Vitetta E. S., Uhr J. W., Capra J. D. Structural studies on the murine Ia alloantigens. V. Evidence that the structural gene for the I-E/C beta polypeptide is encoded within the I-A subregion. J Exp Med. 1979 Apr 1;149(4):981–986. doi: 10.1084/jem.149.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daynes R. A., Novak J. M., Cole B. C. Comparison of the cellular requirements for human T cell transformation by a soluble mitogen derived from Mycoplasma arthritidis and concanavalin A. J Immunol. 1982 Sep;129(3):936–938. [PubMed] [Google Scholar]
- Dietz J. N., Cole B. C. Direct activation of the J774.1 Murine macrophage cell line by mycoplasma arthritidis. Infect Immun. 1982 Aug;37(2):811–819. doi: 10.1128/iai.37.2.811-819.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golightly-Rowland L., Cole B. C., Ward J. R., Wiley B. B. Effect of Animal Passage on Arthritogenic and Biological Properties of Mycoplasma arthritidis. Infect Immun. 1970 Jun;1(6):538–545. doi: 10.1128/iai.1.6.538-545.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J. A., Yeh T. J., Overall J. C., Jr Rapid, quantitative, semiautomated assay for virus-induced and immune human interferons. J Clin Microbiol. 1980 Sep;12(3):433–438. doi: 10.1128/jcm.12.3.433-438.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerne P. A., Piguet P. F., Vassalli P. Positively selected Lyt-2+ and Lyt-2- mouse T lymphocytes are comparable, after Con A stimulation, in release of IL 2 and of lymphokines acting on B cells, macrophages, and mast cells, but differ in interferon production. J Immunol. 1983 May;130(5):2225–2230. [PubMed] [Google Scholar]
- Jones P. P., Murphy D. B., McDevitt H. O. Two-gene control of the expression of a murine Ia antigen. J Exp Med. 1978 Oct 1;148(4):925–939. doi: 10.1084/jem.148.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny G. E. Heat-lability and organic solvent-solubility of mycoplasma antigens. Ann N Y Acad Sci. 1967 Jul 28;143(1):676–681. doi: 10.1111/j.1749-6632.1967.tb27713.x. [DOI] [PubMed] [Google Scholar]
- Kenny G. E. Serological comparison of ten glycolytic Mycoplasma species. J Bacteriol. 1969 Jun;98(3):1044–1055. doi: 10.1128/jb.98.3.1044-1055.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Cole B. C., Overall J. C., Jr, Glasgow L. A. Induction of interferon in mice by mycoplasmas. Infect Immun. 1974 Dec;10(6):1296–1301. doi: 10.1128/iai.10.6.1296-1301.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Cole B. C., Overall J. C., Jr, Ward J. R., Glasgow L. A. Induction of interferon in ovine leukocytes by species of mycoplasma and acholeplasma. Proc Soc Exp Biol Med. 1974 Jun;146(2):613–618. doi: 10.3181/00379727-146-38158. [DOI] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Overall J. C., Jr, Cole B. C., Glasgow L. A. Mycoplasma-associated induction of interferon in ovine leukocytes. Infect Immun. 1973 Nov;8(5):796–803. doi: 10.1128/iai.8.5.796-803.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen W. C., Dean J. H., Lucas D. O. Interferon and the cellular immune response: separation of interferon-producing cells from DNA-synthetic cells. Cell Immunol. 1973 Jan;6(1):110–122. doi: 10.1016/0008-8749(73)90011-7. [DOI] [PubMed] [Google Scholar]
- Washburn L. R., Cole B. C., Ward J. R. Chronic arthritis of rabbits induced by mycoplasmas. III. Induction with nonviable Mycoplasma arthritidis antigens. Arthritis Rheum. 1982 Aug;25(8):937–946. doi: 10.1002/art.1780250805. [DOI] [PubMed] [Google Scholar]
- Wiranowska-Stewart M., Stewart W. E., 2nd Determination of human leukocyte populations involved in production of interferons alpha and gamma. J Interferon Res. 1981 Feb;1(2):233–244. doi: 10.1089/jir.1981.1.233. [DOI] [PubMed] [Google Scholar]
- Yowell R. L., Cole B. C., Daynes R. A. Utilization of T cell hybridomas to establish that a soluble factor derived from Mycoplasma arthritidis is truly a genetically restricted polyclonal T cell activator. J Immunol. 1983 Aug;131(2):543–545. [PubMed] [Google Scholar]


