Abstract
Unfolded and misfolded proteins in the endoplasmic reticulum (ER) of eukaryotic cells elicit a highly conserved unfolded protein response (UPR) that leads to an increase in the capacity of the ER to deal with protein folding by hightened expression of enzymes such as chaperone and protein disulfide isomerases. However, cells die by apoptosis if the function of the ER cannot be restored in metazoans. To what extent is this mechanism evolutionarily conserved in plant cells remains to be elucidated. Emerging data from our recent study now provide compelling evidence that a conserved cell death suppressor, BAX inhibitor-1 (BI-1), plays a pivotal role as a survival factor against endoplasmic reticulum stress-mediated programmed cell death (PCD) that likely acts in parallel to the UPR pathway. This finding suggests a clear functional correlation to the predicted ER localization of AtBI1 as well as directly implicating the ER of plant cells as an important modulator of cell death activation. Furthermore, ER stress and its associated cell death in plants can be relieved by administration of chemical chaperones which have been clinically used for treatment of many human diseases linked to neurodegenerative disorders that are triggered by the dysfunction of ER homeostasis. This opens the way for future studies to decipher the mechanisms and pathways of ER-mediated PCD, and function of this pathway in plant development and stress response.
Key words: Arabidopsis, ER stress, programmed cell death, stress tolerance, unfolded protein
PCD is an essential biological process for organisms not only in normal development and ageing but also in maintenance of homeostasis and in response to stresses and pathogen insults.1,2 In animal cells, the best understood type of PCD is apoptosis, which is coordinated by multiple pathways such as mitochondria-dependent (internal), mitochondria-independent (external) and endoplasmic reticulum (ER)-mediated pathways.3 The ER is a highly dynamic organelle that has evolved specific mechanisms to ensure protein synthesis, folding and post-translational modifications as well as the maintenance of its own homeostasis.4 When proteins fold improperly and these misfolded proteins accumulate in the ER, cellular function can become compromised. This condition is referred to as “ER stress”. The UPR or ER stress response is a defense system for dealing with the accumulation of unfolded and misfolded proteins in the ER lumen via a conserved transcriptional response.5,6 However, cells die if they cannot relieve the ER stress caused by excessive and prolonged inputs, and apoptosis is induced via activation of caspases, cytochrome c release and DNA fragmentation.6,7 In animal system, accumulating evidence has suggested that both mitochondria-dependent and -independent cell death pathways likely mediate apoptosis in response to ER stress.7 In addition, members of the BCL-2 protein family are found in multiprotein complexes at the ER, likely regulating diverse cellular processes including autophagy, calcium homeostasis and calcium-dependent cell death, and the unfoldedprotein response.8–12 Thus, BCL-2-related proteins do not only serve as the “anti- or pro-cell death switch”, but they also have alternative functions in essential cellular processes. However, which molecular components of these pathways control plant PCD still remains to be clarified, as plant genomes do not contain any structural homologues to members of the BCL-2 family found in metazoans.
To obtain molecular and physiological insight into the process of ER stress in plants, we used the drug tunicamycin (TM) that is widely used as an inducer of ER stress in animals, fungi and plants. This drug inhibits N-linked glycosylation and disulfide bond formation, thereby leading to the accumulation and aggregation of improperly folded proteins in the ER. Earlier studies showed that treatment with TM can kill suspension cultured cells or young plants rapidly.13–15 However, whether TM kills plants by a necrotic or programmed mechanism (i.e., PCD) remained obscure. We first studied the impact of ER stress on Arabidopsis seedlings and found that TM perturbs root development including elongation of primary and secondary roots and formation of lateral roots and root hair cells in a dose-dependent manner, concomitantly with the loss of cell viability and induction of PCD phenotypes.16 As a consequence, seedlings die within 3 days following TM treatment. Notably, we showed that such lethal effect of TM can be relieved by an administration of two different chemical chaperones, 4-phenyl butyric acid (PBA) and tauroursodeoxycholic acid (TUDCA), even in the presence of a lethal dose of TM (0.5 µg ml−1). These results provide evidence that TM induces root growth defect and PCD via defected protein folding that leads to ER stress. However, PBA was found to cause partial growth arrest of seedlings with yellowish leaves at doses that we used (1 mM or more) in the absence of TM. In contrast, apparent growth defect was not observed with TUDCA even at a higher dose (5 mM). TUDCA would thus appear to be a better agent to dissect the mechanisms of ER stress response and PCD in Arabidopsis. As supporting evidence to the result obtained with TM treatment, we also examined the impact of two other ER stress inducers, cyclopiazonic acid (CPA, a calcium pump inhibitor) and the proline analogue L-azetidine-2-carboxylic acid (AZC), on Arabidopsis seedlings. The data collectively indicated that those ER stress-inducing agents induce root growth defect in Arabidopsis seedlings accompanied by induction of PCD (our unpublished results). Using three types of pharmacological ER stress inducers, we thus presented a better framework for understanding how ER stress affects growth and survival of Arabidopsis seedlings. However, their distinct modes of action likely contribute to quantitative differences in the phenotypes observed.
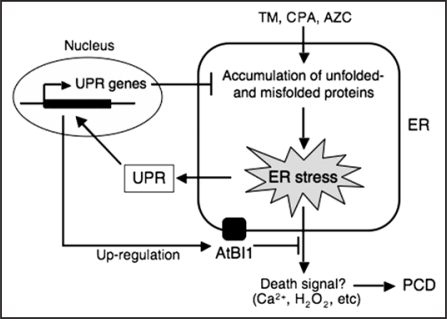
BI-1 is an evolutionally conserved protein that predominantly localizes to the ER membrane and acts as a broad spectrum cell death suppressor in mammals, fungi and plants.17,18 Overexpression of BI-1 proteins from a variety of origins was shown to suppress Bax-induced and abiotic stress-induced cell death in numerous eukaryotes. In Arabidopsis, BI-1 was shown by genetic analysis to play a role as attenuator of mycotoxin- and heat shock stress-induced cell death.19 Our more recent study demonstrated an involvement of AtBI1 in the ER stress response and its related cell death pathway in Arabidopsis.16 Our data collectively suggest that ER stressmediated PCD can be manipulated by the disruption of AtBI1 or overexpressing AtBI1 proteins, resulting in accelerated or attenuated PCD, respectively. Thus, the level of AtBI1 can be thought of serving as a rheostat that functions to gauge the threshold of misfolded proteins in the ER for PCD activation. Here, we propose a simple working model for the role of AtBI1 as a survival factor during ER stress that could account for the results reported in our paper (see Fig. 1). ER stress agents such as TM, CPA and AZC all trigger the accumulation of unfolded and misfolded proteins in the ER lumen. This condition is referred to as “ER stress” and involves the UPR that induce a set of UPR genes such as BIP2 and CRT1 to restore ER homeostasis. BI-1 is also induced transcriptionally through a similar pathway. This upregulation should increase the amount of AtBI1 on the ER membrane and support its biological activity as a survival factor involved in the anti-PCD pathway. However, under persistent or intense ER stress conditions, the UPR will not be sufficient to regain ER homeostasis and misfolded proteins accumulate. Cell death is ultimately activated at a certain threshold of misfolded proteins, likely involving increased calcium release from the ER lumen into the cytosol and accumulation of reactive oxygen species such as H2O2.20–23 In the atbi1 mutants or AtBI1 overexpressors, TM-induced cell death is accelerated or delayed, consistent with the proposed cell death rheostat function for AtBI1 in the ER. Thus, the anti-PCD function of AtBI1 may provide the necessary time for the UPR to remedy stresses perceived in the ER. Since alterations in AtBI1 levels do not appear to significantly impact expression of some of the key genes in the UPR pathway, we concluded that AtBI1 functions in parallel to the UPR and is not involved in the signalling of the UPR per se. Overall, our results indicate that AtBI1 is a critical survival factor for suppression of PCD induced by ER stress, thereby allowing the UPR sufficient time to re-establish proper homeostasis in the cell.
Figure 1.
Working model for a role of AtBI1 in ER stress-induced PCD. See the text for more detail. AtBI1, Arabidopsis Bax inhibitor-1; AZC, L-azetidine-2-carboxylic acid; CPA, cyclopiazonic acid; PCD, programmed cell death; TM, tunicamycin; UPR, unfolded protein response.
In plants, plant PCD is still poorly understood in terms of molecular mechanisms and our current view still largely relies on analogies from animal systems. In addition, the fundamental question regarding how ER stress response pathway is regulated in plant cells still remains to be resolved,24 while the possibility that ER stress signaling may be a common feature for multiple types of plant PCD with different morphotypes have been raised.16 The latter is especially interesting in regard to explaining how AtBI1 may be able to suppress plant PCD activated by biotic and abiotic inducers.19 Further mechanistic analysis of ER stress response and AtBI1-dependent PCD pathways will certainly increase our knowledge in this field.
Abbreviations
- AtBI1
Arabidopsis Bax inhibitor-1
- AZC
L-azetidine-2-carboxylic acid
- BI-1
Bax Inhibitor-1
- CPA
cyclopiazonic acid
- ER
endoplasmic reticulum
- PBA
4-phenylbutyric acid
- PCD
programmed cell death
- TM
tunicamycin
- TUDCA
tauroursodeoxycholic acid
- UPR
unfolded protein response
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/5709
References
- 1.Lawen A. Apoptosis-an introduction. BioEssays. 2003;25:888–896. doi: 10.1002/bies.10329. [DOI] [PubMed] [Google Scholar]
- 2.Lam E. Controlled cell death, plant survival and development. Nature Rev Mol Cell Biol. 2004;5:305–315. doi: 10.1038/nrm1358. [DOI] [PubMed] [Google Scholar]
- 3.Danial NN, Kormeyers SJ. Cell death: Critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 4.Csala M, Banhegyi G, Benedtti A. Endoplasmic reticulum: A metabolic compartment. FEBS Lett. 2006;580:2160–2165. doi: 10.1016/j.febslet.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 5.Winter J, Jacob U. Beyond transcription—New mechanisms for the regulation of molecular chaperones. Crit Rev Biochem Mol Biol. 2004;39:297–317. doi: 10.1080/10409230490900658. [DOI] [PubMed] [Google Scholar]
- 6.Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 7.Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–373. doi: 10.1038/sj.cdd.4401817. [DOI] [PubMed] [Google Scholar]
- 8.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: A control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 9.Zong WX, Li C, Hatzivassiliou G, Lindsten T, Yu QC, Yuan J, Thompson CB. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hetz C, Bernasconi P, Fisher J, Lee AH, Bassi MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Climcher LH, Kormeyer SJ. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1a. Science. 2006;312:572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 11.Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Akha AA, Raden D, Kaufman RJ. Adaptation of ER stress is mediated by different stabilities of prosurvival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:2024–2041. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 13.Koizumi N, Ujino T, Sano H, Chrispeels MJ. Overexpression of a gene that encodes the first enzyme in the biosynthesis of asparagine-linked glycans makes plants resistant to tunicamycin and obviates the tunicamycin-induced unfolded protein response. Plant Physiol. 1999;121:353–361. doi: 10.1104/pp.121.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crosti P, Malerba M, Bianchetti R. Tunicamycin and brefeldin A induce in plant cells a programmed cell death showing apoptotic features. Protoplasma. 2001;216:31–38. doi: 10.1007/BF02680128. [DOI] [PubMed] [Google Scholar]
- 15.Iwata Y, Koizumi N. Unfolded protein response followed by induction of cell death in cultured tobacco cells treated with tunicamycin. Planta. 2005;220:804–807. doi: 10.1007/s00425-004-1479-z. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe N, Lam E. BAX inhibitor-1 modulates endoplasmic reticulum-mediated programmed cell death in Arabidopsis. J Biol Chem. 2008;283:3200–3210. doi: 10.1074/jbc.M706659200. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe N, Lam E. Recent advance in the study of caspase-like proteases and Bax-inhibitor 1 in plants: their possible roles as regulator of programmed cell death. Mol Plant Pathol. 2004;5:65–70. doi: 10.1111/j.1364-3703.2004.00206.x. [DOI] [PubMed] [Google Scholar]
- 18.Hückelhoven R. BAX Inhibitor-1, an ancient cell death suppressor in animals and plants with prokaryotic relatives. Apoptosis. 2004;9:299–307. doi: 10.1023/b:appt.0000025806.71000.1c. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe N, Lam E. Arabidopsis Bax inhibitor-1 functions as an attenuator of biotic and abiotic types of cell death. Plant J. 2006;45:884–894. doi: 10.1111/j.1365-313X.2006.02654.x. [DOI] [PubMed] [Google Scholar]
- 20.Chae HJ, Kim HR, Xu C, Bailly-Maitre B, Krajewska M, Banares S, Cui J, Digicalioglu M, Ke N, Kitada S, Monosov E, Thomas M, Kress CL, Babendure JR, Tsien RY, Lipton SA, Reed JC. BI-1 regulates an apoptosis pathway linked to endoplasmic reticulum stress. Mol Cell. 2004;15:355–366. doi: 10.1016/j.molcel.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 21.Bailly-Maitre B, Fondevila C, Kaldas F, Droin N, Luciano F, Ricci JE, Croxton R, Krajewska M, Zapata JM, Kupiec-Weglinski JW, Farmer D, Reed JC. Cytoprotective gene bi-1 is required for intrinsic protection from endoplasmic reticulum stress and ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2006;103:2809–2814. doi: 10.1073/pnas.0506854103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee GH, Kim HK, Chae SW, Kim DS, Ha KC, Cuddy M, Kress C, Reed JC, Kim HR, Chae HJ. Bax inhibitor-1 regulates endoplasmic reticulum stress-associated reactive oxygen species and heme oxygenase-1 expression. J Biol Chem. 2007;282:21618–21628. doi: 10.1074/jbc.M700053200. [DOI] [PubMed] [Google Scholar]
- 23.Ihara-Ohori Y, Nagano M, Muto S, Uchimiya H, Kawai-Yamada M. Cell death suppressor Arabidopsis Bax inhibitor-1 is associated with calmodulin binding and ion homeostasis. Plant Physiol. 2007;143:650–660. doi: 10.1104/pp.106.090878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urade R. Cellular response to unfolded proteins in the endoplasmic reticulum of plants. FEBS J. 2007;274:1152–1171. doi: 10.1111/j.1742-4658.2007.05664.x. [DOI] [PubMed] [Google Scholar]