Abstract
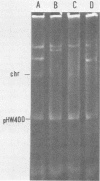
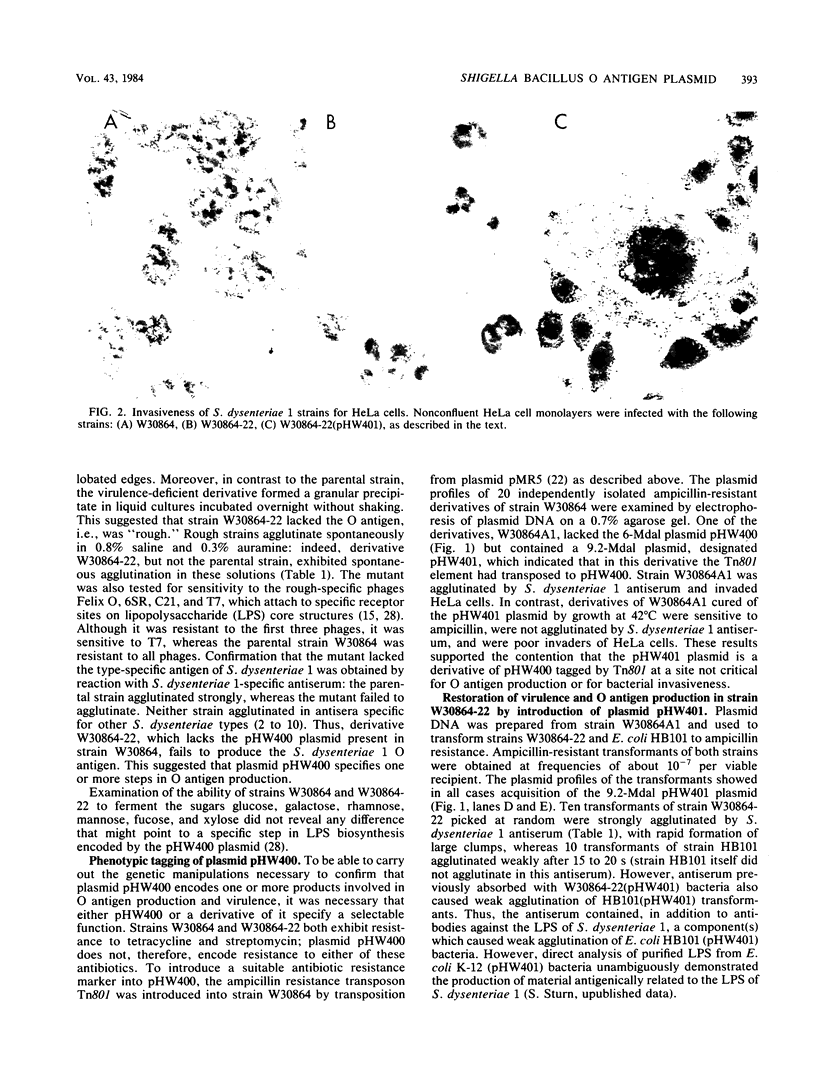
The role of plasmids in the virulence of Shigella dysenteriae 1 W30864, which contains at least five species, was investigated. By means of a standard plasmid-curing procedure, that is, bacterial cultivation at an elevated temperature, five virulence-deficient derivatives were obtained. One of these lacked a small, 6-megadalton plasmid, designated pHW400, exhibited reduced invasiveness for HeLa cells, and failed to produce the somatic antigen. Transposon tagging of the pHW400 plasmid to produce pHW401 and the transfer of this derivative into a variant of strain W30864 lacking pHW400 confirmed the conclusion that the pHW400 plasmid encodes one or more functions involved in O antigen (lipopolysaccharide) biosynthesis and bacterial virulence. A plasmid of similar size was detected in all of the three other strains of S. dysenteriae 1 examined.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER M. L., KELLER H. M., WALTERS E. W. Microscopic characteristics of colonies of Shigella flexneri 2a and 2b and their relation to antigenic composition, mouse virulence and immunogenicity. J Immunol. 1957 Mar;78(3):160–171. [PubMed] [Google Scholar]
- Elwell L. P., Shipley P. L. Plasmid-mediated factors associated with virulence of bacteria to animals. Annu Rev Microbiol. 1980;34:465–496. doi: 10.1146/annurev.mi.34.100180.002341. [DOI] [PubMed] [Google Scholar]
- FORMAL S. B., LABREC E. H., KENT T. H., FALKOW S. ABORTIVE INTESTINAL INFECTION WITH AN ESCHERICHIA COLI-SHIGELLA FLEXNERI HYBRID STRAIN. J Bacteriol. 1965 May;89:1374–1382. doi: 10.1128/jb.89.5.1374-1382.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Gemski P., Baron L. S., Labrec E. H. A Chromosomal Locus Which Controls the Ability of Shigella flexneri to Evoke Keratoconjunctivitis. Infect Immun. 1971 Jan;3(1):73–79. doi: 10.1128/iai.3.1.73-79.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Jr, Sheahan D. G., Washington O., Formal S. B. Virulence of Shigella flexneri hybrids expressing Escherichia coli somatic antigens. Infect Immun. 1972 Aug;6(2):104–111. doi: 10.1128/iai.6.2.104-111.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Sansonetti P. J., Schad P. A., Austin S., Formal S. B. Characterization of virulence plasmids and plasmid-associated outer membrane proteins in Shigella flexneri, Shigella sonnei, and Escherichia coli. Infect Immun. 1983 Apr;40(1):340–350. doi: 10.1128/iai.40.1.340-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhar M., Nuchamowitz Y., Mirelman D. Adherence of Shigella flexneri to guinea pig intestinal cells is mediated by a mucosal adhesion. Infect Immun. 1982 Mar;35(3):1110–1118. doi: 10.1128/iai.35.3.1110-1118.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecko D. J., Washington O., Formal S. B. Genetic and physical evidence for plasmid control of Shigella sonnei form I cell surface antigen. Infect Immun. 1980 Jul;29(1):207–214. doi: 10.1128/iai.29.1.207-214.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A. Bacteriophage receptors. Annu Rev Microbiol. 1973;27:205–241. doi: 10.1146/annurev.mi.27.100173.001225. [DOI] [PubMed] [Google Scholar]
- MITSUHASHI S., HARADA K., KAMEDA M. On the drug-resistance of enteric bacteria. 6. Spontaneous and artificial elimination of transmissible drug-resistance factors. Jpn J Exp Med. 1961 Apr;31:119–123. [PubMed] [Google Scholar]
- Okamura N., Nagai T., Nakaya R., Kondo S., Murakami M., Hisatsune K. HeLa cell invasiveness and O antigen of Shigella flexneri as separate and prerequisite attributes of virulence to evoke keratoconjunctivitis in guinea pigs. Infect Immun. 1983 Feb;39(2):505–513. doi: 10.1128/iai.39.2.505-513.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada Y., Nakajo M., Ogawa H. Application of experimental keratoconjunctivitis shigellosa in chemotherapeutic evaluation of rifampicin to bacillary dysentery. Jpn J Microbiol. 1972 Jul;16(4):329–332. doi: 10.1111/j.1348-0421.1972.tb00665.x. [DOI] [PubMed] [Google Scholar]
- Osada Y., Une T., Ikeuchi T., Ogawa H. Divalent cation stimulation of the cell infectivity of Shigella flexneri 2a. Jpn J Microbiol. 1975 Apr;19(2):163–166. doi: 10.1111/j.1348-0421.1975.tb00863.x. [DOI] [PubMed] [Google Scholar]
- Robinson M. K., Bennett P. M., Falkow S., Dodd H. M. Isolation of a temperature-sensitive derivative of RP1. Plasmid. 1980 May;3(3):343–347. doi: 10.1016/0147-619x(80)90047-5. [DOI] [PubMed] [Google Scholar]
- SERENY B. Experimental keratoconjunctivitis shigellosa. Acta Microbiol Acad Sci Hung. 1957;4(4):367–376. [PubMed] [Google Scholar]
- Sansonetti P. J., Hale T. L., Dammin G. J., Kapfer C., Collins H. H., Jr, Formal S. B. Alterations in the pathogenicity of Escherichia coli K-12 after transfer of plasmid and chromosomal genes from Shigella flexneri. Infect Immun. 1983 Mar;39(3):1392–1402. doi: 10.1128/iai.39.3.1392-1402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Shigella sonnei plasmids: evidence that a large plasmid is necessary for virulence. Infect Immun. 1981 Oct;34(1):75–83. doi: 10.1128/iai.34.1.75-83.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Mise K., Hashimoto H. Recombination between two IS/s flanking the r-determinant of R100-1: involvement of dor and recA gene functions in Salmonella typhimurium. J Bacteriol. 1982 Apr;150(1):113–121. doi: 10.1128/jb.150.1.113-121.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]