Abstract
Second messengers have a key role in linking environmental stimuli to physiological responses. One such messenger, cGMP, has long been known to be critical to many different processes in higher plants while guanylyl cyclases (GCs), enzymes that catalyse the formation of cGMP from GTP have largely remained elusive. This is somewhat surprising considering that the unicellular green alga Chlamydomonas reinhardtii contains >90 annotated GCs. We have recently shown (PLoS ONE 2(5): e449) that a recombinant cytoplasmic domain of the Arabidopsis brassinosteroid receptor AtBRI has GC activity in vitro. This finding may suggest that other leucine-rich receptor kinases such as the phystosulfokine receptor may also confer GC activity as it has a high degree of similarity in the domain that has been delineated as essential for catalysis. In addition, the discovery of increasing complexities in the molecular architecture of higher plant nucleotide cyclases (NCs) is entirely compatible with findings in Chlamydomonas where such domains appear in >20 different combinations suggesting a role in highly diverse and complex signaling events.
Key Words: nucleotide cyclase, guanylyl cyclase, cGMP, signal transduction, Arabidopsis thaliana, Chlamydomonas reinhardtii
Introduction
It is becoming increasingly clear that both cyclic nucleotides, cGMP and cAMP, and hence the generating enzymes guanylyl and adenylyl cyclases play important roles in many diverse biological processes.1–3 Here we shall focus mainly on GCs and cGMP. The latter is implicated in responses to both abiotic and biotic stresses,4,5 the gating of channels,6,7 plant hormone signal transduction8 nitric oxide (NO) dependent signaling9–11 as well as the regulation of transcription.12 We hypothesize that other processes that are also critically dependent on the second messenger cGMP remain to be discovered and that catalytic domains capable of generating cGMP from GTP are part of a growing family of highly diverse multi-domain enzymes.
Nucleotide Cyclase Domain Organizations
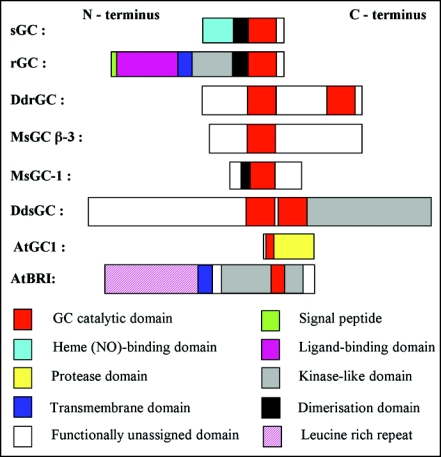
We have learnt from animal GCs that several distinctly different GC domain architectures exist (Fig. 1) that can be divided in two groups, the soluble GCs and the particulate GCs. The former typically have a highly conserved NO binding site13–15 and play a key role in NO sensing and signal transduction; the latter serve as transmembrane receptors where the GC domain located next to a kinase domain is in the cytosol. One of the best studied examples of the latter type are the Atrial Natriuretic Peptide (ANP) receptors.16 To-date there are two experimentally confirmed GCs reported in higher plants, the first is soluble but not NO sensitive.17 In this molecule the GC domain combines with a cysteine protease domain,18 a combination that is also found in Chlamydomonas reinhardtii. The second GC in higher plants19 is particulate and the brassinosteroid receptor (AtBRI1). AtBRI1 contain a leucine rich ligand binding domain, a transmembrane domain and intracellular GC and kinase domains reminiscent of ANP receptors.16 Incidentally, the wall-associated receptor kinase-like 10 precursor (At1g79680) that we have identified as a candidate GC17 has a domain architecture similar to AtBRI1, but here in place of the leucine rich ligand binding domain is the extracellular wall-binding anchor.
Figure 1.
Schematic representation of domain organization of major established GCs. sGC indicates soluble and rGC reticulate GCs. MsGC-\β\3 is a NO independent soluble GC from Manduca sexta that does not require dimerisation. DdrGC is a monomeric reticulate Dictyostelium discoideum GC with two catalytic domains and DdsGC is a monomeric soluble GC. MsGC-I shows highest sequence identity with receptor GCs throughout its catalytic and dimerisation domains but does not contain the ligand-binding, transmembrane, or kinase-like domains typical of receptor GCs. AtGC1 (At5g05930) is one of three Arabidopsis proteases with N-terminal GC catalytic motifs. Organization of the AtBRI1 (At4g39400) with the three major domains—the extracellular leucine rich repeats, the transmembrane domain and the cytosolic kinase and GC domains.
It appears that GC domains tend to combine with other domains to make multifunctional enzymes. A number of enzymes have been found to be “moonlighting” proteins with dual functions20 and it is interesting to note that a cysteine protease was recently isolated from tomato that specifically binds to a cis-element mediating elicitor induction of 1-aminocyclopropane-1-carboxylic acid synthase expression.21 A dual mechanism for the molecule was proposed whereby the protease acts enzymatically in the cytoplasm and as a signaling molecule in the nucleus.21
Further support for NC multi-domain organization comes from the unicellular green alga Chlamydomonas reinhardtii with over 100 annotated NCs. In them 22 different domain architectures with 13 different partners are found (as inspected from supfam.mrc-lmb.cam.ac.uk/SUPERFAMILY22). The partners include H-NOX, periplasmic binding protein, GAF-like, protein kinase-like domain, ATPase domain of HSP90, RNI-like, ribonuclease-H, duplicated hybrid motif, CheY-like, family A G protein-coupled receptors, homodimeric domain of SIG, SAM/pointed domain and cysteine proteinase. While experimental evidence for GC function in Chlamydomonas is lacking, it appears likely that at least some of the multi-domain GCs have cGMP dependent signaling roles and it is conceivable that many more Chlamydomonas receptors and signal transducing molecules with GC domains remain to be described. Such an expansion of functional GCs would make cGMP a second messenger capable of transmitting and/or modulating highly diverse and complex signals.
In higher plants, the current situation is different; while we have functional evidence for the biological roles and mechanisms of action of many cGMP-dependent processes, to date only two GCs have been shown to have catalytic activity. These two molecules can not plausibly account for the number or diversity of cGMP-dependent responses known in higher plants. The identification of additional GCs in higher plants is further complicated by the fact that BLAST searches with GC domains from either higher or lower eukaryotes do not yield any positive hits suggesting that higher plants have evolved unique NCs where only the catalytic centre23 may show a degree of conservation.17,19
The Family Structure of Chlamydomonas Nuceotide Cyclases
It appears that Chlamydomonas NCs are not closely related to proteins in Arabidopsis thaliana. The best e-value of a Chlamydomonas NC protein (or protein fragment) compared against Arabidopsis proteins using BLAST is 0.009 for a putative ethylene-responsive DEAD box RNA helicase (At5G63120.2). Another three Chlamydomonas NC proteins have BLAST e-values less than 0.05 but greater than 0.01. This makes the evolution of NC from Chlamydomonas to Arabidopsis a matter of speculation only.
Can we say something about the relationships between the Chlamydomonas NCs themselves? We carried out an all-against-all comparison of the set of Chlamydomonas protein fragments, annotated as NCs. These proteins showed evidence of homology (BLAST e-values < 0.01) with between 6 and 101 other proteins from the set. However, if homology was inferred transitively (i.e., “A” homologous to “B” and “B” homologous to “C”, implies “A” homologous to “C”), then all of the protein fragments in the list could be linked, suggesting that they all belong to a single, though highly diverse family.
Because some of the proteins are fragments or very distantly related (as indicated by the fact that they do not show evidence of homology in the BLAST comparison) it was not possible to investigate the relationships between them using standard phylogenetic methods. However, it was possible to make use of the BLAST results to gain a general picture of the relationships between the proteins in the family. The following analysis, which we consider could be applied to many diverse protein families, relies on the fact that proteins, or protein fragments that are closely related are likely to have similar score profiles when compared to all other proteins in the family using BLAST. For each pair of protein fragments in the set, we calculated a Pearson correlation coefficient to measure the extent to which the score profiles of the two proteins are correlated. By the end of this process each protein can be associated with a vector, showing the extent to which its BLAST score profile is correlated with the BLAST score profile of each other protein in the set. We then used an unsupervised learning technique (a self-organizing map; SOM) and hierarchical clustering, to find patterns among the vectors associated with each protein fragment.
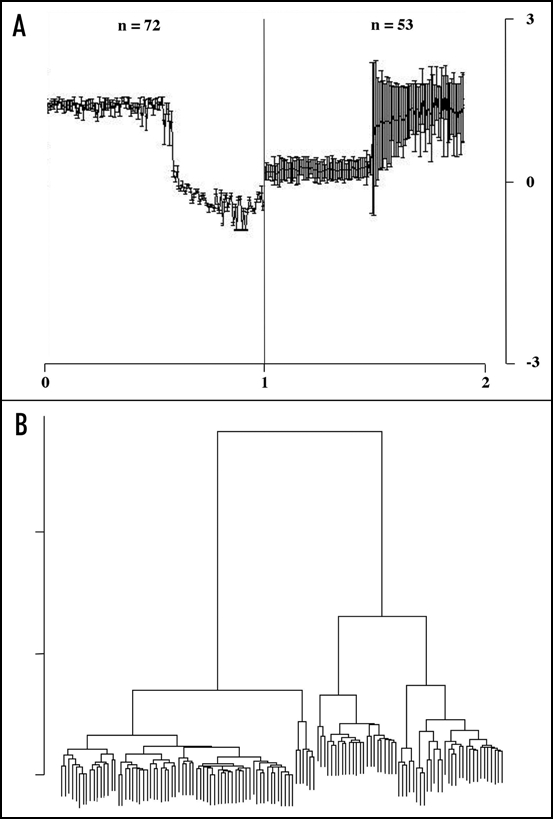
The result of the analysis indicates that although Chlamydomonas NCs show many different domain combinations, essentially they belong to just two distantly related clusters (Fig. 2). All of the protein fragments are arrayed on the x-axis of each panel (Fig. 2A). The BLAST correlation profiles of proteins on the left correspond to proteins that are closely related to the first 72 proteins on the x-axis and less closely related to the remainder. The profile on the right is reversed. These proteins have BLAST profiles that are poorly correlated with the first 72 proteins (and are therefore not likely to be closely related to these proteins) and well correlated with the remainder. The patterns discovered using the SOM are consistent with the dendrogram (Fig. 2B) inferred by applying hierarchical clustering to the vectors of BLAST profile correlations.
Figure 2.
All-against-all comparison of the Chlamydomonas protein and dendrogram. Relationships between Chlamydomonas proteins and protein-fragments annotated as NCs, inferred using BLAST scores. (A) BLAST score profiles discovered using a self-organizing map. All 125 Chlamydomonas NCs are arrayed along the x-axis on the left hand and again on the right hand panel. The left hand panel shows 72 proteins or protein fragments that all have similar BLAST score profiles (i.e., the BLAST scores of these proteins are correlated with one another). This pattern signifies high BLAST score correlation with the first 72 proteins on the x-axis, and low score correlation compared with the remainder. The remaining proteins conform to the opposite pattern (shown on the right hand panel). (B) Resulting dendrogram of hierarchical clustering applied to the BLAST score data. The cluster on the left corresponds to the 72 proteins that have the pattern shown on the right panel of (A).
No Riddle in Higher Plants
Apart from the difficulty of elucidating the evolution of higher plant NCs, one of the major outstanding questions in plant signal transduction is the link between NO sensing and the subsequent activation of a GC domain causing the generation of cGMP. In animals, soluble GCs can function as heme sensors that selectively bind NO and do so by the highly conserved H-NOX family (heme nitric oxide/oxygen-binding domain).14,15 The core H-NOX signature is: Hx{12}Px{14,16}YxSxR, where “x” stands for any amino acid and the number in the curly brackets determine the length of the gap. There are four Arabidopsis proteins that contain such a motif —At1g62580, At4g01160, At5g19160 and At5g57690—one of which (At1g62580) is annotated as chloroplast mono-oxygenase and hence implicated in gas sensing. What is more, At1g62580 also contains the core catalytic GC signature ([RKS]x[GCTHS]x{6}[TNVIL]x{3}[KR]; The residue in position 1 does the hydrogen bonding with the guanine, the amino acid in position 3 confers substrate specificity and the residues in positions 10 and 14 stabilise the transition from GTP to cGMP) that is seen in many annotated soluble GCs. It will be interesting to test if this molecule is indeed the missing links between NO and cGMP-dependent response in higher plants.
In summary, GCs in higher plants are a growing family of increasingly complex molecules and we predict that their roles will prove critical for highly diverse functions in plant growth and development as well as responses to the environment.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4788
References
- 1.Newton R, Roef L, Witters E, Van Onckelen H. Tansley review no. 106—Cyclic nucleotidesin higher plants: The enduring paradox. New Phytol. 1999;143:427–455. doi: 10.1046/j.1469-8137.1999.00478.x. [DOI] [PubMed] [Google Scholar]
- 2.Schaap P. Guanylyl cyclases across the tree of life. Front Biosci. 2005;10:1485–1498. doi: 10.2741/1633. [DOI] [PubMed] [Google Scholar]
- 3.Meier S, Gehring C. Emerging roles in plant biotechnology for the second messenger cGMP—Guanosine 3′, 5′-cyclic monophosphate. Afr J Biotech. 2006;5:1687–1692. [Google Scholar]
- 4.Donaldson L, Ludidi N, Knight MR, Gehring C, Denby K. Salt and osmotic stress cause rapid increases in Arabidopsis thaliana cGMP levels. FEBS Lett. 2004;569:317–320. doi: 10.1016/j.febslet.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Durner J, Wendehenne D, Klessig D. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci USA. 1998;95:10328–10333. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoshi T. Regulation of voltage dependence of the Kat1 channel by intracellular factors. J Gen Physiol. 1995;105:309–328. doi: 10.1085/jgp.105.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leng Q, Mercier R, Yao W, Berkowitz G. Cloning and first functional characterisation of a plant cyclic nucleotide-gated cation channel. Plant Physiol. 1999;121:753–761. doi: 10.1104/pp.121.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penson SP, Schuurink RC, Fath A, Gubler F, Jacobsen JV, Jones RL. cGMP is required for gibberellic acid-induced gene expression in barley aleurone. Plant Cell. 1996;8:2325–2333. doi: 10.1105/tpc.8.12.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neill SJ, Desikan R, Hancock JT. Nitric oxide signalling in plants. New Phytol. 2003;159:11–35. doi: 10.1046/j.1469-8137.2003.00804.x. [DOI] [PubMed] [Google Scholar]
- 10.Prado AM, Porterfield DM, Feijo JA. Nitric oxide is involved in growth regulation and reorientation of pollen tubes. Development. 2004;131:2707–2714. doi: 10.1242/dev.01153. [DOI] [PubMed] [Google Scholar]
- 11.Delledonne M. NO news is good news for plants. Curr Opin Plant Biol. 2005;8:390–396. doi: 10.1016/j.pbi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Maathuis FJM. CGMP modulates gene transcription and cation transport in Arabidopsis roots. Plant J. 2006;45:700–711. doi: 10.1111/j.1365-313X.2005.02616.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, Brandish P, Ballou D, Marletta M. A molecular basis for nitric oxide sensing by soluble guanylate cyclase. Proc Natl Acad Sci USA. 1999;96:14753–14758. doi: 10.1073/pnas.96.26.14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boon E, Huang S, Marletta M. A molecular basis for NO selectivity in soluble guanylate cyclase. Nat Chem Biol. 2005;1:53–59. doi: 10.1038/nchembio704. [DOI] [PubMed] [Google Scholar]
- 15.Boon EM, Marletta MA. Ligand discrimination in soluble guanylate cyclase and the H-NOX family of heme sensor proteins. Curr Opin Chem Biol. 2005;9:441–446. doi: 10.1016/j.cbpa.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Chinkers M, Garbers DL, Chang MS, Lowe DG, Chin H, Goeddel DV, Schulz S. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature. 1989;388:78–83. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- 17.Ludidi N, Gehring C. Identification of a novel protein with guanylyl cyclase activity in Arabidopsis thaliana. J Biol Chem. 2003;278:6490–6494. doi: 10.1074/jbc.M210983200. [DOI] [PubMed] [Google Scholar]
- 18.Ginalski K, Zemojtel T. ECEPE proteins: A novel family of eukaryotic cysteine proteinases. Trends Biochem Sci. 2004;29:524–526. doi: 10.1016/j.tibs.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Kwezi L, Meier S, Mungur L, Ruzvidzo O, Irving H, Gehring C. The Arabidopsis thaliana brassinosteroid receptor (AtBRI1) contains a domain that functions as a guanylyl cyclase in vitro. PloS One. 2007;5:e449. doi: 10.1371/journal.pone.0000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeffery CJ. Moonlighting proteins: Old proteins learning new tricks. Trends Genet. 2003;19:415. doi: 10.1016/S0168-9525(03)00167-7. [DOI] [PubMed] [Google Scholar]
- 21.Matarasso N, Schuster S, Avni A. A novel plant cysteine protease has a dual function as a regulator of 1-aminocyclopropane-1-carboxylic acid synthase gene expression. Plant Cell. 2005;17:1205–1216. doi: 10.1105/tpc.105.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madera M, Vogel C, Kummerfeld SK, Chothia C, Gough J. The SUPERFAMILY database in 2004: Additions and improvements. Nucl Acids Res. 2004;32:D235–D239. doi: 10.1093/nar/gkh117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Ruoho A, Rao V, Hurley J. Catalytic mechanisms of the adenyl and guanylyl cyclases: Modelling and mutational analysis. Proc Natl Acad Sci USA. 1997;94:13414–13419. doi: 10.1073/pnas.94.25.13414. [DOI] [PMC free article] [PubMed] [Google Scholar]