Abstract
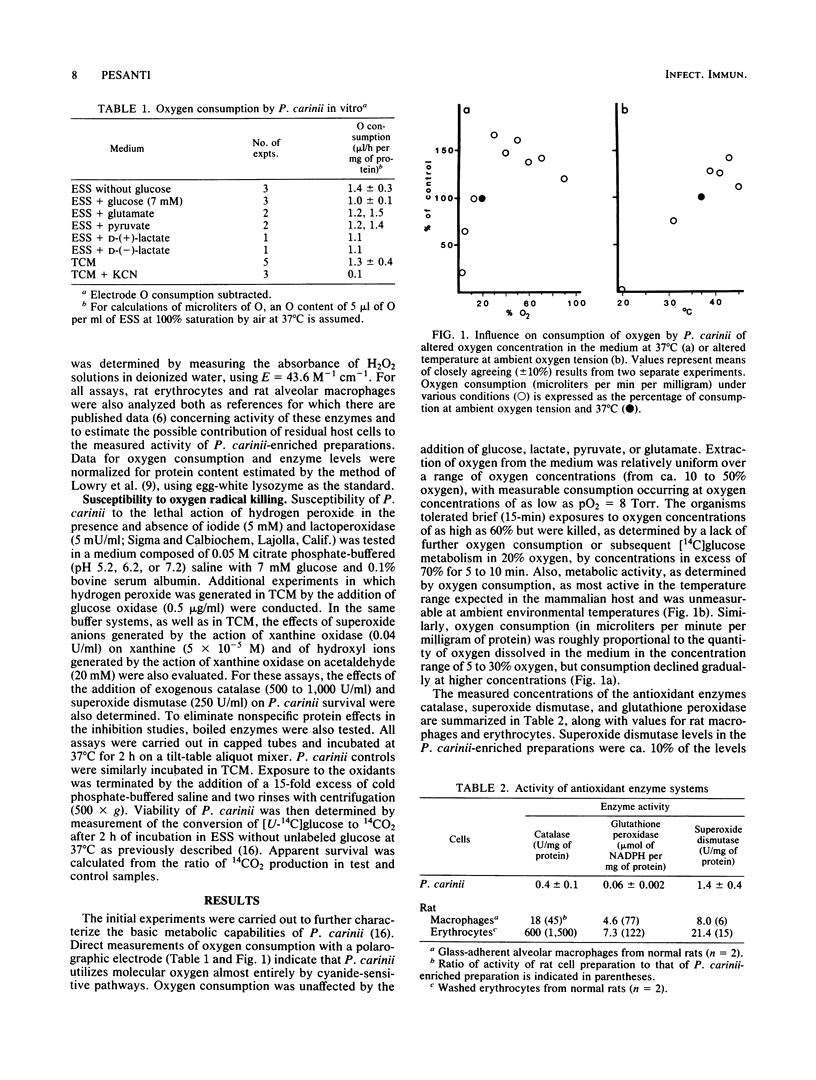
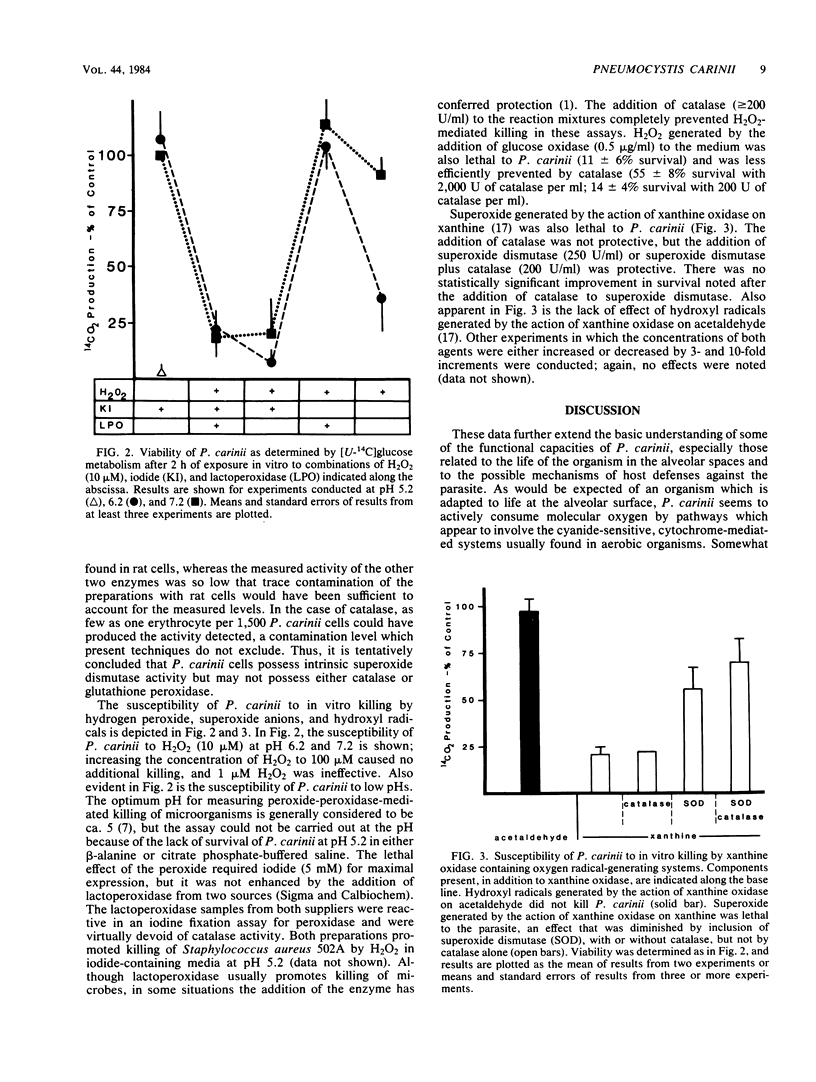
The ability of Pneumocystis carinii obtained by alveolar lavage of rats with glucocorticoid-induced pneumocystosis to utilize molecular oxygen, the concentrations of selected antioxidant enzymes, and the susceptibility of P. carinii to in vitro killing by oxygen radical-generating systems have been evaluated. As expected of an organism which has been found to convert radiolabeled glucose to CO2, the parasite utilizes molecular oxygen. No evidence for pathways of oxygen utilization other than the cytochrome pathway was found; cyanide virtually abolished oxygen consumption. Although readily detectable levels of the antioxidant enzymes superoxide dismutase, catalase, and glutathione peroxidase were present in the P. carinii preparations, only superoxide dismutase was present at levels that suggested that the activity was indeed a property of the parasite. Almost certainly, P. carinii does not possess effective concentrations of catalase. In addition, it was found that P. carinii is susceptible to the lethal actions of hydrogen peroxide and superoxide, but the parasite seems to be resistant to the effects of a hydroxyl radical-generating system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson M., Carlsson J. Lactoperoxidase and thiocyanate protect bacteria from hydrogen peroxide. Infect Immun. 1982 Jan;35(1):20–24. doi: 10.1128/iai.35.1.20-24.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff A. D., Johnston R. B., Jr, Dolen J., South M. A. Chronic granulomatous disease and Pneumocystis carinii pneumonia. Pediatrics. 1982 Jan;69(1):133–134. [PubMed] [Google Scholar]
- Chinchilla M., Frenkel J. K. Mediation of immunity to intracellular infection (Toxoplasma and Besnoitia) within somatic cells. Infect Immun. 1978 Mar;19(3):999–1012. doi: 10.1128/iai.19.3.999-1012.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig S. M., Smithwick E. M., Suntharalingam K., Good R. A. Fibroblast nitroblue tetrazolium test and the in-utero diagnosis of chronic granulomatous disease. Lancet. 1980 Jan 5;1(8158):18–19. doi: 10.1016/s0140-6736(80)90554-1. [DOI] [PubMed] [Google Scholar]
- Gottlieb M. S., Schroff R., Schanker H. M., Weisman J. D., Fan P. T., Wolf R. A., Saxon A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981 Dec 10;305(24):1425–1431. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- Higgins C. P., Baehner R. L., McCallister J., Boxer L. A. Polymorphonuclear leukocyte species differences in the disposal of hydrogen peroxide (H2O2). Proc Soc Exp Biol Med. 1978 Jul;158(3):478–481. doi: 10.3181/00379727-158-40230. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanken P. N., Minda M., Pietra G. G., Fishman A. P. Alveolar response to experimental Pneumocystis carinii pneumonia in the rat. Am J Pathol. 1980 Jun;99(3):561–588. [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal--leukocyte interaction. J Clin Invest. 1975 Mar;55(3):561–566. doi: 10.1172/JCI107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Nathan C. F. Secretion of oxygen intermediates: role in effector functions of activated macrophages. Fed Proc. 1982 Apr;41(6):2206–2211. [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967 Jul;70(1):158–169. [PubMed] [Google Scholar]
- Pedersen F. K., Johansen K. S., Rosenkvist J., Tygstrup I., Valerius N. H. Refractory Pneumocystis carinii infection in chronic granulomatous disease: successful treatment with granulocytes. Pediatrics. 1979 Dec;64(6):935–938. [PubMed] [Google Scholar]
- Pesanti E. L., Cox C. Metabolic and synthetic activities of Pneumocystis carinii in vitro. Infect Immun. 1981 Dec;34(3):908–914. doi: 10.1128/iai.34.3.908-914.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesanti E. L. Effects of bacterial pneumonitis on development of pneumocystosis in rats. Am Rev Respir Dis. 1982 Jun;125(6):723–726. doi: 10.1164/arrd.1982.125.6.723. [DOI] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Bactericidal activity of a superoxide anion-generating system. A model for the polymorphonuclear leukocyte. J Exp Med. 1979 Jan 1;149(1):27–39. doi: 10.1084/jem.149.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Inhibition of the growth of Rickettsia prowazekii in cultured fibroblasts by lymphokines. J Exp Med. 1983 Mar 1;157(3):974–986. doi: 10.1084/jem.157.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Behren L. A., Pesanti E. L. Uptake and degradation of Pneumocystis carinii by macrophages in vitro. Am Rev Respir Dis. 1978 Dec;118(6):1051–1059. doi: 10.1164/arrd.1978.118.6.1051. [DOI] [PubMed] [Google Scholar]
- Walzer P. D., Perl D. P., Krogstad D. J., Rawson P. G., Schultz M. G. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974 Jan;80(1):83–93. doi: 10.7326/0003-4819-80-1-83. [DOI] [PubMed] [Google Scholar]
- Walzer P. D., Rutledge M. E. Serum antibody responses to Pneumocystis carinii among different strains of normal and athymic mice. Infect Immun. 1982 Feb;35(2):620–626. doi: 10.1128/iai.35.2.620-626.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., Schnelle V., Armstrong D., Rosen P. P. Nude mouse: a new experimental model for Pneumocystis carinii infection. Science. 1977 Jul 8;197(4299):177–179. doi: 10.1126/science.301657. [DOI] [PubMed] [Google Scholar]
- Yoneda K., Walzer P. D. Interaction of Pneumocystis carinii with host lungs: an ultrastructural study. Infect Immun. 1980 Aug;29(2):692–703. doi: 10.1128/iai.29.2.692-703.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Ikai T. Pneumocystis carinii pneumonia: epidemiology in Japan, and cyst concentration method. Zentralbl Bakteriol Orig A. 1979 Jul;244(2-3):405–410. [PubMed] [Google Scholar]


