Abstract
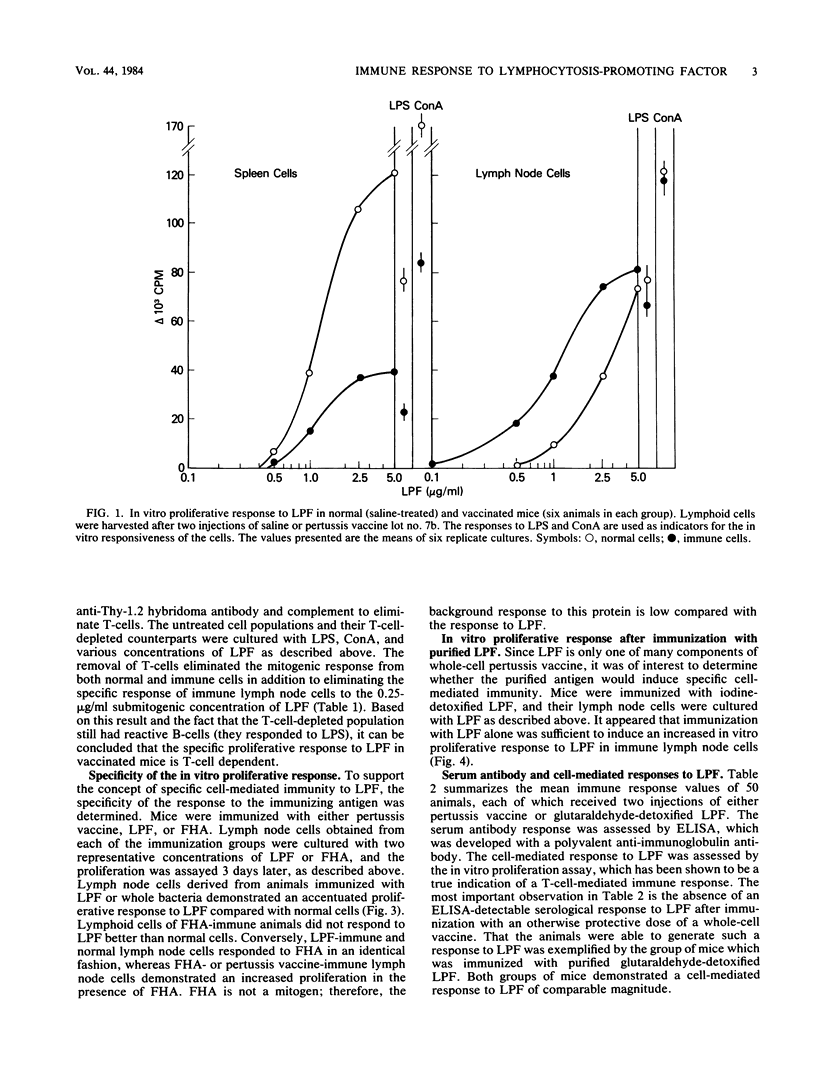
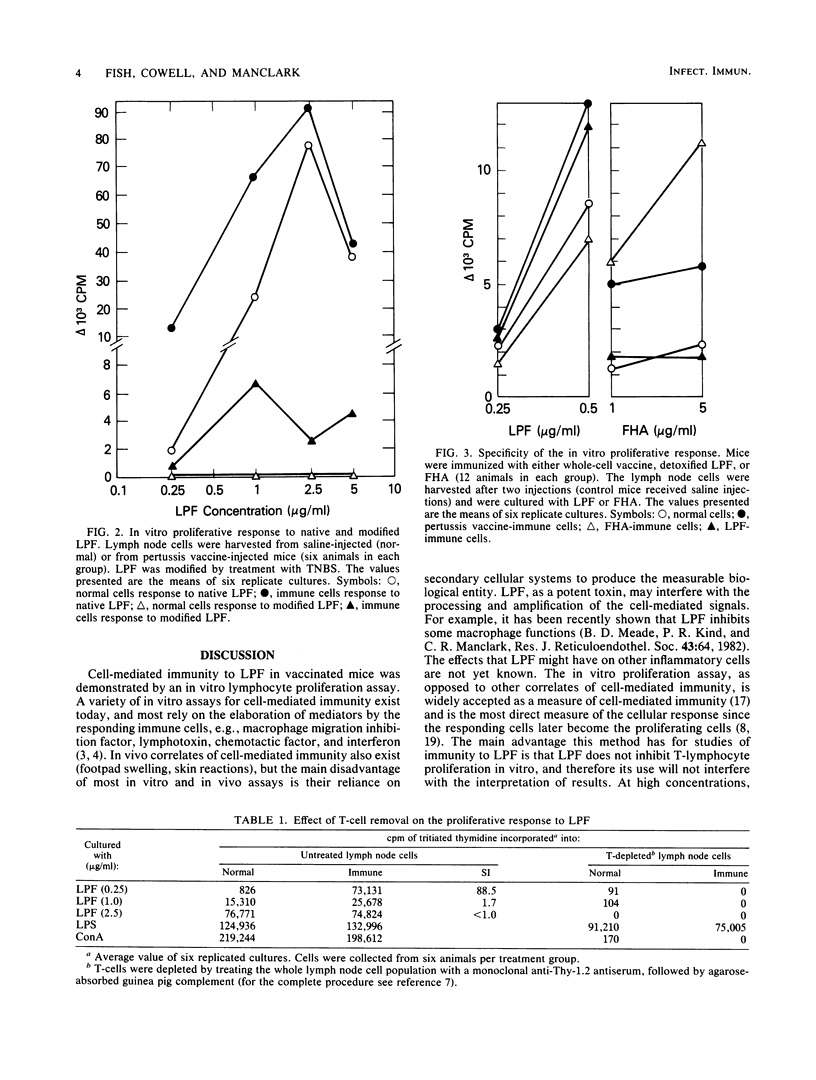
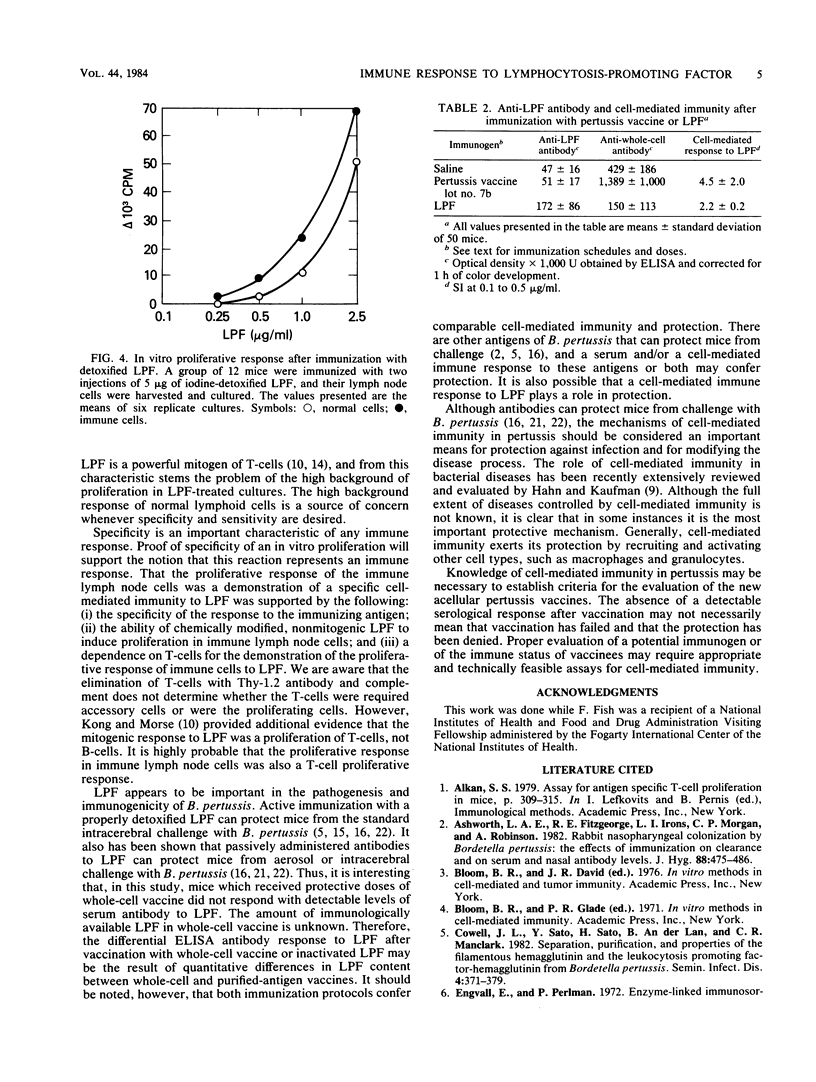
Immunization of mice with a whole-cell pertussis vaccine or with the purified, detoxified lymphocytosis-promoting factor (LPF) of Bordetella pertussis resulted in an increased in vitro proliferative response to LPF in immune lymph node cells. The proliferative response was detected above the nonspecific mitogenic activity of LPF. That the proliferative response of the immune lymph node cells was a demonstration of a specific cell-mediated immunity to LPF was supported by the following: (i) the specificity of the response to the immunizing antigen; (ii) the ability of chemically modified, nonmitogenic LPF to induce proliferation in immune lymph node cells; and (iii) a dependence on T-cells for the demonstration of the proliferative response of immune cells to LPF. Immunization of mice with protective doses of detoxified LPF resulted in serum antibody and cell-mediated responses to LPF. Immunization of mice with protective doses of whole-cell pertussis vaccine resulted in a cell-mediated response but not a detectable antibody response to LPF. The LPF of B. pertussis is believed to play an important role in pathogenesis and immunity in pertussis, and the demonstration of a cell-mediated immune response to LPF suggests a possible role for cell-mediated immunity to LPF in protection from pertussis disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashworth L. A., Fitzgeorge R. B., Irons L. I., Morgan C. P., Robinson A. Rabbit nasopharyngeal colonization by Bordetella pertussis: the effects of immunization on clearance and on serum and nasal antibody levels. J Hyg (Lond) 1982 Jun;88(3):475–486. doi: 10.1017/s0022172400070339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish F., Ziff M. The in vitro and in vivo induction of anti-double-stranded DNA antibodies in normal and autoimmune mice. J Immunol. 1982 Jan;128(1):409–414. [PubMed] [Google Scholar]
- Frbes J. T., Nakaw Y., Smith R. T. Tumor-secific immunity to chemically induced tumors. Evidence for immunologic specificity and shared antigenicity in lymphocyte responses to soluble tumor antigens. J Exp Med. 1975 May 1;141(5):1181–1200. doi: 10.1084/jem.141.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Kong A. S., Morse S. I. The in vitro effects of Bordetella pertussis lymphocytosis-promoting factor on murine lymphocytes: II. Nature of the responding cells. J Exp Med. 1977 Jan 1;145(1):163–174. doi: 10.1084/jem.145.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnroth I., Holmgren J. Protein reagent modification of cholera toxin: characterization of effects on antigenic, receptor-binding and toxic properties. J Gen Microbiol. 1975 Dec;91(2):263–277. doi: 10.1099/00221287-91-2-263. [DOI] [PubMed] [Google Scholar]
- Morse J. H., Kong A. S., Lindenbaum J., Morse S. I. The mitogenic effect of the lymphocytosis promoting factor from Bordetella pertussis on human lymphocytes. J Clin Invest. 1977 Sep;60(3):683–692. doi: 10.1172/JCI108820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J. J., Arai H., Bergman R. K., Sadowski P. L. Biological activities of crystalline pertussigen from Bordetella pertussis. Infect Immun. 1981 Sep;33(3):820–826. doi: 10.1128/iai.33.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J. J., Arai H., Cole R. L. Mouse-protecting and histamine-sensitizing activities of pertussigen and fimbrial hemagglutinin from Bordetella pertussis. Infect Immun. 1981 Apr;32(1):243–250. doi: 10.1128/iai.32.1.243-250.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman M. Pertussis toxin: the cause of the harmful effects and prolonged immunity of whooping cough. A hypothesis. Rev Infect Dis. 1979 May-Jun;1(3):401–412. doi: 10.1093/clinids/1.3.401. [DOI] [PubMed] [Google Scholar]
- STANDFAST A. F. The comparison between field trials and mouse protection tests against intranasal and intracerebral challenges with Bordetella pertussis. Immunology. 1958 Apr;1(2):135–143. [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Cowell J. L., Sato H., Burstyn D. G., Manclark C. R. Separation and purification of the hemagglutinins from Bordetella pertussis. Infect Immun. 1983 Jul;41(1):313–320. doi: 10.1128/iai.41.1.313-320.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Izumiya K., Sato H., Cowell J. L., Manclark C. R. Aerosol infection of mice with Bordetella pertussis. Infect Immun. 1980 Jul;29(1):261–266. doi: 10.1128/iai.29.1.261-266.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekura R. D., Fish F., Manclark C. R., Meade B., Zhang Y. L. Pertussis toxin. Affinity purification of a new ADP-ribosyltransferase. J Biol Chem. 1983 Dec 10;258(23):14647–14651. [PubMed] [Google Scholar]


